Abstract
Neurogranin (NRGN) is the main postsynaptic protein regulating the availability of calmodulin-Ca(2+) in neurons. NRGN is expressed exclusively in the brain, particularly in dendritic spines and has been implicated in spatial learning and hippocampal plasticity. Genetic variation in rs12807809 in the NRGN gene has recently been confirmed to be associated with schizophrenia in a meta-analysis of genome-wide association studies: the T-allele was found to be genome-wide significantly associated with schizophrenia. Cognitive tests and personality questionnaires were administered in a large sample of healthy subjects. Brain activation was measured with functional magnetic resonance imaging (fMRI) during an episodic memory encoding and retrieval task in a subsample. All subjects were genotyped for NRGN rs12807809. There was no effect of genotype on personality or cognitive measures in the large sample. Homozygote carriers of the T-allele showed better performance in the retrieval task during fMRI. After controlling for memory performance, differential brain activation was evident in the anterior cingulate cortex for the encoding and posterior cingulate regions during retrieval. We could demonstrate that rs12807809 of NRGN is associated with differential neural functioning in the anterior and posterior cingulate. These areas are involved in episodic memory processes and have been implicated in the pathophysiology of schizophrenia in structural and functional imaging as well as postmortem studies.
Keywords: NRGN, fMRI, memory, cingulate
Introduction
Neurogranin (NRGN) is the main postsynaptic protein regulating the availability of calmodulin-Ca(2+) in neurons, by binding to calmodulin in the absence of calcium.1 NRGN is expressed exclusively in the brain.2 It is abundantly expressed in brain regions involved in cognitive functioning and especially enriched in CA1 pyramidal neurons in the hippocampus3 and has been shown to play a role in long-term potentiation,4 spatial learning, and hippocampal plasticity.5 Furthermore, the NRGN gene has been implicated in schizophrenia6,7 and the T-allele of the single nucleotide polymorphism (SNP) rs12807809 (C/T) located upstream of the NRGN has recently been shown to be genome-wide significantly associated with schizophrenia in a meta-analysis of genome-wide association studies.8
Although the exact etiology of schizophrenia still remains uncertain, abnormalities in brain structure and function along with a strong genetic component have consistently been implicated in the disorder. Furthermore, several cognitive domains are impaired, among which episodic memory appears to be one of the most severely affected.9 Recent functional magnetic resonance imaging (fMRI) studies on healthy participants document that variation in susceptibility genes for schizophrenia, among others NRG1,10 G72,11 DTNBP1,12 and ZNF804A 13 modulate the neural activation patterns associated with cognitive processing.
Among the cognitive domains impaired in schizophrenia, episodic memory deficits show high effect sizes (d = 0.74 in Heinrichs and Zakzanis9), while other domains such as executive functions, working memory, and verbal fluency also show marked levels of impairment.9,14 Episodic memory processing has been linked to the hippocampus, cingulate, and frontal and temporal cortical regions.15
Besides memory impairment in patients,9 relatives,16 and subjects at high risk,17 many studies have shown structural alterations in patients with schizophrenia in the medial temporal cortex/hippocampal formation.18–20 These alterations can also be found in subjects with an at risk mental state.21,22 Further functional imaging has demonstrated dysactivation during episodic memory encoding (eg, ref. 23–25) and retrieval26 in the hippocampal cortex in patients. These alterations of medial temporal structures have also been demonstrated in relatives27–29 and high-risk subjects,30,31 which underlines the importance of these structures in the aetiology of schizophrenia.
The cingulate cortex (especially the anterior part) has been shown to be hyperactivated in patients with schizophrenia compared with controls during both encoding and retrieval tasks.32–34 In patients, the anterior commissural line (AC) volume is decreased 35 and the posterior cingulate cortex is smaller in both patients and their healthy relatives compared with controls.36
In animal models, NRGN has been shown to exert a profound influence on memory formation: It could be demonstrated that NRGN is involved in long-term potentiation memory formation and it enhances synaptic strength in the hippocampus.3,37–40 In addition, NRGN null mice exhibit anxiety related behavior.41 During development in the rat brain, the hippocampus and the AC are among the first structures to express NRGN.42
Genetic risk variants for schizophrenia with high allele frequencies and limited effects are also present in a large proportion of the healthy population. It has been shown that investigating the impact of a risk variant in healthy individuals on objectively measurable phenotypes such as performance in fMRI-based neuropsychological paradigms constitutes a successful approach.13 Investigating the influence of genetic variation in healthy subjects circumvents possible confounders such as medication status as well as possible influence of the disorder on brain structure and function. We therefore tested the influence of rs12807809 on cognition and personality in healthy subjects. Furthermore, as NRGN has been implicated in memory processes and anxiety related traits in animals,3,41 the neural correlates of episodic memory encoding and retrieval were investigated. We hypothesized that NRGN risk genotype would be associated with impaired cognitive functioning and—based on behavior observed in NRGN deficient mice—higher neuroticism. Based on prior findings on the influence of NRGN on memory processes in animals and functional imaging studies on episodic memory in patients with schizophrenia and their relatives, it was hypothesized that the influence of genotype on the neural correlates of memory encoding and retrieval would manifest in the cingulate cortex as well as the hippocampal formation.
