Abstract
Social cognitive deficits are associated with psychotic symptoms, but the nature of this association remains unknown. This study uses a genetically sensitive cross-trait cross-sibling design to investigate the nature of the overlap between both phenotypes. A sample of 1032 patients, 1017 of their healthy siblings, and 579 control subjects were recruited within the Dutch Genetic Risk and Outcome in Psychosis (GROUP) study. Participants completed a battery of cognitive tests, including 2 social cognitive tests on theory of mind (ToM) and emotion recognition. Within siblings, symptoms were assessed with the Structured Interview for Schizotypy—Revised. The Positive and Negative Syndrome Scale was used to assess patients’ symptoms. Within patients, social cognitive performance was consistently and significantly associated with disorganized and, to a lesser degree, with negative symptoms. Associations with positive symptoms were significant, but smaller. Suggestive of a shared etiology, both social cognitive factors showed significant familial clustering. The associations between patients’ ToM and subclinical symptoms in siblings were nonsignificant, suggesting that their overlap within patients is due to individual rather than shared familial factors. Indicative of a shared etiology, familial covariation was present between patients’ emotion recognition ability and disorganized and, albeit to a lesser degree, positive but not negative subclinical symptoms in siblings.
Keywords: schizophrenia/social cognition/family study/cross-sibling design
Introduction
Schizophrenia is accompanied by significant functional impairment in different social cognitive domains.1,2 The impairments are associated with psychotic symptoms.3–5 This association may reflect a shared etiopathology with a possible genetic basis. Alternatively, both traits may be on a causal pathway such that the psychotic symptoms are the consequence of impaired social cognition (or vice versa) or both traits may be secondary to another, possibly disease-related factor (eg, general cognitive impairment). These explanations are not mutually exclusive, and the present study set out to investigate the evidence for a shared familiar etiology. If symptoms and social cognition covary because of a similar familiar etiology, social cognitive abnormalities could be useful intermediate phenotypes in the search for the genetic causes of the symptoms of psychosis.
There is some evidence in favor of a genetic etiology of the social cognitive impairment in psychosis. For example, higher rates of social cognitive impairment have been reported in first-degree relatives as compared to the general population.6–10 However, higher rates of subclinical symptoms in relatives and familial clustering of symptoms have also been reported.11 There is evidence that in nonclinical individuals higher positive schizotypy is associated with worse theory of mind (ToM) performance.12 Other studies suggested that associations between social cognitive performance and symptoms are also present during prodromal states and remission of the illness.13,14 Studies to date have not been able to rule out alternative explanations for the symptom-cognition association, for example that the presence of social cognitive deficits is secondary to subclinical or residual psychotic experiences. Therefore, the current study applied a genetically sensitive cross-trait cross-sibling design to investigate the nature of the association between social cognitive abnormalities and psychotic symptoms.
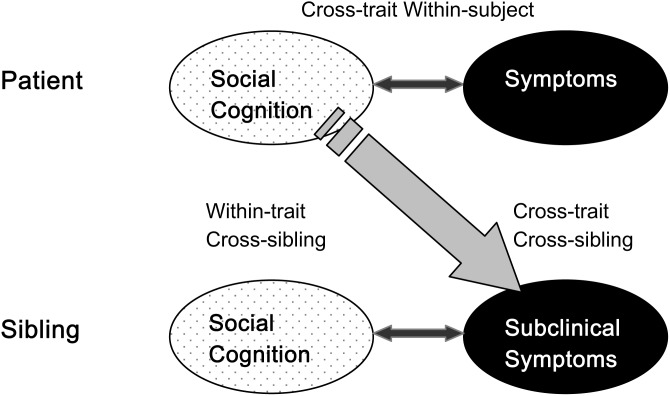
Social cognition is a multidimensional construct,15 and the nature of the associations between symptoms and social cognition may differ across social cognitive domains.16 Here, we focused on 2 core domains of social cognition that are impaired in schizophrenia and have previously been suggested to play a role in the formation of psychotic symptoms: (1) ToM and (2) emotion processing.7,16 We used a cross-trait cross-sibling design (see figure 1) in a large sample of patients with nonaffective psychosis, their unaffected siblings and healthy controls to investigate social cognitive impairment and psychotic symptoms and the nature of their association. First, the associations between the different social cognitive functions and symptom clusters were analyzed within patients and their relatives to confirm the assumption of overlap between the 2 domains. Second, familial clustering of social cognitive functioning was investigated using within-trait cross-sibling analyses. Finally, we investigated all cross-trait cross-sibling associations between subclinical symptomatic expression in siblings and social cognitive functioning in patients. The presence of such associations suggests a common familial etiology of both traits. Alternatively, finding symptom-cognition associations within affected individuals only suggests that the frequently reported overlap between symptoms and social cognitive deficits is due to individual (eg, illness related) factors rather than shared familial etiology.
