Abstract
Schizophrenia is generally characterized by various positive and negative symptoms that are accompanied by significant social dysfunction. Various researchers investigated the functional impairments in schizophrenia including impaired theory of mind (TOM), poor integration of affective and cognitive information, and malfunctioning of adaptive and strategic learning process. However, most of the studies were limited to simplified cognitive tests or computerized choice games that exclude real social interaction. The aim of the current study was to investigate human strategies based on the incentives and particularly the cognitive and emotional motivations of free riding. We examined the decision patterns of 41 healthy subjects (HSs) and 37 schizophrenia patients (SZ) during the public goods game (PGG), one of the games simulating human cooperation and free riding in group interactions. Strategic decision processes during the iterative binary PGG were assessed in terms of cognitive understanding, loss sensitivity, and TOM. We found that greed and loss sensitivity both motivated free-riding behavior in the HS, but that they were more vulnerable to greedy incentives than to possible loss. More significantly, the SZ clearly displayed a lower prevalence of free riding and distinct decision patterns from HS. Nonstrategic and unexpectedly low free ridings in the SZ likely arise from poor integration of cognitive and affective information. We suggest that loss sensitivity and TOM as well as cognitive understanding are involved in regulation of the free riding and cooperative behavior.
Keywords: free riding, cooperation, public goods game, strategic decision making, schizophrenia
Introduction
Schizophrenia is generally characterized by symptoms such as visual or auditory hallucinations, paranoid or bizarre delusions, and disorganized speech and thinking and is commonly accompanied by significant social dysfunction.1 In the last 2 decades, various researchers have administered cognitive tasks to individuals with schizophrenia to reveal the mechanism of their functional impairments, including impaired theory of mind (TOM)2–8and emotion processing,9–11 poor integration of affective and cognitive information,12 and anomalies in strategic decision making.13,14 However, previous studies had critical limitations in that the tasks they used were restricted to computerized choice games or simple cognitive tests that do not require any social interaction. To our knowledge, only 2 recent studies have examined the social decision-making performance of schizophrenia patients (SZ) in a game paradigm that involves human-human interaction.14,15 Both of these studies used the Ultimatum Game, one of the classical bargaining games that involve 2-person interaction but observed different response patterns to social signals from the partner (ie, unfair offer). Hence, little is known about the effect of SZ’s functional impairments on the social decision-making process (eg, cooperation or free riding).
In the field of social decision making, the public goods game (PGG) is often used to simulate human cooperation and free riding as part of a group.16,17 The proportion each player invests between private and public accounts represents the amount of the player’s cooperation. The allocation of the gathered public goods is equal regardless of the amount of each individual’s investment. Thus, in the game-theoretic view, a dominant strategy in this game to maximize one’s own profit is to free ride (ie, invest none of one’s endowment in the public account) and earn the extra money shared from the public account, while a Pareto-efficient outcome (the condition where no change in the allocation of goods can make some individuals’ payoff higher without any other individual being made worse off) is attained by all participants who invest their entire endowments in the public account.16–19 Thus, in theory, 0% of cooperation should be observed. However, various empirical studies using diverse designs (single-trial/repeated-trials/repeated single-trial; linear/binary PGG; different group sizes; and different marginal returns) of the PGG have consistently shown that people choose to contribute (cooperate) 20–40%, on average, in the first round and tend to free ride more in the later rounds (converged to nearly 0%).16–24 This emergence of nonkin cooperation has been broadly investigated, and reciprocity, group selection, and coevolutionary rule have been suggested as possible mechanisms of evolution of cooperation.25,26 Apart from the moderately observed cooperation at the start of the repeated trials, it has been emphasized that participants’ degrading cooperation through iteration might change mainly according to their strategic decisions.16,18,20 The strategic decision making between free riding and cooperation requires high cognitive functions including both social and nonsocial cognition. Thus, investigating the motivations to free ride and the internal decision process triggering cooperation and free riding is essential to estimate the group behavior and induce optimal or suboptimal solutions for allocating public goods.