Methods
Participants
All subjects were recruited from the University of Aachen, Germany. Five hundred and twenty-one subjects underwent neuropsychological and personality assessment and genotyping. Inclusion criteria were age (18–55 years), right-handedness (as assessed by the Edinburgh Inventory43), no psychiatric disorders according to ICD-10, and Western or Middle European descent. A subsample of 94 subjects (66 men) was included in the present study for fMRI scanning procedures. After a complete description of the procedure, subjects provided written informed consent to participating in the study. The protocol was approved by the local ethics committee according to the declaration of Helsinki. The subjects’ characteristics are given in table 1. Genotyping (see below) took place after behavioral testing and fMRI scanning, thus subjects and investigators were blinded with regard to genotype status.
Table 1.
Subjects’ Characteristics: Sex, Age, Education, Cognitive, and Personality Assessment and Performance During the fMRI Recognition Task. Differences in Gender Distribution and Memory Performance were Accounted for in the Statistical Model (See Method Section)
| NRGN Status | T/T | T/C + C/C | t Value | P |
| Whole sample | ||||
| Number of subjects | 359 | 162 | ||
| Sex ratio (men/women) | 190/169 | 78/84 | χ2 = 1.02 | .31 |
| Age (y) | 24.7 ± 5.8 | 24.8 ± 6.0 | 0.14 | .88 |
| Education (y) | 15.7 ± 2.6 | 15.4 ± 2.8 | 1.2 | .25 |
| IQ | 110.2 ± 12.4 | 109.5 ± 11.7 | 0.53 | .59 |
| Cognitive measures | ||||
| Attention | 195.1 ± 36.8 | 191.9 ± 39.7 | 0.88 | .38 |
| Verbal working memory | 16.5 ± 2.6 | 16.4 ± 2.5 | 0.46 | .65 |
| Spatial working memory | 19.2 ± 3.0 | 18.8 ± 2.8 | 1.2 | .23 |
| Executive functioning | 61.3 ± 19.1 | 64.0 ± 20.3 | 1.5 | .14 |
| Semantic verbal fluency | 31.8 ± 9.2 | 30.1 ± 9.1 | 2.0 | .046 |
| NEO-FFI | ||||
| Neuroticism | 1.6 ± .62 | 1.6. ± .63 | 0.33 | .74 |
| Extraversion | 2.4 ± .46 | 2.4 ± .45 | 0.58 | .57 |
| Openness | 2.7 ± .48 | 2.7 ± .48 | 0.39 | .70 |
| Agreeableness | 2.6 ± .48 | 2.6 ± .48 | 0.42 | .67 |
| Conscientiousness | 2.7 ± .55 | 2.7 ± .59 | 0.07 | .95 |
| SPQ-B | ||||
| Cognitive perceptional deficits | 1.7 ± 1.5 | 1.8 ± 1.5 | 0.87 | .39 |
| Interpersonal deficits | 2.2 ± 1.8 | 2.2 ± 1.8 | 0.21 | .83 |
| Disorganization | 1.5 ± 1.6 | 1.4 ± 1.4 | 1.1 | .28 |
| fMRI sample | ||||
| Number of subjects | 67 | 27 | ||
| Sex ratio (men/women) | 53/14 | 13/14 | χ2 = 8.8 | .003 |
| Age (y) | 23.3 ± 3.0 | 23.0 ± 2.8 | 0.53 | .6 |
| Education (y) | 15.7 ± 2.8 | 15.4 ± 1.8 | 0.59 | .6 |
| IQ | 112.3 ± 11.7 | 112.9 ± 11.7 | 0.19 | .8 |
| % correct gender identification (fMRI task) | 98.14 ± 2.3 | 98.8 ± 1.2 | 1.3 | .18 |
| Correctly recognized faces (fMRI task) | 24.5 ± 2.9 | 22.9 ± 3.2 | 2.25 | .027 |
Note: NRGN, Neurogranin; SPQ-B, brief version of the schizotypal personality questionnaire; fMRI, functional magnetic resonance imaging.
Because of the scarcity of homozygous C-allele carriers (n = 9 in the main sample), heterozygous carriers were grouped with homozygous C-allele carriers. All subsequent analyses were therefore performed with 2-sample t tests.
Cognitive Tests and Personality Questionnaires
The following tests were administered in all subjects: A brief verbal IQ assessment,44 the d2 test for attention,45 the letter-number span,46 spatial span,47 the TMT-B,48 and semantic verbal fluency.49 In addition, all subjects completed the NEO-FFI50 and the brief version of the schizotypal personality questionnaire (SPQ-B)51 with the scales cognitive perceptional deficits, interpersonal deficits and disorganization.
Genotyping
Genomic DNA was extracted from ethylenediaminetetraacedic acid anticoagulated venous blood according to standard procedures.52 The SNP rs12807809 was genotyped on an Applied Biosystems 7900HT Fast Real-Time PCR System, using a TaqMan 5′ nuclease assay (TaqMan SNP Genotyping Assay ID C_32029000_20 Applied Biosystems). Genotyping accuracy was assessed by running 15% of the sample in duplicates. Reproducibility was 100%.