Fig. 1.
Cross-trait Cross-sibling design.
Method
Procedure and Sample
The data pertain to baseline measures of the ongoing longitudinal multicenter study “Genetic Risk and Outcome in Psychosis” (GROUP). The sample was recruited in the Netherlands and Belgium. Participants with nonaffective psychosis were identified via clinicians working in regional psychotic disorder mental health services. Family members were recruited through participating patients. Healthy volunteers were recruited through random community mailings in the catchment area. The full GROUP sample consisted of 1120 patients with a nonaffective psychotic disorder, 1057 of their siblings, 919 of their parents, and 590 unrelated controls from the general population. The current inclusion criteria were (1) age between 16 and 60, (2) good command of the Dutch language, and (3) being able and willing to give informed consent. Patients had to meet the DSM-IV-TR17 criteria for a nonaffective psychotic disorder, as assessed by the Comprehensive Assessment of Symptoms and History Interview.18 Additional inclusion criteria for the control group were not having a (1) lifetime psychotic disorder and/or (2) a first-degree family member with a lifetime psychotic disorder. The project was approved by the local Research Ethics Committee, and all participants gave written informed consent in accordance with the committee’s guidelines.
Measures of Social Cognition
Degraded Facial Affect Recognition Task.
The Degraded Facial Affect Recognition Task (DFAR)19 uses photographs of 4 different actors (2 male and 2 female) depicting the 4 emotions: angry, happy, fearful, and neutral. The task comprises 64 trials consisting of 16 face presentations in each emotion category. The emotions were shown with 100% and 75% intensity in order to increase the difficulty of the task. Subjects were asked to indicate the emotional expression of each face with a button press and to respond as accurately as possible. Outcomes were the proportion correctly recognized as neutral, happy, fearful, and angry emotions and the overall proportion correct.
Hinting Task.
ToM was assessed with the Hinting Task (HT).6,9,20 The task tests the ability of subjects to infer the real intentions behind indirect speech utterances. It comprises 10 short passages presenting an interaction between 2 characters that end with 1 of the characters dropping a hint. The subject is then asked what the character really meant. Correctly identified hints are scored with 2 points. In cases of an incorrect response, a more obvious hint is added. A subsequent correct response is scored with 1 point; an incorrect response is scored as 0. The outcome range is 0–20.
Measures of Neurocognition
Benton Facial Recognition Test.
The short form of the Benton Facial Recognition Test,21 a measure of the ability to match unfamiliar faces, was used to assess whether deficits in facial affect recognition are not mediated by differences in general facial-recognition ability.
Wechsler Adult Intelligence Scale.
The Arithmetic, Digit Symbol-Coding, Block Design, and Information subtests of the Wechsler Adult Intelligence Scale III were administered as an indicator of IQ.22,23
Symptom Assessment
The Positive and Negative Syndrome Scale.
The Positive and Negative Syndrome Scale (PANSS24) has been used to assess symptoms in patients. Originally, the PANSS consisted of a positive and negative syndrome scale and a general psychopathology scale. However, a specific model has been formulated on the social cognitive basis of disorganized symptoms25 and research to date shows the most robust association between poor mental state attribution and disorganization symptoms.26,27 Recently, Van der Gaag and colleagues28 developed a more fine-grained model of symptoms. Among other factors, the model captures disorganized symptoms. The positive, negative, and disorganized symptom factors of the model were used in the current analyses.
Structured Interview for Schizotypy—Revised.
The Structured Interview for Schizotypy—Revised (SIS-R)29,30 was administered to assess subclinical symptoms in siblings. It is a semistructured interview that contains 20 schizotypal symptoms and 11 schizotypal signs. Guided by our a-priori theoretical considerations for the classification of symptoms within patients and previous research,25 we reduced the item scores to the 3 dimensions of (1) positive (referential thinking, magical ideation, illusions, and suspiciousness), (2) negative (social isolation, social anxiety, introversion, and restricted affect), and (3) disorganization schizotypy (goal directness of thinking, loosening of associations, and oddness).
