Abstract
Objective: There is increasing evidence that schizophrenia patients have difficulties in the hedonic appraisal of odors. In a prior study, we assessed olfactory hedonic perception birhinally and found that males with schizophrenia failed to attach the appropriate hedonic valence to a pleasant odor, despite correctly perceiving changes in odor intensity. Female patients, in contrast, exhibited normal responses. The current study extends this work by examining odor valence processing in unaffected first-degree relatives of schizophrenia patients, to determine the extent to which this abnormality may be genetically mediated. We also examine odor valence processing unirhinally, rather than birhinally, to probe possible lateralized differences in patients’ hedonic processing deficits. Method: Individuals with schizophrenia (n = 54), first-degree unaffected family members (n = 22), and demographically matched controls (n = 45) were administered the Suprathreshold Amyl Acetate Odor Intensity and Odor Pleasantness Rating Test. Results: In contrast to family members and controls, both male and female schizophrenia probands underevaluated the hedonic characteristics of amyl acetate at lower concentrations and overevaluated its pleasantness at concentrations perceived as unpleasant by both controls and relatives. These patient-specific differences could not be explained by differences in smoking habit, medication use, or subjective ratings of odor intensity. However, they were associated with increased levels of anhedonia/asociality and negative symptomatology. Conclusions: Our findings suggest that both male and female schizophrenia patients have difficulties in the unirhinal appraisal of hedonic valence. Normal responses in unaffected first-degree relatives suggest that this is an environmentally, rather than genetically, mediated abnormality denoting negative symptomatology.
Keywords: smell, olfactory, valence processing, pleasantness, emotion, olfaction
Introduction
Anhedonia, the decreased capacity to experience pleasure, is an enduring and treatment-resistant negative symptom in the presentation of schizophrenia.1,2 Increased social anhedonia is thought to be a marker of transition to psychosis3 and, in patients, is associated with poor premorbid adjustment, decreased social functioning, and poor long-term outcome.4,5 Therefore, understanding the neural correlates of anhedonia may contribute to elucidating the pathophysiology of schizophrenia and help identify first-episode patients and at-risk individuals early in the course of illness.
Among neurobehavioral probes of hedonic capacity, olfactory measures may have a unique advantage owing to direct and reciprocal connections between brain regions modulating olfaction and emotion processing, including the orbitofrontal cortex (OFC), amygdala, and hippocampus. In schizophrenia, structural and functional abnormalities in the olfactory-orbitofrontal circuitry have been consistently observed (for a review, see Turetsky et al6), and reduced activity in the OFC and putamen/ventral striatum is inversely associated with anhedonia in patients.7 Behaviorally, increased olfactory anhedonia is associated with a higher level of negative symptoms8,9 and is more profound in patients with deficit syndrome schizophrenia.10 Taken together, these data suggest that similar neural circuitry may underlie odor hedonic processing and trait anhedonia.
Current reports on olfactory anhedonia have indicated that patients have difficulties in the appraisal of odor valence despite intact intensity perception,11,12 though how these abnormalities manifest have varied across studies. Several reports have noted attenuated pleasantness ratings for pleasant but not unpleasant odors in patients.11−13 (Note: Data from the Hudry et al12 report were reanalyzed separately for pleasant and unpleasant odor valences, and results were reported in Plailly et al13). Conversely, others have reported increased pleasantness ratings in patients8,14 or pleasantness ratings restricted to the higher end of the pleasantness spectrum in contrast to more variability observed in controls.9 While these studies collectively noted disruptions in odor hedonic processing, the use of disparate odors with varying chemical compositions, trigeminal contributions, and dimensions of edibility, familiarity, and pleasantness, may have introduced potential confounds into the analysis. In an attempt to minimize these confounds, our group ascertained pleasantness and intensity ratings following birhinal presentation of 1 odor, amyl acetate, at 4 suprathreshold concentrations.15 While groups did not differ in their appreciation of intensity differences across odor concentrations, healthy individuals rated amyl acetate as more pleasant at weaker concentrations and increasingly unpleasant as the concentration increased. Female patients showed a pattern of ratings that was similar to controls, whereas male patients rated the odor as significantly more pleasant at higher concentrations. Sexually dimorphic pleasantness ratings are consistent with reports that male patients experience more enduring negative symptoms, earlier illness onset, and decreased social, cognitive, and premorbid functioning compared with their female counterparts (for a review, see Leung et al16).
Despite the growing body of literature on olfactory hedonic processing in schizophrenia, no studies have examined possible odor hedonic disturbance in unaffected family members of patients. Given the familial basis for increased social anhedonia17,18 and olfactory impairments,19,20 the aim of the current study was to examine odor valence processing in relatives of schizophrenia patients. We also sought to extend prior work by assessing odor valence processing unirhinally (separately for each nostril), as opposed to birhinally as we did in our previous study.15 Unirhinal odor presentation allows the examination of potentially important lateralized differences in hedonic processing. It also enables us to assess hedonic processing in the absence of birhinal facilitation, whereby olfactory performance is enhanced through secondary integration of left and right nostril inputs.