Encoding and Retrieval fMRI Paradigm
The paradigm consisted of an encoding and a retrieval task performed in different sessions. Both sessions were divided by a break of approximately 3 minute. During this time, subjects stayed in the scanner.
Encoding Task.
During the encoding phase, either single pictures of neutral faces (encoding condition) or the symbol “#” (baseline condition) were presented on a black background for 4000 ms in a pseudorandomized order using Presentation software package (Neurobehavioral Systems Inc, San Francisco, CA). Following this, stimuli were replaced by a blank screen for another 1000 ms completing one trial. During face encoding, participants were instructed to actively memorize each face for later recognition. In order to ensure continuous attention to the task, participants had to indicate the sex of the displayed person via button press (LUMItouchTM; Lightwave Technologies, Richmond, BC, Canada). During low-level baseline, participants were enforced to press a button with the left index finger every time the symbol # appeared. There were 5 blocks of each condition with 6 responses in each block resulting in 30 faces (half male and half female) to be encoded. Similarly, during baseline, 30 button presses had to be accomplished. Each block lasted for 30 seconds. This task has been applied successfully in different samples previously by our group.25,53–55
Retrieval Session.
After the encoding phase, a recognition phase of equal length and structure was administered. During the retrieval condition, 2 pictures of faces were presented simultaneously side by side, each trial comprising a previously presented face and a new face, randomly positioned at the left or right side. Subjects were requested to select the previously presented face and forced to make a choice by pressing the corresponding button with the left or right index finger. The baseline condition was the same as in the encoding phase.
MRI Data Acquisition
All MRI data were acquired on a 3-Tesla Tim Trio MR scanner (Siemens Medical Systems) at the Research Center Jülich. Functional images were collected with a T2* weighted echo planar imaging sequence sensitive to BOLD contrast (64 × 64 matrix, FOV 200 mm, in plane resolution 3.13 mm, 36 slices, slice thickness 3 mm, TR = 2.25 s, TE = 30 ms, flip angle 90°). Slices covered the whole brain and were positioned transaxially parallel to the anterior-posterior commissural line. One hundred and thirty-seven functional images were collected, and the initial 3 images excluded from further analysis in order to remove the influence of T1 stabilization effects.
FMRI Data Analyses
Analysis of Behavioral Data.
Behavioral data (ie, the number of correctly remembered faces in the retrieval session) were analyzed using an independent t test with rs12807809 status (T/T vs C/T and C/C genotype) as grouping variable.
Analysis of fMRI Data.
SPM5 (www.fil.ion.ucl.ac.uk/spm) standard routines and templates were used for analysis of fMRI data. The functional images were realigned, normalized (resulting voxel size 2 × 2 × 2 mm3), smoothed (8 mm isotropic Gaussian filter), and high-pass filtered (cut off period 120 s).
Statistical analysis was performed in a 2-level mixed-effects procedure. At the first level, the BOLD responses for the activation (encoding and retrieval, respectively) and the baseline condition were modeled by a boxcar function convolved with the canonical hemodynamic response function employed by SPM5. Parameter estimate (ß-) and t statistic images were calculated for each subject. At the second level, the individual ß-contrasts relating to activation differences between the activation and the baseline condition were entered into a t test design with rs12807809 status (T/T vs T/C and C/C status) as grouping variable. First, we calculated group activation maps related to the activation of memory encoding/retrieval (ie, encoding>baseline; retrieval>baseline). Activation maps were thresholded at P < .05, corrected for multiple comparisons (applying the family-wise error correction employed by SPM5). Second, we determined activation differences between the 2 rs12807809 genotype groups. Resulting first-level contrasts were entered in a second level, 2-sample t test. As groups differed with regard to gender distribution and memory performance (see results section and table 1), these 2 parameters were entered as covariates of no interest into the 2-sample t test.
In order to correct for multiple comparisons within a search volume, we applied a cluster extent threshold determined by Monte Carlo simulations.56 For a threshold at the voxel level at P = .001 and spatial properties as present in this study, 10 000 simulations resulted in an extent threshold of 26 resampled voxels. This procedure prevented a false positive rate above 5% due to multiple testing. The anatomical localization of activated brain regions was assessed both by the SPM anatomy toolbox57 and the Talairach atlas.58 Based on effect sizes from previous findings (eg, Kircher et al59), a statistical power of 1−β = .80 resulted with the sample size in the fMRI task for a threshold of P = .001.
As NRGN is implied in memory formation, additional region of interest (ROI) analyses were calculated for the hippocampus proper and the adjacent parahippocampal gyrus (WFU Pickatlas toolbox implemented in SPM5).
Results
Cognitive Functioning and Personality Measures
Homozygous carriers of the T-allele did not differ from C-allele carrieres in either of the administered tests or questionnaires with the sole exception of semantic verbal fluency. Homozygous T-allele carriers produced more words compared with the group of C-allele carriers (31.8 ± 9.2 and 30.1 ± 9.1, respectively, P = .046). This difference did not withstand correction for multiple comparisons.