Analyses
Statistical analyses were performed using STATA version 11.0 statistical software. Linear regression analyses were used to investigate group differences and within-group symptom-cognition associations within siblings and patients. It has been argued that social cognitive impairment in schizophrenia is nonspecific and that any association with symptoms may be due to confounding by neurocognitive impairment.31,32 We therefore adjusted all between group analyses for IQ (for intercorrelations between IQ and the social cognitive tasks see table 1). The potentially confounding factors age and gender were controlled in all between group analyses. Between group linear regression analyses on DFAR performance were also adjusted for general face recognition ability. Some families contributed more than 1 patient or sibling. All possible patient-sibling pairs were included in the analysis. To account for the observations of multiple siblings within 1 family, multilevel random regression analyses (XTREG) were used to analyze within-trait cross-sibling associations, ie, the familial clustering of cognitive performance. Cross-trait cross-sibling associations between cognitive performance in patients and subclinical symptoms in siblings were analyzed using the same routine. All cross-trait cross-sibling analyses were adjusted for the corresponding trait within the patient and sibling group. Effect sizes are expressed as the standardized regression coefficient β for linear regression analyses and the regression coefficient b with the 95% CI's.
Table 1.
Intercorrelations Between the (Social) Cognitive Tasks by Group
| Overall | Controls | Siblings | Patients | |||||
| HT | DFAR | HT | DFAR | HT | DFAR | HT | DFAR | |
| DFAR | .23 | .12 | .10 | .25 | ||||
| IQ | .35 | .21 | .17 | .09 | .23 | .13 | .37 | .22 |
Note: DFAR, Degraded Facial Affect Recognition; HT, Hinting Task.
Results
Sample
The current study incorporated a subset of participants from the full GROUP sample. This subsample included 1032 patients, 1017 of their healthy siblings, and 579 controls. Sample characteristics and test statistics are displayed in table 2.
Table 2.
Demographic and Clinical Sample Characteristics
| Controls | Siblings | Patients | ||||||
| N = 579 | n | N = 1017 | n | N = 1032 | n | Test Statistic | P Value | |
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Age (y) | 30.4 (10.6)a | 579 | 27.8 (8.2)b | 1017 | 27.3 (7.2)c | 1032 | F (2, 2624) = 26.84 | <.001 |
| Male (%) | 45a | 46a | 77b | χ 2(2) = 257.53 | <.001 | |||
| Education (%) | ||||||||
| None/primary only | 2.4 | 576 | 7.4 | 994 | 13 | 1008 | χ 2(16) = 246.54 | <.001 |
| Lower secondary | 14.8 | 19.7 | 30.7 | |||||
| Lower vocational | 15.6 | 22.4 | 17.4 | |||||
| Higher secondary | 31.7 | 20.5 | 25.5 | |||||
| Higher vocational | 25.5 | 18.5 | 9.1 | |||||
| University | 10 | 11.5 | 4.3 | |||||
| IQ | 109.7 (15.1)a | 579 | 102.7 (15.6)b | 1017 | 94.9 (16)c | 1032 | F (4, 2623) = 95.40 | <.001 |
| HT (range 0–20) | 19.1 (1.3)a | 573 | 18.8 (1.7)a | 1009 | 17.5 (2.8)b | 1008 | F (5, 2584) = 114.25 | <.001 |
| DFAR total | 73.2 (9.1)a | 542 | 72.5 (9.4)a | 943 | 68.6 (10.7)b | 934 | F (6, 2398) = 63.61 | <.001 |
| Neutral | 81.3 (15.1) | 80.4 (15) | 77.8 (17.5) | F (6, 2398) = 17.41 | <.001 | |||
| Happy | 87.4 (11.1) | 88.2 (10.7) | 86.8 (12.7) | F (6, 2398) = 14.17 | <.001 | |||
| Angry | 70.5 (18.6) | 68.8 (19.3) | 62.3 (20.9) | F (6, 2398) = 30.61 | <.001 | |||
| Fearful | 53.9 (18.1) | 52.7 (19.7) | 47.5 (19.7) | F (6, 2398) = 34.17 | <.001 | |||
| SIS-R | ||||||||
| Disorganized | .03 (.12)a | 571 | .05 (.19)b | 1006 | F (4, 1573) = 6.78 | <.001 | ||
| Negative | .45 (.43)a* | 571 | .5 (.47)b | 1006 | F (4, 1574) = 1.91 | .005 | ||
| Positive | .31 (.35)a | 571 | .38 (.42)b | 1006 | F (4, 1574) = 14.72 | <.001 | ||
| PANSS | ||||||||
| Disorganized | 16.7 (6.2) | 971 | ||||||
| Negative | 15 (6.6) | 970 | ||||||
| Positive | 13.9 (6.6) | 979 |
Note: Different superscripts indicate significant group differences with P < .05. Group differences on the specific emotion categories of the DFAR are displayed in table 3. *Indicates P < .06. DFAR, Degraded Facial Affect Recognition; HT, Hinting Task; PANSS, Positive and Negative Syndrome Scale; SIS-R, Structured Interview for Schizotypy—Revised.
Social Cognition.