Methods
Participants
Participants in the current study were recruited to the University of Pennsylvania Medical Center into 1 of 3 groups as follows: (1) schizophrenia probands (n = 54) who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) diagnosis of schizophrenia, (2) healthy comparison subjects (n = 45), and (3) first-degree unaffected family members of individuals with schizophrenia (n = 22). Participants underwent a standard clinical assessment using the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient or Nonpatient Edition (SCID21) by trained master’s level clinicians, as well as a detailed medical history including a physical examination and laboratory testing by a licensed psychiatrist (C.G.K., R.E.G., and B.I.T.). Diagnoses were established by consensus conference of psychologists and psychiatrists using best estimate DSM-IV-TR22 diagnoses for schizophrenia. A trained diagnostician rated patients on the Brief Psychiatric Rating Scale (BPRS23), the Scales for Assessment of Negative Symptoms (SANS24) and Positive Symptoms (SAPS25), as well as the Hamilton Psychiatric Rating Scale for Depression (HAM-D26). Family members and controls were also assessed for a DSM-IV Axis II disorder with the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II27) in order to rule out the presence of an Axis I disorder. Raters were trained to a criterion reliability of .90 (intraclass correlation). Individuals were excluded from participation from all groups based on a lack of proficiency in English, history of neurological disorder, loss of consciousness, head trauma, current substance abuse (determined by a urine drug screen), mental retardation, past substance dependence, and the presence of a medical condition that could alter cerebral or olfactory functioning (eg, an upper respiratory infection, common cold, or allergy).
Unaffected family members were first-degree relatives (father, mother, offspring, or full sibling) of an individual affected with the sole diagnosis of schizophrenia. The cohort of unaffected first-degree relatives represented 6 parents, 15 siblings, and 1 offspring. Healthy participants and family members were screened for any current or past history of a DSM-IV Axis I or Axis II Disorder using the SCID—Nonpatient Edition. After providing a complete description of the study to participants, written informed consent was obtained from all participants. All study procedures were approved by the University of Pennsylvania Institutional Review Board and complied with the Declaration of Helsinki’s ethical standards in the treatment of human research participants.
The 3 groups did not differ with regard to ‘age’ (F 2 ,118 = 1.50, P = .23), ‘sex composition’ (χ2 = 1.81, df = 2, P = .40), ‘handedness’ (χ2 = 3.96, df = 4, P = .41), or ‘ethnicity’ (χ2 = 8.26, df = 6, P = .22). Overall group differences in educational attainment were statistically significant (F 2 ,118 = 4.97, P = .01). Healthy comparison subjects had more years of education than patients (F 1 ,118 = 9.66, P = .002), while differences between healthy controls and family members were not statistically significant (F 1 ,118 = 0.41, P = .52). Patients and family members did not differ significantly with regard to educational attainment (F 1 ,118 = 3.20, P = .08). Conversely, groups did not differ with regard to parental education (Wilks’ F 4 ,170 = 1.30, P = .27). Overall group differences in ‘smoking’ (measured as packs per day) were statistically significant (F 2 ,111 = 5.03, P = .01). Patients smoked significantly more than the healthy comparison subjects (F 1 ,111 = 9.55, P = .003) but the same as family members (F 1 ,111 = 0.14, P = .71). Differences between family members and controls trended toward significance (F 1, 111 = 3.59, P = .06).
Of the patients with schizophrenia, 8 individuals were unmedicated and 46 were on a regimen of antipsychotic medications (11 were on first-generation antipsychotics and 35 were on second-generation antipsychotics). Medication dosages were converted to chlorpromazine equivalents using published reference tables.28 Means and standard deviations for sample demographic and clinical data are provided in table 1.
Table 1.