Genotype did not correlate with IQ, age, or years of education (all P > .05). During the fMRI task, there was an effect of NRGN genotype on the number of correctly recognized faces. Homozygous T-allele carriers showed a significantly better performance during retrieval (P = .027). These differences, along with a difference in gender distribution, were accounted for in the statistical model (see Methods section). All results are given in table 1.
fMRI Results
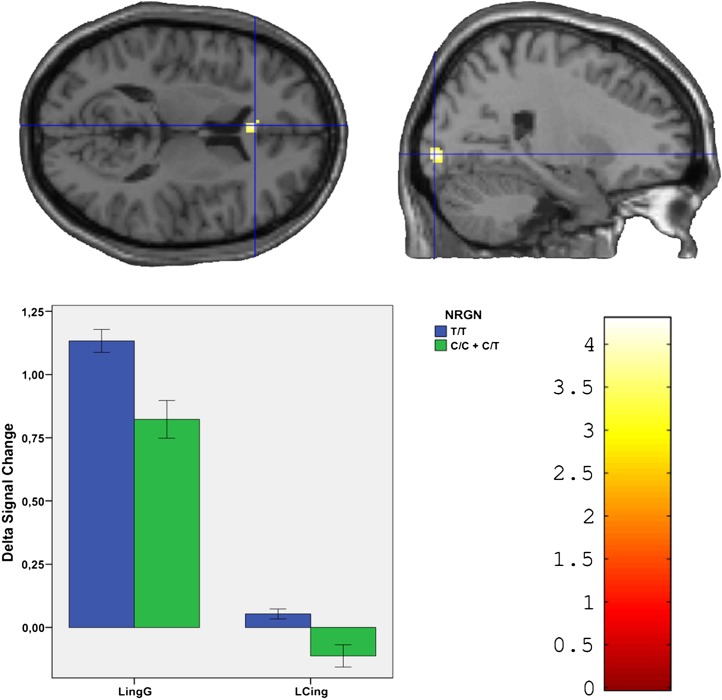
During encoding, homozygous T-allele carriers exhibited stronger activations in the left lingual gyrus (Brodmann areas [BA] 19) and the anterior cingulate cortex (ACC, BA 24) compared with subjects with at least one C-allele (P < .001). There were no significant differences in the gender identification task: homozygous T-allele carriers correctly identified 98.14% (SD = 2.3) of the faces and C-allele carriers correctly identified 98.80% (SD = 1.2) of the faces.
The reversed contrast (T/C + C/C>T/T) did not yield any significant activations. Results are depicted in figure 1 and table 2.
Fig. 1.
Cortical activation during face encoding: top row (brain images) illustrates activations mapped on the standard SPM brain template. Lower left side depicts higher activation of T/T allele carriers compared with T/C and C/C allele carriers in the anterior cingulate (LCing) and the lingual gyrus (LingG) (results: P < .001; Monte Carlo simulated, error bars represent standard error of the mean). Colored bar (bottom right) represents t values. The images are oriented in neurological convention (right hemisphere of the brain corresponds to the right side of the image).
Table 2.
Correlations of rs12807809 of NRGN Status With Neural Activations During Memory Encoding and Retrieval Processes; Only Clusters of At Least 26 Voxels (See Method Section) Are Depicted
| Coordinates | |||||||
| Hemisphere | BA | x | y | z | t Value | Cluster Size (in voxels) | |
| Memory encoding task | |||||||
| (T/T > T/C + C/C) | |||||||
| R | Lingual Gyrus | 18 | 24 | −94 | −2 | 4.28 | 88 |
| L | Anterior Cingulate Cortex | 24 | −4 | 30 | 12 | 3.85 | 35 |
| (C/C + C/T > T/T) | |||||||
| No areas of differential activation | |||||||
| Memory retrieval task | |||||||
| (T/T > T/C + C/C) | |||||||
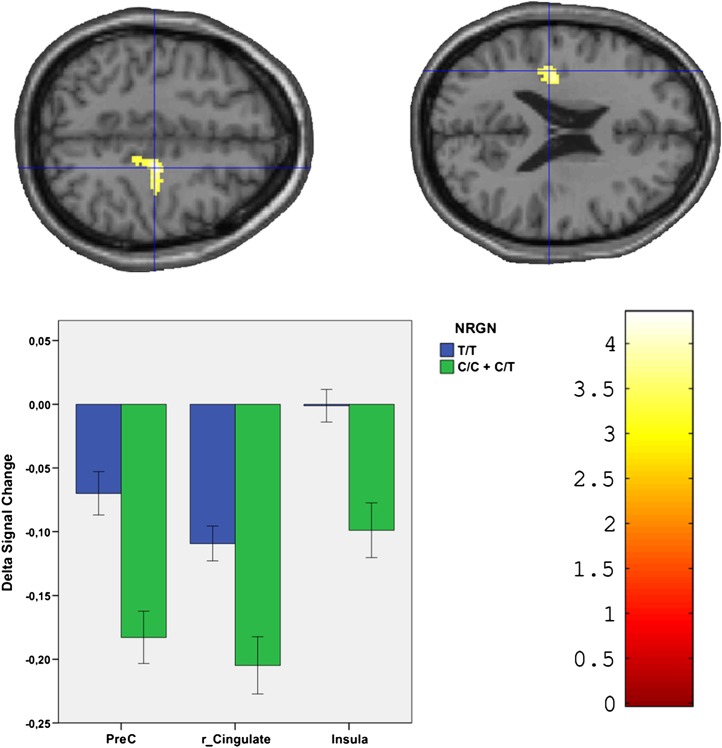
| L | Precentral Gyrus | 4 | −14 | −26 | 64 | 4.22 | 46 |
| R | Cingulate Gyrus | 24 | 20 | −20 | 46 | 4.14 | 161 |
| L | Insula | 13 | −34 | −22 | 24 | 3.99 | 52 |
| (C/C + C/T > T/T) | |||||||
| No areas of differential activation | |||||||
Note: Coordinates are listed in MNI atlas space. BA is the Brodmann area nearest to the coordinate and should be considered approximate. NRGN, Neurogranin.