Patients had a worse HT performance than controls and siblings. The means of controls and siblings differed into the expected direction but this difference was not significant. Patients also performed worse on the DFAR than controls and siblings. Again, the performance of controls and siblings differed into the expected direction but was not significant. All groups recognized happy emotion best, followed by neutral, angry, and fearful emotion with the lowest rate of correct recognitions. Analyses per emotion category showed no differences between controls and siblings for any of the 4 categories. Patients did not perform worse than controls and siblings in recognizing neutral and happy emotions. They did, however, perform significantly worse than controls and siblings with respect to angry and fearful emotions (see table 3). All analyses were controlled for age, gender, and IQ. DFAR analyses were also controlled for general face recognition ability.
Table 3.
Group Differences on the Social Cognitive Tests
| Controls vs Patients | Controls vs Relatives | Patients vs Relatives | ||||
| β | P | β | P | β | P | |
| HT | −.17 | <.001 | .01 | .61 | −.18 | <.001 |
| DFAR | −.13 | <.001 | −.03 | .30 | −.10 | <.001 |
| Neutral | −.04 | .12 | −.01 | .80 | −.04 | .11 |
| Happy | .02 | .51 | .04 | .13 | −.02 | .36 |
| Anger | −.13 | <.001 | −.04 | .12 | −.09 | <.001 |
| Fear | −.10 | <.001 | −.03 | .21 | −.07 | .002 |
Note: β = adjusted for age, gender, IQ, and face recognition ability (for the DFAR only). Abbreviations are explained in the first footnote to table 1.
Cross-Trait Within-Group Analyses
Within patients, HT performance was consistently and significantly associated with disorganized and, to a lesser extent, with negative symptoms. The association with positive symptoms was smaller but also significant. DFAR performance was significantly associated with disorganized and, to a lesser extent, with negative and positive symptoms. Within siblings, HT performance was weakly but significantly associated with subclinical disorganized symptoms only. The associations with subclinical negative and positive symptoms were nonsignificant. DFAR performance was significantly associated with subclinical negative symptoms only. The associations with subclinical disorganized and positive symptoms were nonsignificant (see table 4).
Table 4.
Cross-Trait Within-Patients/Siblings, Within-Trait Cross-Sibling and Cross-Trait Cross-Sibling Analyses
| Analysis | n | Association | β | b | P value | 95% CI: lb/ub | |
| Cross-trait within-patients analysis | 948 | HT-disorganized symptoms | −.33 | −.73 | <.001 | −.91/−.56 | |
| 946 | HT-negative symptoms | −.21 | −.51 | <.001 | −.70/−.32 | ||
| 957 | HT-positive symptoms | −.11 | −.27 | <.001 | −.46/−.07 | ||
| 880 | DFAR-disorganized symptoms | −.21 | −.12 | <.001 | −.17/−.07 | ||
| 875 | DFAR-negative symptoms | −.11 | −.07 | <.001 | −.12/−.02 | ||
| 885 | DFAR-positive symptoms | −.09 | −.05 | .008 | −.11/−.001 | ||
| Cross-trait within-siblings analysis | 1001 | HT-disorganized symptoms | −.09 | −.01 | .006 | −.02/.001 | |
| 1001 | HT-negative symptoms | −.04 | −.01 | .17 | −.04/.01 | ||
| 1001 | HT-positive symptoms | −.04 | −.01 | .22 | −.03/.01 | ||
| 935 | DFAR-disorganized symptoms | −.02 | −.0004 | .55 | −.002/.001 | ||
| 935 | DFAR-negative symptoms | −.07 | −.003 | .04 | −.01/.001 | ||
| 935 | DFAR-positive symptoms | −.02 | −.001 | .60 | −.005/.003 | ||
| Families* | |||||||
| Within-trait cross-sibling analysis | 755 | HT | .08 | <.001 | .02/.13 | ||
| 674 | DFAR | .09 | .002 | .02/.17 | |||
| 766 | IQ | .42 | <.001 | .35/.51 | |||
| Cross-trait cross-sibling analysis | 715 | HT patients-disorganized symptoms siblings | .001 | .87 | −.01/.01 | ||
| 717 | HT patients-negative symptoms siblings | .01 | .30 | −.01/.02 | |||
| 721 | HT patients-positive symptoms siblings | .002 | .75 | −.01/.02 | |||
| 640 | DFAR patients-disorganized symptoms siblings | .001 | .02 | −.0002/.003 | |||
| 639 | DFAR patients-negative symptoms siblings | .003 | .10 | −.002/.01 | |||
| 643 | DFAR patients-positive symptoms siblings | .003 | .06 | −.001/.01 | |||
Note: *All analyses are adjusted for age, gender, IQ, and the respective relevant traits (ie, social cognition in siblings and symptoms in patients); 95% CI, confidence interval for b; lb, lower bound; ub, upper bound; Abbreviations are explained in the first footnote to table 1.