Demographic and Clinical Characteristics
| Characteristics | Schizophrenia Probands (n = 54) | Family Members (n = 22) | Control Group (n = 45) | ||||||
| Mean | SD | % (N) | Mean | SD | % (N) | Mean | SD | % (N) | |
| Age (years) | 40.69 | 8.89 | 45.68 | 16.00 | 40.89 | 13.03 | |||
| Sex | |||||||||
| Males | 57.4 (31) | 40.9 (9) | 55.6 (25) | ||||||
| Females | 42.6 (23) | 59.1 (13) | 44.4 (20) | ||||||
| Ethnicity | |||||||||
| Caucasian | 27.8 (15) | 40.9 (9) | 51.1 (23) | ||||||
| African-American | 68.5 (37) | 59.1 (13) | 42.2 (19) | ||||||
| Latino-American | 1.9 (1) | 0 (0) | 4.4 (2) | ||||||
| Asian-American | 1.9 (1) | 0 (0) | 2.2 (1) | ||||||
| Education level* (years) | 12.57 | 2.08 | 12.95 | 2.26 | 14.04 | 2.66 | |||
| Mother’s education (years) | 12.19 | 2.60 | 11.47 | 2.94 | 13.32 | 2.03 | |||
| Father’s education (years) | 12.15 | 3.76 | 11.71 | 4.36 | 12.69 | 2.76 | |||
| Pack-days* | 0.34 | 0.48 | 0.30 | 0.41 | 0.09 | 0.20 | |||
| Illness duration (years) | 16.89 | 9.50 | |||||||
| Age of onset (years) | 22.76 | 7.71 | |||||||
| BPRS23 total score | 29.42 | 8.01 | |||||||
| SANS24 total score | 26.98 | 15.78 | |||||||
| SAPS25 total score | 21.62 | 15.77 | |||||||
| HAM-D26 total score | 6.22 | 6.88 | |||||||
| Chlorpromazine equivalents | 353.79 | 491.13 | |||||||
Note: BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; HAM-D, Hamilton Psychiatric Rating Scale for Depression.
*Significant group difference (P = .01).
Suprathreshold Amyl Acetate Odor Intensity and Odor Pleasantness Rating Test
Measures of odor intensity and pleasantness were assessed using the Suprathreshold Amyl Acetate Odor Intensity and Odor Pleasantness Rating Test,15 a modified version of a hedonic task created by Doty et al.29 Subjects were presented with 100-ml glass containers with 4 suprathreshold concentrations (−1.00, −2.00, −3.00, and −4.00 log vol/vol, from strong to weak intensity) of amyl acetate, with United States Pharmacopeia grade light mineral oil as the diluent. Odor concentrations were administered unirhinally (ie separately to each nostril). The individual’s contralateral nostril was occluded with Durapore tape (3M Corporation, Minneapolis, Minnesota) in order to minimize retronasal airflow.30 Standardized administration was completed by a trained technician that opened each glass bottle, held it under the unoccluded nostril, and acquired intensity and pleasantness ratings on separate 5-point scales for each odor presentation. The scales used to assess odor intensity and pleasantness were adaptations of the Self-Assessment Manikin (developed by Lang et al31). The 4 odor concentrations were presented 5 times in a counterbalanced order to each nostril, for a total of 20 trials in each rating condition. Previous research has suggested a test-retest reliability of 0.75 or greater for similar tasks.29
Data Analysis
Primary data analysis was performed in STATISTICA (Statsoft Inc., Tulsa, Oklahoma). Overall group differences in age, education, smoking, and parental education were assessed using an ANOVA. Pearson chi-squares were used to determine group differences for sex, handedness, and ethnicity. A MANCOVA was conducted separately for intensity and pleasantness ratings. Left and right nostril ratings across the 4 concentrations (−1.00, −2.00, −3.00, and −4.00 log vol/vol) were repeated measure factors, with group and sex as between-subject factors. Smoking (packs/day) was included as a covariate in all analyses. Post hoc univariate analyses were conducted for significant effects by examining pairwise group contrasts on individual measures.
In order to account for the potential impact of differences in intensity perception on hedonic ratings, group differences were also examined using the Generalized Linear Latent and Mixed Models algorithm implemented in Stata 9.0 (StataCorp., College Station, Texas), with subject as a random-effects factor. Group (patient/relative/control), nostril, odor concentration, and sex were included as fixed-effects predictors of response. Individual intensity ratings and smoking were included as covariates in the analysis. Significance levels were assessed using the Wald test statistic with χ2 distribution.
Within the patient group, relationships between pleasantness ratings and clinical assessments (duration of illness, age of onset, negative and positive symptoms, and chlorpromazine equivalents) were measured using Pearson’s correlations (r).
Results
Intensity Ratings
Analysis of intensity ratings revealed a significant main effect of concentration (F 3 ,321 = 426.94, P < .0001) with all participants rating stronger concentrations as more intense than adjacent weaker concentrations (all P’s < .0001). No effect of nostril (F 1 ,107 = 0.01, P = .94), sex (F 1 ,107 = 0.03, P = .87), or group-by-sex interaction (F 2 ,107 = 1.39, P = .25) was observed.