During retrieval, homozygous T-allele carriers showed less deactivations compared to C-allele carriers in the left precentral gyrus, right cingulate gyrus (expanding to the right precentral gyrus), and the left insula (P < .001; see figure 2). Homozygous T-allele carriers showed better performance (correctly recognized faces) compared with the other group (P = .027; this was taken into account as a covariate of no interest, see above).
Fig. 2.
Cortical activation during face retrieval: top row (brain images) illustrates activations (left precentral gyrus not shown) mapped on the standard SPM brain template. Lower left side depicts parameter estimates derived from clusters of higher activation (due to less deactivation) in T/T allele carriers as compared with T/C and C/C allele carriers in left insula (Insula), left precentral gyrus (PreC), and the cingulate cortex (r_Cingulate; expanding to the right precentral gyrus) (results: P < .001; Monte Carlo simulated, error bars represent standard error of the mean). Colored bar (bottom right) represents t values. The images are oriented in neurological convention (right hemisphere of the brain corresponds to the right side of the image).
For both tasks, ROI analyses neither revealed significant activations within the hippocampus proper nor in the parahippocampal gyrus.
Discussion
This is the first report to describe the influence of a recently discovered genome-wide significant schizophrenia variant8 in NRGN on cognition and personality traits as well as neural correlates of episodic memory encoding and retrieval that was investigated in a large sample of healthy subjects. While no differences in personality dimensions were found in our study, homozygous T-allele carriers showed a trend toward higher performance in a semantic verbal fluency task. During fMRI scanning, homozygous T-allele carriers showed better performance with respect to correctly recognized faces. When controlling for performance and gender distribution, this group showed higher activations in the ACC and the lingual gyrus during encoding and less deactivation in the left insula, left precentral gyrus, and the cingulate gyrus during retrieval. It could previously be demonstrated that NRGN is differentially expressed in the prefrontal cortex in patients with schizophrenia.6 Furthermore, it is involved in long-term potentiation.4 A modulation of its function through genetic variation may therefore be a plausible explanation of our findings at the functional level.
Cognitive Functions and Personality
In the large sample, no differences in cognitive performance or any measures of personality could be detected after correction for multiple testing. This is in line with recent evidence from a study investigating a different German sample.60 However, there was a trend in differences in semantic verbal fluency. Importantly, it has to be noted that homozygous T-allele carriers showed a better performance than C-allele carriers. This result is somewhat surprising with regard to findings in schizophrenia where patients show impairments in semantic verbal fluency.9 However, there are several studies that also show these resulting patterns: in 2 studies investigating cognitive domains in healthy subjects, similar results have been demonstrated with respect to G72.11,61 In these studies, subjects with the risk diplotype showed a higher performance in working memory and attention than subjects without the risk diplotype. In a related fashion, Stefanis et al62 found that healthy carriers of the minor allele in Dysbindin rs1018381 scored lower on the paranoid factor of the SPQ. Since these studies demonstrated that risk allele carriers also showed better results in a variety of variables, the present study may point to possible further investigations into semantic processing associated with NRGN genotype variations.
fMRI Results
Encoding.
The ACC has consistently been implied in control and decision-making processes (eg, Kennerley et al63) as well as episodic memory (eg, Svoboda et al64). Particularly, the BOLD signal increase in this region has been shown to correlate with subsequent memory performance,65 demonstrating its importance in encoding strategies. In schizophrenia, meta-analytical findings demonstrated higher ACC activations in patients as compared with healthy controls during episodic memory encoding.33 A similar activation pattern has also been reported for executive processing comparing patients with schizophrenia to healthy controls.66 Meta-analyses additionally confirmed a volume reduction in the ACC in patients compared with controls (see also Introduction section).67 Taken together, these findings implicate the ACC as an important structure in memory encoding as well as the pathophysiology of schizophrenia. Our T/T risk allele carriers exhibited stronger activation in the ACC during encoding new material. This may indicate that they must exert higher cognitive control in order to reach a similar performance level as the nonrisk carrier group.
The lingual gyrus (BA 19) is being activated when subjects process features of faces rather than the configuration of a face as a whole.68 It could thus be argued that homozygous T-allele carriers may focus more on particular single facial components while encoding novel faces. This is a strategy that has been attributed to patients with schizophrenia.69,70
Retrieval.
The cingulate gyrus, especially the posterior part, has been implied as a part of a “core” network subserving episodic memory,64 especially recognition of previously encoded material.71 As such, it could be hypothesized that homozygous T-allele carriers rely more on this region in order to adequately perform memory processes.