Therefore, the aim of the current study was to investigate the strategic mechanism by which healthy subjects (HSs) and SZ decide to cooperate or to free ride during the iterative binary PGG. Although participants have shown similar behavioral trends in previous studies of the PGG for the last 2 decades,16–23,27,28 a well-controlled version of the PGG is critically required to investigate the motivations for cooperation and free riding and to find solutions for the social dilemma posed by the game. In this study, first, the options for cooperation were simplified by utilizing a simple binary PGG21 and providing only 2 alternatives of cooperation for simplicity: cooperation, in which a player’s entire endowment is invested in the public account, or free riding, in which a player’s entire endowment is kept for himself or herself. This form of the game involves not only binary decisions but also binary results, ie, success when the group has at least as many cooperators as the preset threshold or failure when fewer participants cooperate. Second, the major incentives for free riding can be modified and differentiated in a multiple-condition design,21 which introduced 3 differentiated conditions of PGG, emphasizing 2 main motivations of free riding: fear of being “suckered” (ie, losing money) and a desire to earn more money than others. Since the PGG itself requires a complex decision process, the researchers could assess the substitutional environments (ie, half of the social dilemma) by controlling each of them separately and found a dominant effect of greed on free-riding behavior.21 Third, to assess the involvement of TOM, affective or cognitive motivations in the mechanisms of cooperation and free riding, patients with schizophrenia were recruited to conduct the PGG. (The rationale for the experimental design was described in detail in the online supplementary material).
Methods
Subjects
Fifty SZ (mean age: 37.9 ± 6.8; M/F: 30/20) and 60 HS (mean age: 35.8 ± 7.9; M/F: 34/26) were initially recruited for the current study. The SZ were inpatients from Bugok National Hospital, Gyeongnam, South Korea, and the HS were independently recruited using the Internet and local newspaper advertisements. A comprehensive review of medical records and the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth edition, (DSM-IV) Axis I disorders29 were completed by specialists under the supervision of 2 psychiatrists. All patients met the DSM-IV criteria for schizophrenia, except for 3 patients. Among them, we excluded data obtained from 13 SZ and 19 HS who did not match the age, education, and DSM-IV criteria (see online supplementary material). The patients who were included were medicated with stable dosages of atypical antipsychotics. Positive and Negative Syndrome Scales (PANSS) positive, negative, general, and total scales were administered to all participants to assess the severity of their positive and negative symptoms.30
According to these exclusion and inclusion criteria, we finally used data from 37 SZ and 41 HS. The 2 groups were matched in terms of age (t 76 = −0.336, P = .738), sex (χ2 1 = 0.307, P = .579), and education length (t 73.567 =1.865, P = .066). The socioeconomic status difference between the 2 groups was not considered based on a previous study that showed that individual-level economic variables (including wealth) cannot explain cooperation patterns.31 Table 1 shows the demographic properties of and clinical information for the 2 groups.
Table 1.
Demographic and Clinical Characteristics of Schizophrenia Patients and Healthy Subjects
| Variables | Healthy Subjects (n = 41) | Schizophrenia (n = 37) | Significance Level | ||
| Mean | SD | Mean | SD | ||
| Age (years) | 36.1 | 7.5 | 36.6 | 6.0 | t 76 = −0.336, P = .738 |
| Sex (male/female) | 29/12 | 24/13 | χ2 1 = 0.307, P = .579 | ||
| Education (years) | 13.7 | 2.1 | 12.9 | 1.6 | t 73.567 = 1.865, P = .066 |
| Duration of illness (years) | 12.1 | 6.5 | |||
| Onset (year) | 24.5 | 6.3 | |||
| PANSS-P | 19.0 | 3.6 | |||
| PANSS-N | 20.0 | 3.4 | |||
| PANSS-G | 41.1 | 5.3 | |||
| PANSS-T | 80.1 | 9.0 | |||
Note: PANSS, Positive and Negative Syndrome Scales; P, positive; N, negative; G, general; and T, total.
Written informed consent was obtained from all participants after a description of the experimental procedure. The current study protocol and the consent forms were reviewed and approved by both the Bugok National Hospital institutional review board and the KAIST institutional review board (KH2008-01).