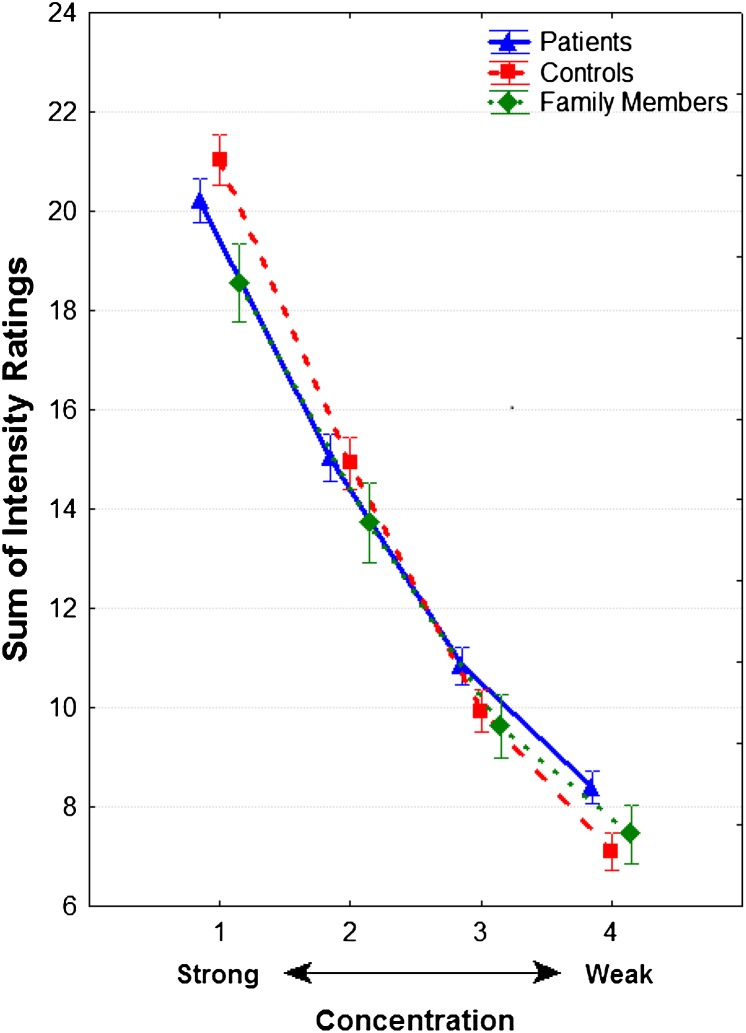
However, a significant group-by-concentration interaction (F 6 ,321 = 2.51, P = .02) was noted. In paired contrasts, this interaction was significant for both patients vs controls (F 3 ,273 = 3.25, P = .02) and family members vs controls (F 3 ,168 = 4.67, P = .004) but not for patients vs family members (F 3 ,198 = 0.21, P = .89). As illustrated in figure 1, control subjects rated the strongest (1.00 log vol/vol) concentration as more intense and the weakest (–4.00 log vol/vol) concentration as less intense than patients and family members.
Fig. 1.
Odor intensity ratings (±SE) for suprathreshold concentrations of amyl acetate in patients with schizophrenia, first-degree unaffected relatives, and healthy comparison subjects. Note: Participants were presented with 4 odor concentrations (from strongest to weakest: −1.00, −2.00, −3.00, and −4.00 log vol/vol) to each nostril 5 times in counterbalanced order for a total of 40 trials
Pleasantness Ratings
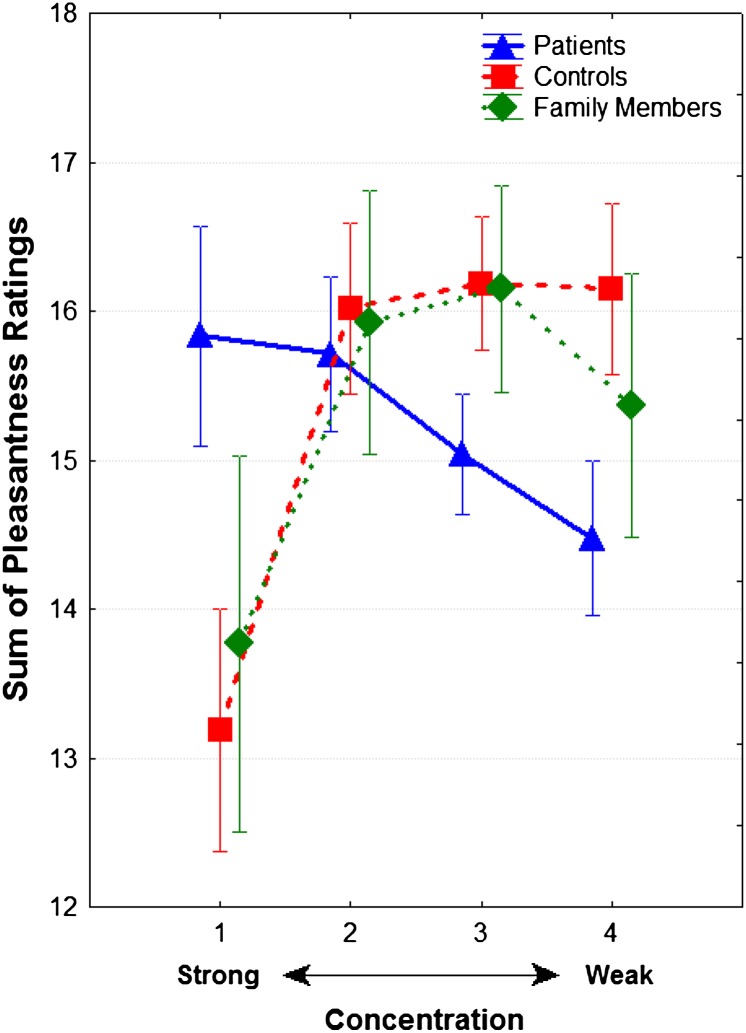
A statistically significant group-by-concentration interaction was observed (F 6 ,321 = 3.35, P = .003). In paired contrasts, this interaction was significant only for patients vs controls (F 3 ,273 = 5.85, P < .001), although there was also a trend toward significance (F 3 ,198 = 2.43, P = .06) for patients vs family members. Family members and controls were indistinguishable (F 3 ,168 = 0.07, P = .98). As seen in figure 2, controls and family members showed parallel changes in odor pleasantness ratings, with the strongest odor concentration being rated as the least pleasant. Schizophrenia patients, in contrast, showed a linear increase in pleasantness ratings with increasing odor concentration; they rated weaker odors as less pleasant and the strongest odor as more pleasant than both controls and family members. The statistically significant concentration-by-group interaction persisted after included intensity ratings as a covariate (χ2 = 41.88, df = 3, P < .0001), and intensity ratings did not significantly predict pleasantness ratings (Z = 1.06, P = .29).
Fig. 2.
Odor pleasantness ratings (±SE) for suprathreshold concentrations of amyl acetate in patients with schizophrenia, first-degree unaffected relatives, and healthy comparison subjects. Note: Participants were presented with 4 odor concentrations (from strongest to weakest: −1.00, −2.00, −3.00, and −4.00 log vol/vol) to each nostril 5 times in counterbalanced order for a total of 40 trial