Within-Trait Cross-Sibling Analyses
The analyses between at least 674 patient-sibling pairs showed significant within-trait familial clustering of HT performance, DFAR performance, and IQ (see table 4). All within-trait cross-sibling analyses were controlled for age, gender, and in case of the HT and DFAR for IQ.
Cross-Trait Cross-Sibling Analyses
None of the associations between HT performance in patients and subclinical symptoms in siblings were significant. DFAR performance in patients and subclinical disorganized symptoms in siblings were significantly associated. The associations between patients’ DFAR performance and siblings’ subclinical symptoms were marginally significant for positive subclinical symptoms and nonsignificant for negative subclinical symptoms (see table 4). All cross-trait cross-sibling analyses were controlled for age, gender, and IQ and the respective symptom domain and cognitive task across siblings.
Discussion
Our results showed no familial covariation of the associations between ToM performance and psychotic symptoms across siblings, suggesting that the overlap between the 2 phenotypes, which is seen in patients, does not reflect a shared familial etiology. However, indicative of a shared etiology, familial covariation was present between patients’ emotion recognition ability and disorganized and, to a lesser degree, positive, but not negative subclinical symptoms in siblings.
Social Cognitive Impairment Over the Psychosis Continuum
In line with earlier evidence,27 a worse performance in ToM and emotion recognition ability was weakly associated with a higher psychosis risk. Patients performed worse on the HT than siblings and controls and siblings mean values were intermediate. Yet, in contrast to what had been expected on the basis of previous research,6,8,9,33,34 the difference between siblings and controls was small and not significant. A similar pattern of results was present for emotion recognition. Despite their overall impairment, the valence-related performance pattern of patients was similar to that of siblings and controls, with a superior recognition of happy and neutral emotions as compared to fearful and angry emotions.35 Analyses per emotion category indicated that the overall effect was mainly driven by differences in the recognition of angry and fearful emotion. The finding of unimpaired recognition of neutral and happy affect but impaired recognition of negative affect is consistent with previous reports on the disproportionate impairment in the identification of negative emotions and lends further support to emotion-specific processing deficits in schizophrenia.33,34,36–38
Symptoms and Social Cognition
To investigate the nature of the associations between symptoms and social cognitive impairment in patients and siblings, we carried out cross-trait within-group analyses first. Within patients, poorer ToM performance was consistently and significantly associated with all 3-symptom clusters. The strongest association was present with disorganized symptoms. The association with negative symptoms was intermediate, and the weakest association was present with positive symptoms. Emotion recognition was significantly associated with disorganized and negative symptoms, albeit to a lesser degree than ToM. Within siblings, significant associations were present between ToM and subclinical disorganized symptoms but not negative symptoms. Emotion recognition, in turn, was significantly associated with subclinical negative symptoms but not disorganized symptoms. Associations between both social cognitive domains and positive symptoms were entirely specific to patients.
In the next step, we investigated whether social cognitive performance clusters within families. The within-trait cross-sibling analyses revealed considerable familial clustering of ToM and emotion recognition, although to a slightly lesser degree. The social cognitive clusters remained significant when controlled for IQ, supporting a substantial independence from the neurocognitive domain.15
The cross-trait cross-sibling investigations revealed no significant associations between ToM in patients and any subclinical symptoms in their siblings. No significant association was present between patients’ emotion recognition and negative symptoms in siblings. However, indicative of a common etiological transmission, patients’ emotion recognition ability was significantly associated with siblings’ disorganized subclinical symptoms and, although to a lesser degree, with their positive subclinical symptoms.