Experimental Procedures: PGG
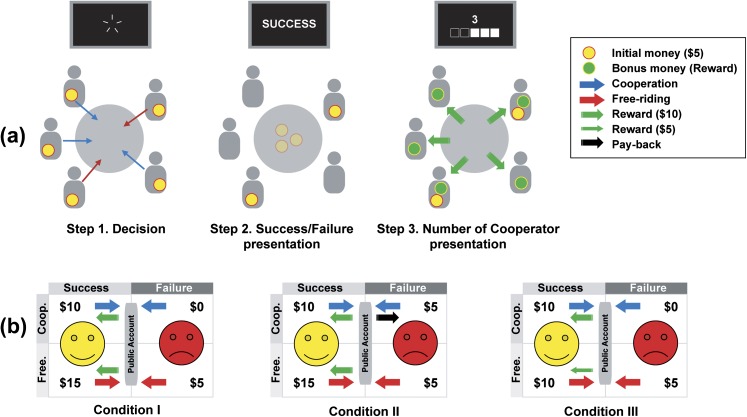
In the binary PGG, the participants were given a certain endowment and had 2 alternative choices, to cooperate or to free ride (figure 1a). The players could invest their money in the public or the private good. Five participants were assigned to a group for the PG game (SZ were only assigned to groups with other patients). For the current study, cards with “5000” or “0” marked on one side were used. At the end of the game procedures, each card with a 5000 mark was considered as $0.50 (500 Korean won), and each participant received an honorarium in Korean money according to the number of cards he gathered. All participants were seated facing each other and were provided with instructions. Each condition was repeated for 10 trials, and $5 of promissory notes was given to each participant before starting each trial. Each player had to choose either to cooperate or to free ride for the group benefit. A bonus of $50 was distributed equally to all players, regardless of each one’s decision, if 3 or more of the 5 players cooperated (ie, extra $10 each). The participants were instructed to turn in their cards simultaneously after a countdown from 5 to 0, which were displayed on a monitor. The number of cooperators and whether the group had received a bonus were displayed on the monitor sequentially. Only if the group succeeded was a bonus distributed by means of the cards to maintain a realistic environment.
Fig. 1.
Schematic diagram of the public goods game (PGG). (a) The game consists of 3 steps. Step 1. After receiving the initial money from the experimenter, all participants anonymously decide to cooperate or to free ride. Step 2. Whether the group earned the reward is presented (success/failure). Step 3. The number of cooperators is presented and if the group succeeded, an equal amount of bonus money is distributed to the group members. (b) Three tables display the payoff matrix of each of the 3 conditions. Each of the 2 main motivations to free ride were removed from the standard PGG (condition I). Possible monetary loss was controlled (removed) in condition II (fear-free; right-top in condition II) and greed was controlled in condition III (greed-free; left-bottom in condition III). Free: free ride; Coop.: cooperate.
We employed 2 methods of modifying incentives in social dilemmas (from condition I explained above; figure 1b) that were introduced in previous studies.21,32 One was to assure the participants that they would not lose their money, even if the group failed to earn a bonus (condition II); the other was to guarantee them that all group members would be provided with a fair share of the money (condition III). By analyzing strategy differences (mean free-riding rates and responses to the presented results in the preceding trial) between conditions, we expected to uncover affective and social motivations to free ride that are hidden under and interact with greedy motivation to maximize one’s profit (see online supplementary material for details). The order of the 3 conditions was counterbalanced, and instructions for each condition were provided just before it started. After the instruction, all players were provided with 4-question questionnaires for each condition to check their understanding of the game rules. We explained the game rules repeatedly and provided examples in cases in which the participants incorrectly responded to the condition questionnaires. All decisions made by the players and the results of each trial for each group were recorded for further analysis.
Data Analysis
The participants were provided with information about group cooperation, but not about individual decisions from each other player to preserve anonymity. Although 5 participants played as a group, we analyzed each participant as an independent player under the assumption that the group characteristics other than cooperation performance (eg, age, education, or IQ of other participants) did not affect the choices made during the game due to the conserved anonymity of the game. We used repeated measures ANOVA to assess performance differences among the 3 conditions in each group and one-way ANOVA to assess group differences in each condition and subcase. The alpha level was set at .05 for all statistical tests (see online supplementary material for details on statistical analysis).