When comparing valence ratings between the strongest and weakest concentration within each group, healthy controls had significantly higher pleasantness ratings for the weakest compared with the strongest concentration (F 1 ,107 = 5.93, P = .01). Neither patients (F 1 ,107 = 1.39, P = .24) nor family members (F 1 ,107 = 1.15, P = .28) exhibited a significant difference between the ratings for the strongest and weakest concentrations. Family members’ ratings were in the same direction as controls, while patients’ ratings were in the opposite direction.
In addition, a main effect of nostril (F 1 ,107 = 4.13, P = .03) and a significant nostril-by-concentration interaction (F 3 ,321 = 3.96, P = .01) were observed. Individuals across all groups reported significantly higher pleasantness ratings for odors presented to the left nostril, and this was especially true at the lower odor concentrations which were rated the most pleasant. A main effect of sex was also observed across all odor concentrations such that females, regardless of group status, rated odors as more pleasant than males (F 1 ,107 = 4.873, P = .03). Contrary to our previous results using birhinal stimulation (Moberg et al15), sex did not modify or interact with the observed group differences in pleasantness ratings across odor concentrations.
Pleasantness Ratings and Illness Characteristics
In patients, an inverse relationship was observed between SANS total score (sum of all items) and left nostril pleasantness ratings, such that patients with higher negative symptoms reported lower left nostril ratings of pleasantness for the 2 weakest concentrations (−3.00 log vol/vol: r = −.37, P = .01 and −4.00 log vol/vol: r = −.44, P = .002). In particular, the SANS anhedonia/asociality subscale (calculated as the sum of all anhedonia/asociality items) was inversely associated with left nostril ratings for the 3 weaker concentrations (−2.00 log vol/vol: r = −.40, P < .01; −3.00 log vol/vol: r = −.33, P = .02; and −4.00 log vol/vol: r = −.32, P = .03). After controlling for HAM-D total scores, correlations between the SANS anhedonia/asociality subscale and left nostril hedonic ratings for all odor concentrations were statistically significant (−1.00 log vol/vol: r = −.34, P = .05; −2.00 log vol/vol: r = −.50, P = .002; −3.00 log vol/vol: r = −.44, P = .01; and −4.00 log vol/vol: r = −.34, P = .05). Overall associations between SANS indices and odor valence ratings are presented in table 2.
Table 2.