Activations of the left insula in patients with schizophrenia during episodic memory processes are a common finding in fMRI studies (meta-analysis eg, Ragland et al33). In addition, left insular volume is decreased in schizophrenia compared with healthy subjects. These changes are already obvious in first-episode schizophrenia (meta-analysis in Ellison-Wright et al72).
Other studies also found higher activations in the left precentral gyrus in patients compared with controls during memory retrieval (eg, ref. 33,73,74). The differences in activation occurred while controlling for performance in the statistical analyses. It could be argued that homozygous T-allele carriers show less deactivation in the reported regions and thus compensating behavioral underperformance. Overrecruitment of task-related brain regions is a phenomenon found in the ageing brain75 and schizophrenia76 but has also been shown to be present in risk allele carriers of NRG1 in healthy subjects.10 Following this logic, one could hypothesize that fine tuning of cortical activations during memory performance is disturbed in homozygous T-allele carriers.
In contrast to our hypotheses, we could not detect an influence of NRGN genotype on hippocampal activation in our ROI analyses, neither for encoding nor retrieval. The encoding paradigm was developed to particularly activate the hippocampus and we could previously detect activation differences in these areas between healthy subjects and patients with schizophrenia55 and Alzheimer’s Disease.53 It is unlikely that we missed differences in the current study. It seems that this common variant—even though associated with memory functions as evidenced by our fMRI task—exerts an influence on a widespread neural network that is implied in memory formation, such as the ACC and the insula, but not the hippocampal formation. Variations in other genes associated with schizophrenia have been shown to impact on hippocampal structure and functioning during memory processes.77 As such, it is suggested that some, but not all, risk polymorphisms involved in schizophrenia may impact on hippocampal formation processes. Consequently, different risk variants act on different neural networks, their prominent locus of expression depending on their relative function during brain development. Among other factors, this might explain the heterogeneous symptomatology and course of the disorder.
In sum, when correcting for multiple comparisons, the present findings demonstrate that rs12807809 does not seem to influence domains of cognition or personality dimensions in this sample of healthy subjects, which replicates the results of a recent study.60 However, there was a trend toward differences in semantic verbal fluency performance and in episodic memory functions, which warrant further investigation. The main finding was that rs12807809 exerts a profound influence on the cortical activation/deactivation pattern evident during episodic memory processes. In this study, we could show that variation in rs12807809 does not necessarily influence memory processes orchestrated by the hippocampus proper but rather impacts on cortical regions that have been implicated in this function. Thus these findings suggest that rs12807809 has a pleiotropic effect on different brain networks. As this is the first study on NRGN rs12807809 replications of our findings are warranted. Even though we studied a large sample of healthy subjects, it still remains the possibility that these are chance findings.
Funding
This work was supported by grants from the Federal Ministry of Education and Research (BMBF grant no. 01GO0204) and within the context of the German National Genome Research Network (NGFN-2 and NGFN-Plus FKZ 01GS08147).
Acknowledgments
A.K. and S.K. share joint first authorship and M.R. and T.K. share joint senior authorship. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Baudier J, Deloulme JC, Van Dorsselaer A, Black D, Matthes HW. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem. 1991;266:229–237. [PubMed] [Google Scholar]
- 2.Martinez de Arrieta C, Perez Jurado L, Bernal J, Coloma A. Structure, organization, and chromosomal mapping of the human neurogranin gene (NRGN) Genomics. 1997;41:243–249. doi: 10.1006/geno.1997.4622. [DOI] [PubMed] [Google Scholar]
- 3.Huang FL, Huang KP, Boucheron C. Long-term enrichment enhances the cognitive behavior of the aging neurogranin null mice without affecting their hippocampal LTP. Learn Mem. 2007;14:512–519. doi: 10.1101/lm.636107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhabotinsky AM, Camp RN, Epstein IR, Lisman JE. Role of the neurogranin concentrated in spines in the induction of long-term potentiation. J Neurosci. 2006;26:7337–7347. doi: 10.1523/JNEUROSCI.0729-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pak JH, Huang FL, Li J, et al. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc Natl Acad Sci U S A. 2000;97:11232–11237. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broadbelt K, Ramprasaud A, Jones LB. Evidence of altered neurogranin immunoreactivity in areas 9 and 32 of schizophrenic prefrontal cortex. Schizophr Res. 2006;87:6–14. doi: 10.1016/j.schres.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Ruano D, Aulchenko YS, Macedo A, et al. Association of the gene encoding neurogranin with schizophrenia in males. J Psychiatr Res. 2008;42:125–133. doi: 10.1016/j.jpsychires.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 10.Krug A, Markov V, Eggermann T, et al. Genetic variation in the schizophrenia-risk gene neuregulin1 correlates with differences in frontal brain activation in a working memory task in healthy individuals. Neuroimage. 2008;42:1569–1576. doi: 10.1016/j.neuroimage.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 11.Jansen A, Krach S, Krug A, et al. A putative high risk diplotype of the G72 gene is in healthy individuals associated with better performance in working memory functions and altered brain activity in the medial temporal lobe. Neuroimage. 2009;45:1002–1008. doi: 10.1016/j.neuroimage.2008.12.054. [DOI] [PubMed] [Google Scholar]