The results confirmed previously observed associations between symptoms and social cognitive performance within the patients. Only 2 significant cognition-symptom associations were present within the sibling group. This finding may be due to constricted variation within siblings. Alternatively, other symptom-cognition associations may only come into effect once the disorder has been developed. Our findings corroborated the potential role of both social cognitive functions as intermediary phenotypes of the illness. Familial clustering indicates a possible shared a etiological basis underlying impaired social cognition in siblings and patients. Obviously, the 2 phenotypes may also have been acquired in a shared environment (eg, parental neglect). However, research suggests that the familial liability to schizophrenia strongly represents the influence of shared genes, so a partly genetic transmission of the phenotypes is therefore likely.39,40
The differential cross-trait cross-sibling associations between social cognitive functioning and the specific symptom clusters point toward partly differential etiological substrates. The etiology of emotion recognition deficits and symptoms seems to vary between the 3 clusters. Emotion recognition deficits may be useful endophenotypes in the search for the genetic causes of disorganized symptoms and possibly to a lesser degree of positive but not negative symptoms. Alternatively, it could be argued that these differences are due to a lower sensitivity of the SIS-R to positive and even more strongly to negative symptoms. However, previous evidence proved the SIS-R is sensitive to family-specific variation in positive and negative subclinical symptoms.41
Our results did not substantiate a shared etiology of ToM deficits and any of the 3 symptom clusters. In this case, the overlap seems to originate at an individual level. In line with previous research,15 the present findings suggest a substantial heterogeneity and multicomponent structure of social cognition. Analogous to other (genetic) predispositions that appear in the phenotype under particular conditions (eg, sunburn and skin cancer), manifold factors may bring the associations between ToM impairment and symptoms to expression. A dynamic interplay of symptoms, cognitive processes, behavioral, and environmental factors may offer a suitable explanation for our findings. Patients’ ToM deficits may play a role in the formation, exacerbation and maintenance of psychotic symptoms.42,43 Specifically, impaired ToM could lead to a paranoid interpretation of other persons’ intentions as malevolent. Also, impaired emotion recognition, although possibly to a lesser degree, may cause negative misinterpretations of social-emotional cues. Misperceptions may lead to the avoidance of social situations or contacts and problematic social behavior, which may be partially reflected in negative symptoms. Disorganized symptoms and social cognitive deficits, moreover, could be associated on a level of conceptual understanding rather than misinterpretation of social situations.
Strengths and Limitations
The current study used a uniquely large sample with adequate power to detect even delicate effects. Also, the patient–sibling based design has the important advantage of automatically controlling for confounds that are associated with the illness, such as residual symptoms or the effects of antipsychotic medication. Sibling-based designs also have the advantage of low confounding by unobserved factors that may affect case–control comparisons in unrelated subjects, such as shared socioeconomic and developmental conditions.
The findings should be considered keeping the following limitations in mind. First, our data cannot completely clarify whether any abnormalities in social cognition are present prior to the illness onset (ie, potentially causal) or whether they are covarying epiphenomena of the clinical picture. However, in line with previous research, our results indicate that the association between social cognitive impairment and symptoms is not only present during acute psychosis.27 Second, we had to use different measures to assess the clinical phenotypes in patients and siblings. The SIS-R is not suitable for the use in patients because it may underlie ceiling effect. The PANSS interview, in turn, may be subject to floor effects when used in relatives. However, we aimed to establish concurrent validity between the 2 measures by structuring the items along the same symptom dimensions. Third, as reported by previous research, the social cognitive performance differences between siblings and controls were subtle.34,44 This may partly be due to the nature of the social cognitive tasks that we employed. The HT is specifically prone to ceiling effects. It also needs to be noted that our measure may only reflect a part of the broader domain that it belongs to. Other ToM tasks possibly tap into different mentalizing capacities and future studies should aim to include more tasks to get a better representation of the domain. Another possible explanation for the relatively small effects could be a self-selection bias, in which only relatively stable patients volunteer to participate in demanding research. Also, biased answering of siblings who are highly aware of the symptoms of psychosis and who may want to appear healthy may have reduced the effects. Obviously, it is difficult to translate statistical effect sizes and P values into clinical significance one to one. Even though effect sizes on some tasks are small, the work of our group and others45,46 has shown that social cognitive performance in the domains of ToM and emotion perception and processing is associated with functional outcome, possibly to a higher degree than many other cognitive factors. These findings imply a substantial clinical significance of the degree of social cognitive impairment that is typically seen in schizophrenia.
Conclusions
ToM and emotion recognition impairments are associated with the liability to nonaffective psychosis and cluster within families. Our findings support the idea that both traits could be suitable intermediary phenotypes for genetic studies. The cross-trait cross-sibling analyses did not support a familial continuity between subclinical symptoms and ToM. However, a shared familial etiology may underlie the overlap between emotion recognition deficits and disorganized and positive psychotic symptoms. Emotion recognition deficits could therefore be useful intermediate phenotypes in the identification of the familial causes of specific symptoms of psychosis.
Funding
The infrastructure for the GROUP study is funded by the Geestkracht program of the Dutch Health Research Council (ZON-MW, 10-000-1002) and matching funds from participating universities and mental health care organizations (Site Amsterdam: Academic Psychiatric Centre AMC, Ingeest, Arkin, Dijk en Duin, Rivierduinen, Erasmus MC, GGZ Noord Holland Noord; Site Utrecht: University Medical Centre Utrecht, Altrecht, Symfora, Meerkanten, Riagg Amersfoort, Delta; Site Groningen: University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGZ De Grote Rivieren and Parnassia Bavo Groep; Site Maastricht: Maastricht University Medical Center, GGZ Eindhoven en de Kempen, GGZ Midden-Brabant, GGZ Oost-Brabant, GGZ Noord-Midden Limburg, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem).The research leading to these results has received funding from the European Community's Seventh Framework Program under grant agreement No. HEALTH-F2-2009-241909 (Project EU-GEI). Preparation of this article was supported by a VIDI grant from the Netherlands Organization for Scientific Research (to Dr Lydia Krabbendam).