Correlations Between Odor Valence Ratings and Negative Symptoms in Schizophrenia
| SANS24 Indices | Valence Ratings | |||||||
| Right Nostril | Left Nostril | |||||||
| Odor Concentration (log vol/vol) Strong↔Weak | Odor Concentration (log vol/vol) Strong↔Weak | |||||||
| −1.00 | −2.00 | −3.00 | −4.00 | −1.00 | −2.00 | −3.00 | −4.00 | |
| Total score | .04 | –.04 | –.22 | –.23 | .07 | –.25 | –.37** | –.44** |
| Affective flattening | –.12 | –.15 | –.19 | –.19 | .03 | –.22 | –.27 | –.24 |
| Alogia | .14 | –.03 | –.05 | –.03 | .11 | –.05 | –.27 | –.29* |
| Avolition-apathy | .20 | .16 | –.06 | –.17 | .16 | .00 | –.08 | –.21 |
| Anhedonia-asociality | –.03 | –.06 | –.16 | –.16 | −.15 | –.40** | –.33* | –.32* |
| Attention | .06 | .03 | –.23 | –.18 | .18 | –.02 | –.21 | –.43** |
Note: SANS, Scale for the Assessment of Negative Symptoms. Values are Pearson correlations.
*Statistically significant P <.05; **P <.01.
For the SAPS, a significant association was observed between the SAPS Bizarre Behavior subscale and right nostril pleasantness ratings of the strongest odor concentration (−1.00 log vol/vol: r = .30, P = .04), such that patients with more bizarre symptomatology rated these odors as more pleasant (ie, more abnormal in comparison with controls). Similarly, the SAPS Positive Formal Thought Disorder subscale was positively associated with right nostril pleasantness ratings for the 2 strongest odors (−1.00 log vol/vol: r = .31, P = .03 and −2.00 log vol/vol: r = .43, P < .01). Pleasantness ratings in the patient group were not significantly associated with age, education, illness duration, age of onset, HAM-D scores, or BPRS scores (all P’s > .09). Furthermore, the SANS symptoms subscales, including the SANS anhedonia subscale, were not associated with HAM-D scores, nor were total SANS scores (all P’s > .13).
Patients were further subdivided according to medication status. Unmedicated and medicated patients did not differ significantly with respect to ratings of either pleasantness or intensity (all P’s > .10). Further subdividing medicated patients into those taking first-generation antipsychotics and those taking second-generation antipsychotic medications similarly revealed no significant main effects or interactions (all P’s > .20). Finally, no relationships were observed between chlorpromazine equivalent dosage and ratings of pleasantness (all P’s > .42).
Discussion
In the current study, we extend prior research on olfactory anhedonia in schizophrenia by examining unirhinal hedonic and intensity ratings in males and females with schizophrenia, as well as unaffected first-degree family members. Our results indicate, first, that both male and female patients demonstrate abnormalities in perceived olfactory hedonic valence. Specifically, patients undervalued the pleasantness of amyl acetate at low concentrations and did not appreciate its increasing unpleasantness at high concentrations, as experienced by healthy control subjects. This finding replicates our previous results for male patients.15 However, in contrast to our previous finding that female patients had normal hedonic valence perception, the female patients in the current study also experienced a disturbance in the appreciation of hedonic valence. One notable explanation for this discrepancy in findings between the current and prior study was the method of odor presentation. Compared with Moberg’s15 study, the current study employed unirhinal assessment whereby each nostril was tested in isolation. While few studies have compared differences between unirhinal and birhinal assessment, at least 1 study noted that the female advantage for birhinal odor identification disappears during unirhinal testing.32 As birhinal odor assessment is thought to involve interhemispheric facilitation compared with unirhinal conditions,30,33 it is possible that unirhinal testing suppressed the compensation of the contralateral hemisphere, and without this advantage female patients exhibited the same hedonic disturbance as male patients. Further research directly comparing birhinal and unirhinal conditions with measures for interhemispheric transfer times could help elucidate these differences.
The unaffected family members of schizophrenia patients, in contrast, did not show any disruption in odor hedonic perception. This was unexpected because previous investigations have noted both increased anhedonia18 and deficits in olfactory task performance in family members with schizophrenia.19,20 In addition, significant associations between physical anhedonia and neuropsychological probes of executive function have been noted in unaffected siblings of schizophrenia patients.34 Given these findings, we anticipated that family members would also exhibit a disturbance in attaching hedonic valence to odors. However, the current literature on heritability for olfactory ability is complex because heritability may vary depending on the olfactory task type and odorants assessed. Finkel and colleagues35 noted modest heritability for odor identification and odor intensity perception but found that ratings for the perceived pleasantness of odorants did not show significant genetic mediation in a non-clinical sample. Knaapila et al36 reported that both intensity and pleasantness perception are predominantly environmentally mediated, with minimal genetic contribution. Still others have noted that heritability of hedonic perception may depend on the nature and chemical composition of the particular odorant being assessed because hedonic perception of certain odorants (eg, androstenone, cinnamon) appear to be genetically mediated.37,38 Our findings suggest, though, that the odor hedonic abnormalities associated with schizophrenia are not genetically mediated but rather represent a functional deterioration associated with the illness state.