- 12.Markov V, Krug A, Krach S, et al. Genetic variation in schizophrenia-risk-gene dysbindin 1 modulates brain activation in anterior cingulate cortex and right temporal gyrus during language production in healthy individuals. Neuroimage. 2009;47:2016–2022. doi: 10.1016/j.neuroimage.2009.05.067. [DOI] [PubMed] [Google Scholar]
- 13.Esslinger C, Walter H, Kirsch P, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 15.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 16.Whyte MC, McIntosh AM, Johnstone EC, Lawrie SM. Declarative memory in unaffected adult relatives of patients with schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2005;78:13–26. doi: 10.1016/j.schres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Seidman LJ, Giuliano AJ, Meyer EC, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 19.Seidman LJ, Pantelis C, Keshavan MS, et al. A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophr Bull. 2003;29:803–830. doi: 10.1093/oxfordjournals.schbul.a007048. [DOI] [PubMed] [Google Scholar]
- 20.Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 21.Borgwardt SJ, McGuire PK, Aston J, et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry. Suppl. 2007;51:s69–s75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- 22.Lawrie SM, McIntosh AM, Hall J, Owens DG, Johnstone EC. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull. 2008;34:330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyer P, Phillips JL, Rousseau FL, Ilivitsky S. Hippocampal abnormalities and memory deficits: new evidence of a strong pathophysiological link in schizophrenia. Brain Res Rev. 2007;54:92–112. doi: 10.1016/j.brainresrev.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 25.Leube DT, Erb M, Grodd W, Bartels M, Kircher TT. Differential activation in parahippocampal and prefrontal cortex during word and face encoding tasks. Neuroreport. 2001;12:2773–2777. doi: 10.1097/00001756-200108280-00035. [DOI] [PubMed] [Google Scholar]
- 26.Jessen F, Scheef L, Germeshausen L, et al. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am J Psychiatry. 2003;160:1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- 27.Goldman AL, Pezawas L, Mattay VS, et al. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry. 2008;63:475–483. doi: 10.1016/j.biopsych.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Ho BC, Magnotta V. Hippocampal volume deficits and shape deformities in young biological relatives of schizophrenia probands. Neuroimage. 2010;49:3385–3393. doi: 10.1016/j.neuroimage.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thermenos HW, Seidman LJ, Poldrack RA, et al. Elaborative verbal encoding and altered anterior parahippocampal activation in adolescents and young adults at genetic risk for schizophrenia using FMRI. Biol Psychiatry. 2007;61:564–574. doi: 10.1016/j.biopsych.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 30.Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh AM, Owens DC, Moorhead WJ, et al. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biol Psychiatry. 2011;69:953–958. doi: 10.1016/j.biopsych.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Koch K, Wagner G, Nenadic I, et al. Fronto-striatal hypoactivation during correct information retrieval in patients with schizophrenia: an fMRI study. Neuroscience. 2008;153:54–62. doi: 10.1016/j.neuroscience.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 33.Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- 35.Haznedar MM, Buchsbaum MS, Hazlett EA, Shihabuddin L, New A, Siever LJ. Cingulate gyrus volume and metabolism in the schizophrenia spectrum. Schizophr Res. 2004;71:249–262. doi: 10.1016/j.schres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Calabrese DR, Wang L, Harms MP, et al. Cingulate gyrus neuroanatomy in schizophrenia subjects and their non-psychotic siblings. Schizophr Res. 2008;104:61–70. doi: 10.1016/j.schres.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong L, Cherry T, Bies CE, Florence MA, Gerges NZ. Neurogranin enhances synaptic strength through its interaction with calmodulin. EMBO J. 2009;28:3027–3039. doi: 10.1038/emboj.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang FL, Huang KP, Wu J, Boucheron C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J Neurosci. 2006;26:6230–6237. doi: 10.1523/JNEUROSCI.1182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang KP, Huang FL, Jager T, Li J, Reymann KG, Balschun D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J Neurosci. 2004;24:10660–10669. doi: 10.1523/JNEUROSCI.2213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J, Huang KP, Huang FL. Participation of NMDA-mediated phosphorylation and oxidation of neurogranin in the regulation of Ca2+- and Ca2+/calmodulin-dependent neuronal signaling in the hippocampus. J Neurochem. 2003;86:1524–1533. doi: 10.1046/j.1471-4159.2003.01963.x. [DOI] [PubMed] [Google Scholar]
- 41.Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez-Bolado G, Rodriguez-Sanchez P, Tejero-Diez P, Fairen A, Diez-Guerra FJ. Neurogranin in the development of the rat telencephalon. Neuroscience. 1996;73:565–580. doi: 10.1016/0306-4522(96)00061-9. [DOI] [PubMed] [Google Scholar]
- 43.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 44.Lehrl S, Triebig G, Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand. 1995;91:335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x. [DOI] [PubMed] [Google Scholar]