Acknowledgments
We are grateful for the generosity of time and effort by the families who make this GROUP project possible. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Appendix
Genetic Risk and Outcome in Psychosis (GROUP) Investigators: René S. Kahn, MD, PhD, Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, Utrecht, the Netherlands; Don H. Linszen, MD, PhD, Department of Psychiatry, Academic Medical Centre, University of Amsterdam, Amsterdam, the Netherlands; Jim van Os, MD, PhD, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht University Medical Centre, Maastricht, the Netherlands, and King's College London, King's Health Partners, Department of Psychosis Studies, Institute of Psychiatry, London, United Kingdom; Durk Wiersma, PhD, Department of Psychiatry, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands; Richard Bruggeman, MD, PhD, Department of Psychiatry, University Medical Center Groningen, University of Groningen; Wiepke Cahn, MD, PhD, Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht; Lieuwe de Haan, MD, PhD, Department of Psychiatry, Academic Medical Centre, University of Amsterdam; Lydia Krabbendam, PhD, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht University Medical Centre; and Inez Myin-Germeys, PhD, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht University Medical Centre.
References
- 1.Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 3.Shean G, Murphy A, Meyer J. Social cognition and symptom dimensions. J Nerv Ment Dis. 2005;193:751–755. doi: 10.1097/01.nmd.0000185868.58587.5b. [DOI] [PubMed] [Google Scholar]
- 4.Marjoram D, Gardner C, Burns J, Miller P, Lawrie SM, Johnstone EC. Symptomatology and social inference: a theory of mind study of schizophrenia and psychotic affective disorder. Cognit Neuropsychiatry. 2005;10:347–359. doi: 10.1080/13546800444000092. [DOI] [PubMed] [Google Scholar]
- 5.Doody GA, Gotz M, Johnstone EC, Frith CD, Owens DG. Theory of mind and psychoses. Psychol Med. 1998;28:397–405. doi: 10.1017/s003329179700648x. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Krabbendam L, Jolles J, van Os J. Alterations in theory of mind in patients with schizophrenia and non-psychotic relatives. Acta Psychiatr Scand. 2003;108:110–117. doi: 10.1034/j.1600-0447.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 7.de Achával D, Costanzo EY, Villarreal M, et al. Emotion processing and theory of mind in schizophrenia patients and their unaffected first-degree relatives. Neuropsychologia. 2010;48:1209–1215. doi: 10.1016/j.neuropsychologia.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Anselmetti S, Bechi M, Bosia M, et al. ‘Theory’ of mind impairment in patients affected by schizophrenia and in their parents. Schizophr Res. 2009;115:278–285. doi: 10.1016/j.schres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Versmissen D, Janssen I, Myin-Germeys I, et al. Evidence for a relationship between mentalising deficits and paranoia over the psychosis continuum. Schizophr Res. 2008;99:103–110. doi: 10.1016/j.schres.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Addington J, Penn DL, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for psychosis. Br J Psychiatry. 2008;192:67–68. doi: 10.1192/bjp.bp.107.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardno AG, Sham PC, Murray RM, McGuffin P. Twin study of symptom dimensions in psychoses. Br J Psychiatry. 2001;179:39–45. doi: 10.1192/bjp.179.1.39. [DOI] [PubMed] [Google Scholar]
- 12.Pickup G. Theory of mind and its relation to schizotypy. Cognit Neuropsychiatry. 2006;11:177–192. doi: 10.1080/13546800444000236. [DOI] [PubMed] [Google Scholar]
- 13.Langdon R, Coltheart M. Mentalising, schizotypy, and schizophrenia. Cognition. 1999;71:43–71. doi: 10.1016/s0010-0277(99)00018-9. [DOI] [PubMed] [Google Scholar]
- 14.Eack SM, Mermon DE, Montrose DM, et al. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophr Bull. 2010;36:1081–1088. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Hooren S, Versmissen D, Janssen I, et al. Social cognition and neurocognition as independent domains in psychosis. Schizophr Res. 2008;103:257–265. doi: 10.1016/j.schres.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association, ed. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, revised. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 18.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 19.van 't Wout M, Aleman A, Kessels RP, Laroi F, Kahn RS. Emotional processing in a non-clinical psychosis-prone sample. Schizophr Res. 2004;68:271–281. doi: 10.1016/j.schres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating "theory of mind" in people with schizophrenia. Schizophr Res. 1995;17:5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- 21.Benton AL, Sivan AB, Hamsher KdeS, Varney NR, Spreen O. Benton's Test of Facial Recognition. New York, NY: Oxford University Press; 1983. [Google Scholar]