Patients’ abnormal pleasantness ratings could not be attributed to their inability to perceive the odors. Although we observed a statistically significant group difference in odor intensity ratings, the pattern of results was not consistent with decreased patient intensity perception. Rather, control subjects rated the strongest odor concentration as more intense and the weakest odor concentration as less intense. This suggests that control subjects used a greater range in their attribution of odor intensity but were not actually better at perceiving the odors. All groups showed a robust relationship between odor concentrations and intensity ratings, suggesting intact appreciation for basic intensity differences across adjacent concentrations. In addition, intensity ratings were not a significant predictor of pleasantness ratings, and controlling for individual intensity ratings for corresponding pleasantness ratings did not alter the effects.
Patient hedonic ratings were also not associated with antipsychotic medication dosage, illness duration, age of onset, depressive symptomatology, or current smoking behavior. However, a significant relationship was observed between pleasantness perception and anhedonia such that patients with higher levels of anhedonia/asociality showed a blunted perception of amyl acetate pleasantness, specifically at those concentrations that were perceived as most pleasant by control subjects. This association remained significant after controlling for current level of depressive symptomatology, suggesting a relationship between trait anhedonia/asociality and impaired odor valence processing. In contrast, increased disorganization (bizarre behavior and positive thought disorder) was associated with blunted appreciation of amyl acetate unpleasantness at the high concentrations that were perceived by controls as most unpleasant. The association between negative symptoms and impaired odor identification has been noted repeatedly.39,40 A prior study by Malaspina and Coleman41 noted a significant relationship between smell identification deficits and diminished social drive in schizophrenia patients. These data provide a logical and anticipated extension of this relationship into the domain of olfactory hedonic perception. However, the association between disorganized symptoms and impaired unpleasantness perception is not one that was either expected or readily understood. We did previously observe an association between disorganized symptoms and impaired odor identification.42 More recently, a study by Strauss et al10 noted that Nondeficit Syndrome patients (ie, those with positive, but not enduring negative, symptoms) showed a similar selective impairment in judging unpleasant odors to be less unpleasant compared with controls, whereas deficit syndrome (ie, primary and enduring negative symptoms) patients also underrated the pleasantness of pleasant odors. Collectively, these findings suggest that negative symptoms of anhedonia/asociality and positive symptoms of disorganization are independently associated with the perception of opposing valence characteristics in schizophrenia. Further studies comparing patient groups within these symptoms clusters are needed to elucidate the complexity of odor hedonic perception in schizophrenia.
This relationship between anhedonia and olfactory hedonic deficits was limited to odor presentations to the left nostril. This is consistent with the overall effect we observed in which all subjects tended to rate odors as more pleasant when presented to the left, rather than the right, nostril. While the neural basis for odor hedonic abnormalities is unclear, odor valence processing in healthy people, particularly for pleasant odors, has shown left hemisphere dominance.43 In schizophrenia, reduced hedonic ratings for pleasant odors has been associated with decreased activation in left temporo-limbic and left orbital olfactory regions13 and increased physical anhedonia has been linked with decreased activation in the amygdala.44 The authors of the latter report note that aberrant amygdala activation results in a failure to mark the salience of pleasant events. If one were to extrapolate these findings to the current analysis, it is possible that aberrant processing in OFC and amygdala networks results in reduced representation of affective value and a failure to signify the pleasantness of amyl acetate as salient. Further investigation of aberrant processing in these brain regions as neural correlates of anhedonia is warranted.
Prior laboratory studies in schizophrenia using olfactory stimuli have repeatedly noted abnormalities in valence ratings,8–13,15 while studies examining subjective ratings of stimuli from other modalities (eg, pictures, film clips) have not consistently elicited patient-control differences in subjective hedonic reactions (for a review, see Cohen and Minor45). It is possible that the relative integration that exists between olfactory and limbic circuitry, coupled with the evolutionary salience of olfaction to social behavior, makes olfactory stimuli especially sensitive probes of hedonic perception. While this is of course speculative, there is evidence for an evolutionary role of olfactory cues in directing humans toward food, identifying mates and offspring, and signaling threat or danger. Furthermore, olfaction is distinct from other senses because only 1 synapse lies between olfactory receptors and olfactory cortex, providing a direct pathway between the sensory environment and cortex, bypassing the thalamus. Therefore, olfactory probes may offer a unique advantage over other forms of emotional stimuli when probing emotional disturbance and hedonic capacity in schizophrenia.