Results
Free-Riding Behavior in the PGG
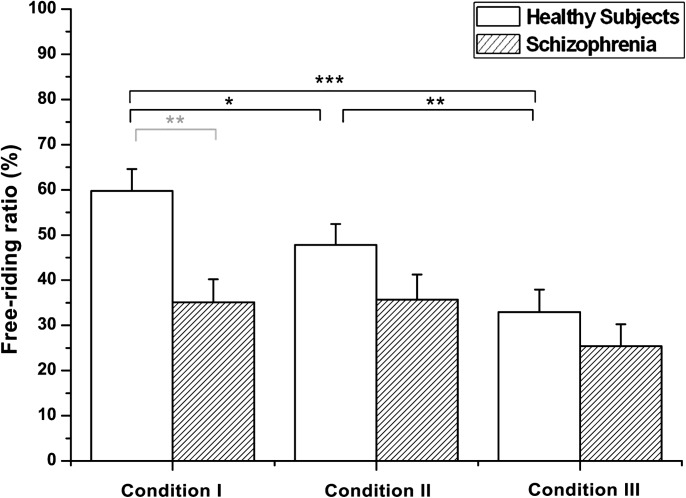
To investigate how the differences in performance were affected by the major incentives to free ride, we firstly measured cooperation ratios in each condition for the HS. Not only enforcing cooperation but also removing the possibilities of loss significantly lessened the free-riding rates of the participants (condition I, II > III; figure 2). The significantly larger amount of decrease on free riding induced by omitting the greed factor than by omitting the fear factor (condition II > III) indicates that greed takes precedence over fear in the free-riding decisions of HS.
Fig. 2.
Mean free-riding ratios in each condition. Healthy subjects exhibited significantly different free-riding rates in each condition (condition I > II > III). Schizophrenia patients showed comparable free-riding rates across all 3 conditions, but showed relatively lower rates of free riding than the healthy group. Black asterisk: within-group difference; gray asterisk: between-group difference. SEs of each condition are represented as error bars. **P < .01; ***P < .001
We used the same measurement (free-riding ratio) for the SZ to investigate if the incentives, ie, fear and greed, affect their free-riding performance in the same way that they affected the HS. The SZ showed relatively low free riding, on average, in all 3 conditions compared with the HS, around 35% in condition I (35.1 ± 5.1%) and condition II (35.7 ± 5.6%) and around 25% in condition III (25.4 ± 4.9%). However, they did not show any statistical difference between conditions (F 1.63, 58.68 = 3.014, P = .067). Neither of the incentive modifications, eliminating the fear factor in condition II and the greed factor in condition III had a significant effect on changing SZ’s decisions. Compared with the behavioral differences observed in the HS throughout the 3 conditions, these results indicate that few SZ were affected by the fear of losing money or greed.
The free-riding ratios of the 2 groups were compared with examine their behavioral differences in each condition. The SZ showed a relatively lower rate of free riding than the HS for all conditions, but this difference was only significant for the first condition (F1 = 12.338, P < .01; figure 2). We did not observe any statistical differences between groups in conditions II and III, in which both the SZ and the HS showed lower rates of free riding than in condition I. These results provide statistical evidence that the SZ were not only less affected by fear and greed but also more cooperative in the condition with both motivations to free ride.
Sequence Effect on Free-Riding Performance
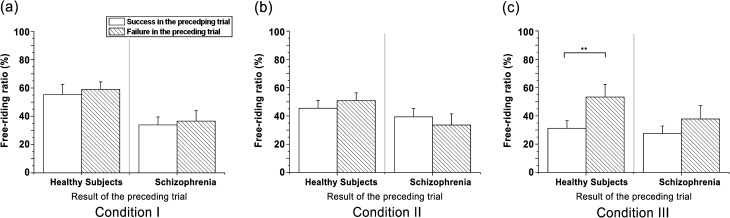
We could estimate motivation underneath revealed free-riding behavior by analyzing sequential choices that respond to the result in the preceding trial. To compare strategies between 2 groups, we categorized free-riding decisions according to the result obtained for whether the group succeeded or failed to earn a bonus in the preceding trial. We compared categorized free-riding ratios for success vs failure. No statistical differences were found between the 2 outcomes for HS in conditions I (figure 3a) and II (figure 3b). Interestingly, in condition III only, the HS free-rode less in the trial after earning a bonus compared with the opposite case (ie, after failing to earn a bonus; F 1 = 5.085, P < .05; figure 3c). Unlike in condition II, which includes guaranteed pay-back in failed cases, cooperators among the participants risked losing their invested money in conditions I and III. The increased free riding of the HS after a failure in condition III, the only condition of the 3 that is independent of greed as a motivation, indicates that the HS were averse to losing their money during the game.
Fig. 3.