- 22.Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale (3rd ed.) Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 23.Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46:209–215. doi: 10.1016/s0920-9964(00)00017-7. [DOI] [PubMed] [Google Scholar]
- 24.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 25.Hardy-Baylé MC, Sarfati Y, Passerieux C. The cognitive basis of disorganization symptomatology in schizophrenia and its clinical correlates: toward a pathogenic approach to disorganization. Schizophr Bull. 2003;29:459–471. doi: 10.1093/oxfordjournals.schbul.a007019. [DOI] [PubMed] [Google Scholar]
- 26.Brüne M, Schaub D, Juckel G, Langdon R. Social skills and behavioral problems in schizophrenia: the role of mental state attribution, neurocognition and clinical symptomatology. Psychiatry Res. doi: 10.1016/j.psychres.2010.03.015. In press. doi:10.1016/j.psychres.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia: a meta-analysis. Br J Psychiatry. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- 28.Van der Gaag M, Hoffman T, Remijsen M, et al. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006;85:280–287. doi: 10.1016/j.schres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Kendler KS, Liebermann JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15:559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- 30.Vollema M, Ormel J. The reliability of the Structured Interview for Schizotypy-Revised. Schizophr Bull. 2000;26:619–629. doi: 10.1093/oxfordjournals.schbul.a033482. [DOI] [PubMed] [Google Scholar]
- 31.Kerr SL, Neale JM. Emotion perception in schizophrenia: specific deficit or further evidence of generalized poor performance? J Abnorm Psychol. 1993;102:312–318. doi: 10.1037//0021-843x.102.2.312. [DOI] [PubMed] [Google Scholar]
- 32.Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: goliath defeats david? Biol Psychiatry. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bediou B, Asri F, Brunelin J, et al. Emotion recognition and genetic vulnerability to schizophrenia. Br J Psychiatry. 2007;191:126–130. doi: 10.1192/bjp.bp.106.028829. [DOI] [PubMed] [Google Scholar]
- 34.Alfimova MV, Abramova LI, Barhatova AI, Yumatova PE, Lyachenko GL, Golimbet VE. Facial affect recognition deficit as a marker of genetic vulnerability to schizophrenia. Span J Psychol. 2009;12:46–55. doi: 10.1017/s1138741600001463. [DOI] [PubMed] [Google Scholar]
- 35.Silver H, Bilker W, Goodman C. Impaired recognition of happy, sad and neutral expressions in schizophrenia is emotion, but not valence, specific and context dependent. Psychiatry Res. 2009;169:101–106. doi: 10.1016/j.psychres.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Edwards J, Jackson HJ, Pattison PE, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res. 2001;48:235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- 37.Kohler CG, Turner TH, Bilker WB, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- 38.van't Wout M, Aleman A, Kessels RPC, Cahn W, de Haan EHF, Kahn RS. Exploring the nature of facial affect processing deficits in schizophrenia. Psychiatry Res. 2007;150:227–235. doi: 10.1016/j.psychres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P. A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry. 2002;159:539–545. doi: 10.1176/appi.ajp.159.4.539. [DOI] [PubMed] [Google Scholar]
- 40.Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 41.Hanssen M, Krabbendam L, Vollema M, Delespaul P, Van Os J. Evidence for instrument and family-specific variation of subclinical psychosis dimensions in the general population. J Abnorm Psychol. 2006;115:5–14. doi: 10.1037/0021-843X.115.1.5. [DOI] [PubMed] [Google Scholar]
- 42.Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31:189–195. doi: 10.1017/s0033291701003312. [DOI] [PubMed] [Google Scholar]
- 43.Laroi F, Fonteneau B, Mourad H, Raballo A. Basic emotion recognition and psychopathology in schizophrenia. J Nerv Ment Dis. 2010;198:79–81. doi: 10.1097/NMD.0b013e3181c84cb0. [DOI] [PubMed] [Google Scholar]
- 44.Baas D, van't Wout M, Aleman A, Kahn RS. Social judgement in clinically stable patients with schizophrenia and healthy relatives: behavioural evidence of social brain dysfunction. Psychol Med. 2008;38:747–754. doi: 10.1017/S0033291707001729. [DOI] [PubMed] [Google Scholar]
- 45.Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]