- 45.Brickenkamp R. Der Aufmerksamkeits-Belastungstest d2 [The d2 test of Attention] Göttingen, Germany: Hogrefe; 2002. [Google Scholar]
- 46.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 47.Wechsler D. Wechsler Memory Scale: Administration and Scoring Manual. San Antonio, TX: Harcourt Brace & Co; 1997. [Google Scholar]
- 48.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 49.Daum I, Graber S, Schugens MM, Mayes AR. Memory dysfunction of the frontal type in normal ageing. Neuroreport. 1996;7:2625–2628. doi: 10.1097/00001756-199611040-00043. [DOI] [PubMed] [Google Scholar]
- 50.Costa PM, McCrae RR. Professional Manual for the Revised NEO Personality Inventory. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 51.Raine A, Benishay D, The SPQ- B. A brief screening instrument for schizotypal personality disorder. J Personal Disord. 1995;9:346–355. [Google Scholar]
- 52.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kircher TT, Erb M, Grodd W, Leube DT. Cortical activation during cholinesterase-inhibitor treatment in Alzheimer disease: preliminary findings from a pharmaco-fMRI study. Am J Geriatr Psychiatry. 2005;13:1006–1013. doi: 10.1176/appi.ajgp.13.11.1006. [DOI] [PubMed] [Google Scholar]
- 54.Leube DT, Erb M, Grodd W, Bartels M, Kircher TT. Successful episodic memory retrieval of newly learned faces activates a left fronto-parietal network. Brain Res Cogn Brain Res. 2003;18:97–101. doi: 10.1016/j.cogbrainres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Leube DT, Rapp A, Buchkremer G, et al. Hippocampal dysfunction during episodic memory encoding in patients with schizophrenia-an fMRI study. Schizophr Res. 2003;64:83–85. doi: 10.1016/s0920-9964(02)00503-0. [DOI] [PubMed] [Google Scholar]
- 56.Slotnick SD, Moo LR, Segal JB, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 57.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 58.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme; 1988. [Google Scholar]
- 59.Kircher T, Krug A, Markov V, et al. Genetic variation in the schizophrenia-risk gene neuregulin 1 correlates with brain activation and impaired speech production in a verbal fluency task in healthy individuals. Hum Brain Mapp. 2009;30:3406–3416. doi: 10.1002/hbm.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donohoe G, Walters J, Morris DW, et al. A neuropsychological investigation of the genome wide associated schizophrenia risk variant NRGN rs12807809. Schizophr Res. 2011;125:304–306. doi: 10.1016/j.schres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Jansen A, Krach S, Krug A, et al. Effect of the G72 (DAOA) putative risk haplotype on cognitive functions in healthy subjects. BMC Psychiatry. 2009;9:60. doi: 10.1186/1471-244X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stefanis NC, Trikalinos TA, Avramopoulos D, et al. Impact of schizophrenia candidate genes on schizotypy and cognitive endophenotypes at the population level. Biol Psychiatry. 2007;62:784–792. doi: 10.1016/j.biopsych.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 63.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 64.Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chee MW, Goh JO, Lim Y, Graham S, Lee K. Recognition memory for studied words is determined by cortical activation differences at encoding but not during retrieval. Neuroimage. 2004;22:1456–1465. doi: 10.1016/j.neuroimage.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 66.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fornito A, Yucel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35:973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lobmaier JS, Klaver P, Loenneker T, Martin E, Mast FW. Featural and configural face processing strategies: evidence from a functional magnetic resonance imaging study. Neuroreport. 2008;19:287–291. doi: 10.1097/WNR.0b013e3282f556fe. [DOI] [PubMed] [Google Scholar]
- 69.Leppanen JM, Niehaus DJ, Koen L, Schoeman R, Emsley R. Allocation of attention to the eye and mouth region of faces in schizophrenia patients. Cognit Neuropsychiatry. 2008;13:505–519. doi: 10.1080/13546800802608452. [DOI] [PubMed] [Google Scholar]
- 70.Loughland CM, Williams LM, Gordon E. Schizophrenia and affective disorder show different visual scanning behavior for faces: a trait versus state-based distinction? Biol Psychiatry. 2002;52:338–348. doi: 10.1016/s0006-3223(02)01356-2. [DOI] [PubMed] [Google Scholar]
- 71.Kircher T, Weis S, Leube D, et al. Anterior hippocampus orchestrates successful encoding and retrieval of non-relational memory: an event-related fMRI study. Eur Arch Psychiatry Clin Neurosci. 2008;258:363–372. doi: 10.1007/s00406-008-0805-z. [DOI] [PubMed] [Google Scholar]
- 72.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ragland JD, Gur RC, Valdez J, et al. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Assaf M, Rivkin PR, Kuzu CH, et al. Abnormal object recall and anterior cingulate overactivation correlate with formal thought disorder in schizophrenia. Biol Psychiatry. 2006;59:452–459. doi: 10.1016/j.biopsych.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 75.Vallesi A, McIntosh AR, Stuss DT. Overrecruitment in the aging brain as a function of task demands: evidence for a compensatory view. J Cogn Neurosci. 2011;23:801–815. doi: 10.1162/jocn.2010.21490. [DOI] [PubMed] [Google Scholar]
- 76.Ragland JD, Gur RC, Valdez JN, et al. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am J Psychiatry. 2005;162:1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Giorgio A, Blasi G, Sambataro F, et al. Association of the SerCys DISC1 polymorphism with human hippocampal formation gray matter and function during memory encoding. Eur J Neurosci. 2008;28:2129–2136. doi: 10.1111/j.1460-9568.2008.06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]