Mean free-riding ratios in the trials preceded by successful or failed trials. Both groups displayed comparable free-riding rates regardless of the result of the preceding trial in (a-b) condition I and II. (c) Only healthy subjects exhibited significantly less free-riding ratios in trials following successful vs failed trials in condition III. SEs of each condition are represented as error bars; **P < .01
In contrast to the HS, the results of the preceding trial did not affect the SZ’s behavior enough to cause any statistical differences, regardless of the type of condition (figure. 3a–c). This result shows that the SZ were insensitive to failure in the preceding trial. Another way to explain this result is that the SZ might not have recognized the possible influence of the preceding result on the current decision.
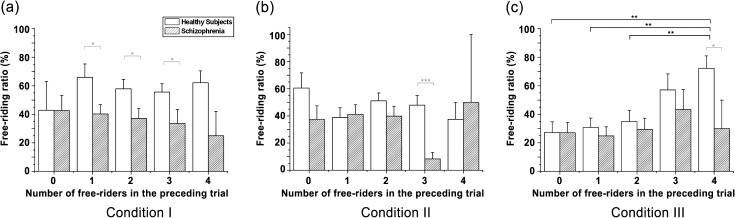
We also compared the free-riding ratios between and within the groups to test whether the number of free riders in the preceding trial affects free-riding behavior. A participant can roughly anticipate the other players’ decisions in a given round from the number of free riders in the preceding round. To go one step further, a player could estimate how the other players would react to the information given and expect him or the group to behave. For comparison purposes, we categorized players’ decisions into 5 subcases based on the number of players among the 5 participants in each group who free-rode in the previous trial; categories ranged from 0 to 4, excluding the player’s own free riding.
In condition I, we found no significant differences within the HS (F 4 = 0.548, P = .701; figure 4a). The free-riding rates in the 5 subgroups were also comparable in condition II (F 4 = 1.075, P = .371; figure 4b). In contrast to the other 2 conditions, we observed that the HS showed statistically significant differences in their decision patterns according to others’ free riding in condition III (F 4 = 3.296, P < .05; figure 4c). The HS showed relatively less, although not statistically different, free riding following trials in which 3 other players free-rode than they did following trials in which all other players free-rode. Their free riding was significantly more frequent following trials in which 4 other players free-rode than they did following trials in which 0, 1, or 2 other players free-rode (t 114 = −2.872, P < .01; t 18.091 = −3.780, P < .01; and t 21.627 = −3.196, P < .01 for 0, 1, and 2 free riding players, respectively). The differentiated free-riding rates in condition III, affected by the number of free riders in the preceding trial in addition to success/failure result, revealed the ability of HS to predict others’ behavior, which can also be thought of as TOM or mentalizing.
Fig. 4.
Mean free-riding ratios in the trial following a trial with the indexed number of free riders. Both groups displayed comparable free-riding rates within their groups in (a-b) condition I and II. (c) In condition III, healthy subjects showed significantly higher free-riding rates when all participants defected than when 0, 1, or 2 players free-rode in the preceding trial. Black asterisk: within-group difference; gray asterisk: between-group difference. SEs of each condition are represented as error bars. *P < .05; **P < .01; ***P < .001
We also recalculated the free-riding rates of the SZ into 5 subcases. In condition I, no statistical difference was detected within the group (F 4 = 0.297, P = .879; figure 4a). The SZ tended to free ride relatively less immediately after trials with 3 free riders than after other subcases in condition II but did not show significant difference (F 4 = 2.233, P = .070; figure 4b). In condition III, compared with the HS group, the SZ showed comparable free-riding rates between subgroups regardless of the number of free riders in the preceding trial (F 4 = 0.468, P = .759; figure 4c). These results indicate that the SZ have difficulties in reading others’ intentions, ie, to cooperate or to free ride. Several local differences between each subcase of the 2 groups were observed that can be considered as by-products that are dependent on the global group behavior (see online supplementary material for details).
Correlation Between Performance and Clinical Features
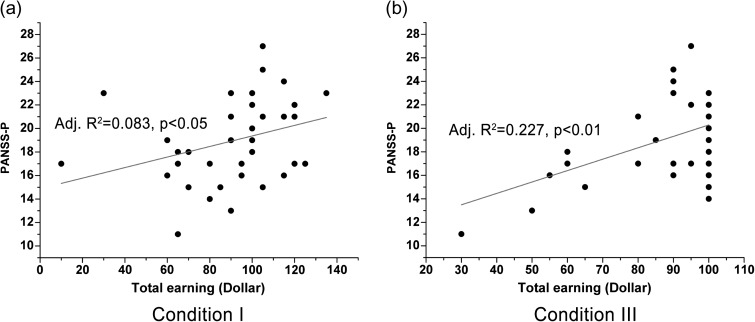
We examined if the patients’ performance (ie, free-riding ratio and total earning) was correlated with their symptom severity using PANSS. No significant correlation was found between the free-riding ratio in each condition and each of the PANSS scales in all pairs. However, we found a positive correlation between the PANSS positive (PANSS-P) and the participants’ total earnings in condition I (Adjusted R 2 = .083, P = .047; figure 5a) and a significant positive correlation with both PANSS-P and PANSS total (PANSS-T) scales in condition III (Adjusted R 2 = .227, P = .002; Adjusted R 2 = .117, P = .022; respectively, figure 5b and figure S6(a) in online supplementary material). No significant correlation was observed between the participants’ total earnings from condition II and each of the PANSS scales. These significant correlations indicate that the patients’ symptom severity might be associated with loss sensitivity. Finally, we found the only significant correlation between the stay rates following failure of the cooperators in condition I and their PANSS negative (PANSS-N) scales (Adjusted R 2 = .236, P = .028; figure S6(b) in online supplementary material). No significant correlations were found between the game performances and other clinical features, such as duration of illness, onset of illness, or medication dosage (see online supplementary material for details).
Fig. 5.
Correlations between total earnings and PANSS scales. (a-b) Positive correlations were observed between total earnings and PANSS-P scale in condition I and III.
Discussion
The objectives of the current study were, first, to unravel the strategic mechanisms by which healthy participants decide whether to invest or not in public goods and, second, to investigate whether SZ show intact performance in a social decision-making task and to clarify the causal deficiency behind any abnormal behavior observed during the PGG. We observed that fear of losing money and greed for earning more money than others both induced HS to free ride. The HS showed notable sensitivity of loss and TOM behavior through repeated trials of the PGG. In contrast, the SZ had low sensitivity to both fear of losing money and greediness—no free-riding rate difference was made with modified incentives—which result in highly cooperative behavior in the PGG. Furthermore, the SZ showed dysfunction of TOM on iterated PGG trials. (The significance of the current study was described in detail in the online supplementary material).
Loss Sensitivity in the PGG
In contrast to HS, we confirmed the absence of loss sensitivity on SZ’s behavior by testing whether they made any changes in their decisions depending on the result of the previous trial. In particular, we observed a significant positive correlation between the symptom severity (ie, PANSS scores) of the SZ and the average total earnings. On average, patients with higher PANSS scores earned greater monetary rewards. Interestingly, these correlations were observed only in conditions I and III, both of which require loss sensitivity to process the circumstances to maximize one’s profit. Because condition II involves no risk of losing, the success/failure result does not affect the participants’ decision in the following trial. In the conditions in which significant correlations were found, patients who earned more also had more severe symptoms. It could be inferred from these results that severely impaired patients mutually cooperated due to reduced sensitivity of loss.
Previous studies have shown loss sensitivity findings that are consistent with the current study.10,12 The current study provided a risky paradigm that requires intact sensitivity of loss and risk to avoid possible wasting of resources (ie, endowment) and to maximize one’s own profit. In general, emotional or social malfunctioning (eg, omitted loss aversion) has a high correlation with the PANSS-N score,30 whereas no significant correlation between PANSS-N and performance was found in the current study. Instead of a correlation with PANSS-N, we found a significant correlation between monetary earning performance and the PANSS-P score, which generally reveals the severity of hallucination and delusion.30 Previously, Suhara et al33 found a significant negative correlation between PANSS-P and dopamine D2 receptor binding in the anterior cingulate cortex (ACC). In other words, SZ with a high PANSS-P score have defective ACC activation. We speculate that SZ with a high PANSS-P might not be able to process the conflict between the given alternatives,34 which is consequently revealed as a weak ability to estimate the risk of loss.
We cannot ignore that there is also some evidence that SZ have intact loss sensitivity. Patients have shown an intact ability to avoid the negative rewards (loss)35 and their neural response to unexpected loss has been demonstrated as intact.36 These previous studies appear to contradict the results in the current study. However, the PGG in the current study required rapid trial-by-trial learning, not gradual learning, which refers to a different aspect of “sensitivity.” Thus, in the point of view of measuring the ability on rapid feedback-driven learning, the behavioral pattern that we observed from SZ in this study is consistent with the results from previous studies.35,37
TOM in the PGG
TOM has been established to be a fundamental ability for social decision making.38–40 In the current study, we were able to see the HSs using TOM during the PGG. However in SZ, we failed to observe TOM during game performance. Not only in condition III, during which the HS exhibited consideration of TOM, but also in conditions I and II, the SZ did not seem to be affected by the preceding rounds (figure S3 in online supplementary material). It could be that the patients’ impaired loss sensitivity, not their defective TOM, might have induced the behavior in condition III. However, we must note that if the SZ had intact TOM functioning during the game, their free-riding rates would have increased through iterations, as we observed in the HS in the pay-back guaranteed condition (ie, condition II) independent to presence of the loss sensitivity. Thus, one can infer that the SZ showed unexpected behavior not only due to reduced loss sensitivity but also as a consequence of TOM malfunction. This result agrees with previous studies suggesting that psychiatric patients with schizophrenia show poor social abilities due to TOM dysfunction.2–8
To the best of our knowledge, this study is the first to investigate free-riding and cooperative behaviors in psychiatric patients and to show that the 3 condition, iterative binary PGG is useful for assessing social decision-making impairments in psychiatric diseases such as schizophrenia, although there are several limitations (see online supplementary material). Nonstrategic and unexpectedly low free riding in the SZ likely arise from poor integration of cognitive and affective information. Future studies should further investigate the dynamic interaction and integration of cognitive and affective origins, such as profit maximizing, TOM, and loss/risk aversion, that underlie complex social decision-making skills. Neuroimaging studies using functional magnetic resonance imaging or electroencephalogram could determine brain regions correlated with enhanced or inhibited motivations and provide more clues to understand the brain mechanisms of patients with schizophrenia and autism who suffer from poor social decision making.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
CHUNG Moon Soul Research Center for Bio Information and Bio Electronics in KAIST; the Korea Science and Engineering Foundation grant funded by the Korea government (R01-2007-000-21094-0, M10644000028-06N4400-02810; 20090093897, 20090083561)
Supplementary Material
Acknowledgments
We thank Youngmin Oh, Hankyul Lee, and Kwangyeol Baek (KAIST) for their assistance in conducting the experiments, and to Dr Dongsu Shin, Kyongsik Yun, Miriam Chun, Anna Jo, and Yoonsol Lee (KAIST) for performing the clinical interviews. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. p. 358. [Google Scholar]
- 2.Abdel-Hamid M, Lehmkamper C, Sonntag C, Juckel G, Daum I, Brune M. Theory of mind in schizophrenia: the role of clinical symptomatology and neurocognition in understanding other people's thoughts and intentions. Psychiatry Res. 2009;165:19–26. doi: 10.1016/j.psychres.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Sprong M, Schothorst P, Vos E, Hox J, Van Engeland H. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- 4.Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Frith CD. The Cognitive Neuropsychology of Schizophrenia. Hove, England: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 6.Brüne M, Abdel-Hamid M, Lehmkamper C, Sonntag C. Mental state attribution, neurocognitive functioning, and psychopathology: what predicts poor social competence in schizophrenia best? Schizophr Res. 2007;92:151–159. doi: 10.1016/j.schres.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Brüne M, Brüne-Cohrs U. Theory of mind evolution, ontogeny, brain mechanisms and psychopathology. Neurosci Biobehav Rev. 2006;30:437–455. doi: 10.1016/j.neubiorev.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Doody GA, Gotz M, Johnstone EC, Frith CD, Cunningham Owens DG. Theory of mind and psychoses. Psychol Med. 1998;28:397–405. doi: 10.1017/s003329179700648x. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Kim YT, Seo E, et al. Dissociation of emotional decision-making from cognitive decision-making in chronic schizophrenia. Psychiatry Res. 2007;152:113–120. doi: 10.1016/j.psychres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Trémeau F, Brady M, Saccente E, et al. Loss aversion in schizophrenia. Schizophr Res. 2008;103:121–128. doi: 10.1016/j.schres.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Kee KS, Horan WP, Salovey P, et al. Emotional intelligence in schizophrenia. Schizophr Res. 2009;107:61–68. doi: 10.1016/j.schres.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Lee D, Shin YM, Chey J. Impaired strategic decision making in schizophrenia. Brain Res. 2007;1180:90–100. doi: 10.1016/j.brainres.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Agay N, Kron S, Carmel Z, Mendlovic S, Levkovitz Y. Ultimatum bargaining behavior of people affected by schizophrenia. Psychiatry Res. 2008;157:39–46. doi: 10.1016/j.psychres.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Wischniewski J, Brune M. Moral reasoning in schizophrenia: an explorative study into economic decision making. Cognit Neuropsychiatry. 2011;1:1–16. doi: 10.1080/13546805.2010.539919. [DOI] [PubMed] [Google Scholar]
- 16.Camerer C. Behavioral Game Theory: Experiments in Strategic Interaction. Princeton, NJ: Princeton University Press; 2003. [Google Scholar]
- 17.Ledyard JO. Public goods: a survey of experimental research. 1994:111–194. Public Economics, EconWPA, http://econpapers.repec.org/RePEc:wpa:wuwppe:9405003. [Google Scholar]
- 18.Andreoni J. Why free ride? Strategies and learning in public goods experiments. J Public Econ. 1988;37:291–304. [Google Scholar]
- 19.Isaac RM, Walker JM, Thomas SH. Divergent evidence on free riding: an experimental examination of possible explanations. Public Choice. 1984;43:113–149. [Google Scholar]
- 20.Andreoni J. Cooperation in public-goods experiments: kindness or confusion? Am Econ Rev. 1995;85:891–904. [Google Scholar]
- 21.Dawes RM, Orbell JM, Simmons RT, Kragt A. Organizing groups for collective action. Am Polit Sci Rev. 1986;80:1171–1185. [Google Scholar]
- 22.Isaac RM, Walker JM. Group size effects in public goods provision: the voluntary contributions mechanism. Q J Econ. 1988;103:179–199. [Google Scholar]
- 23.Palfrey TR, Prisbrey JE. Altruism, reputation and noise in linear public goods experiments. J Public Econ. 1996;61:409–427. [Google Scholar]
- 24.Weimann J. Individual behaviour in a free riding experiment. J Public Econ. 1994;54:185–200. [Google Scholar]
- 25.Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314:1560. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perc M, Szolnoki A. Coevolutionary games. a mini review. Biosystems. 2010;99:109–125. doi: 10.1016/j.biosystems.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Poppe M, Utens L. Effects of greed and fear of being gypped in a social dilemma situation with changing pool size. J Econ Psychol. 1986;7:61–73. [Google Scholar]
- 28.Fischbacher U, Gachter S, Fehr E. Are people conditionally cooperative? Evidence from a public goods experiment. Econ Lett. 2001;71:397–404. [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York, USA: American Psychiatric Press; 1995. [Google Scholar]
- 30.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 31.Henrich J, Boyd R, Bowles S, et al. “Economic man” in cross-cultural perspective: behavioral experiments in 15 small-scale societies. Behav Brain Sci. 2005;28:795–815. doi: 10.1017/S0140525X05000142. [DOI] [PubMed] [Google Scholar]
- 32.Chung D, Yun K, Kim JH, Jang B, Jeong J, Perc M. Different gain/loss sensitivity and social adaptation ability in gifted adolescents during a public goods game. PLoS One. 2011;6:707–718. doi: 10.1371/journal.pone.0017044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suhara T, Okubo Y, Yasuno F, et al. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Am Med Assoc. 2002;59:25–30. doi: 10.1001/archpsyc.59.1.25. [DOI] [PubMed] [Google Scholar]
- 34.Brown JW, Braver TS. A computational model of risk, conflict, and individual difference effects in the anterior cingulate cortex. Brain Res. 2008;1202:99–108. doi: 10.1016/j.brainres.2007.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waltz JA, Schweitzer JB, Gold JM, et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2008;34:1567–1577. doi: 10.1038/npp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 39.Lissek S, Peters S, Fuchs N, et al. Cooperation and deception recruit different subsets of the theory-of-mind network. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. The neural correlates of theory of mind within interpersonal interactions. Neuroimage. 2004;22:1694–1703. doi: 10.1016/j.neuroimage.2004.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.