Limitations of this study include the use of a single odor to ascertain intensity and pleasantness ratings. While the use of 1 odor remedies the potential confound of using disparate odors that differ with respect to chemical makeup and odor dimensions (eg, familiarity, edibility, intensity), it does not allow for generalizability to other classes of odorants or for an examination of genetic mediation across different odor classes. Second, we characterized anhedonia in schizophrenia using a subscale of the SANS. The use of multiple measures of anhedonia, including those that distinguish between social and physical anhedonia (eg, Chapman Scales46), or anticipatory and consummatory pleasure (eg, Temporal Experience of Pleasure Scale47), and relating these measures to odor hedonic capacity would help to elucidate what aspects of anhedonia are associated with odor hedonic capacity in schizophrenia. Though we observed no significant effect of medication status or type of antipsychotic agent used on odor hedonic ratings, our analysis was likely underpowered given the relatively small number of patients within each subgroup. Therefore, these results must be interpreted with caution given the possibility for false-negative findings. Future studies examining odor valence processing in larger groups of medicated and unmedicated patients are warranted. Similarly, the first-degree family member cohort was considerably smaller than the patient and control group. This limited our ability to examine differences in odor hedonic ratings by degree of relationship (eg, sibling vs parent vs offspring) and resulted in a higher degree of variability in their hedonic responses. Thus, follow-up studies on odor hedonic abnormalities in larger family member groups would be especially useful in understanding how odor valence perception is influenced by degree of genetic relationship to schizophrenia probands.
Our findings indicate that individuals with schizophrenia experience difficulty when assigning valence characteristics to amyl acetate and this difficulty is related at opposing ends of the spectrum, to increased anhedonia and increased disorganization. In contrast to our prior report,15 female patients showed a similar abnormality to male patients when hedonic ratings were elicited during unirhinal odor presentation. Unaffected family members of schizophrenia patients did not show a disruption in valence appreciation for amyl acetate, which suggests that this disruption represents an environmentally mediated abnormality. Anhedonia is a treatment-resistant and enduring feature of schizophrenia that is associated with poor functional outcome in patients and individuals at-risk for psychosis. New treatments that target anhedonia in schizophrenia are underway,48 and thus, behavioral measures that have predictive value in identifying those at risk for anhedonia are necessary. The use of olfaction as a probe of hedonic capacity is a novel and promising avenue to explore anhedonia in schizophrenia and could be especially useful in identifying people at-risk for anhedonia and in need of targeted intervention for poor social, emotional, and functional outcomes.
Funding
The National Institutes of Health (MH63381 to P.J.M., MH59852 to B.I.T.); a NARSAD Independent Investigator Award from the National Alliance for Research on Schizophrenia and Depression to P.J.M.
Acknowledgments
We wish to thank Dana Gatto, B.S., and Dana Marchetto, B.A., for their assistance with subject recruitment, task administration and data entry, as well as the Hofmann Trust for their support of this research through the National Alliance for Research on Schizoprenia and Depression.
References
- 1.Blanchard JJ, Horan WP, Brown SA. Diagnostic differences in social anhedonia: a longitudinal study of schizophrenia and major depressive disorder. J Abnorm Psychol. 2001;110:363–371. doi: 10.1037//0021-843x.110.3.363. [DOI] [PubMed] [Google Scholar]
- 2.Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32:259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velthorst E, Nieman DH, Becker HE, et al. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr Res. 2009;109:60–65. doi: 10.1016/j.schres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr Bull. 1998;24:413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- 5.Herbener ES, Harrow M, Hill SK. Change in the relationship between anhedonia and functional deficits over a 20-year period in individuals with schizophrenia. Schizophr Res. 2005;75:97–105. doi: 10.1016/j.schres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Turetsky BI, Hahn CG, Borgmann-Winter K, Moberg PJ. Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophr Bull. 2009;35:1117–1131. doi: 10.1093/schbul/sbp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey PO, Armony J, Malla A, Lepage M. Functional neural substrates of self-reported physical anhedonia in non-clinical individuals and in patients with schizophrenia. J Psychiatr Res. 2010;44:707–716. doi: 10.1016/j.jpsychires.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Cumming AG, Matthews NL, Park S. Olfactory identification and preference in bipolar disorder and schizophrenia. Eur Arch Psychiatry Clin Neurosci. doi: 10.1007/s00406-010-0145-7. Advance Access published on September 6, 2010; doi: 10.1007/s00406-010-0145-7. [DOI] [PubMed] [Google Scholar]
- 9.Doop ML, Park S. On knowing and judging smells: identification and hedonic judgment of odors in schizophrenia. Schizophr Res. 2006;81:317–319. doi: 10.1016/j.schres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Strauss GP, Allen DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgment in patients with deficit syndrome schizophrenia. Schizophr Bull. 2010;36:860–868. doi: 10.1093/schbul/sbn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crespo-Facorro B, Paradiso S, Andreasen NC, et al. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- 12.Hudry J, Saoud M, D'Amato T, Daléry J, Royet JP. Ratings of different olfactory judgements in schizophrenia. Chem Senses. 2002;27:407–416. doi: 10.1093/chemse/27.5.407. [DOI] [PubMed] [Google Scholar]
- 13.Plailly J, d'Amato T, Saoud M, Royet JP. Left temporo-limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. Neuroimage. 2006;29:302–313. doi: 10.1016/j.neuroimage.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 14.Rupp CI, Fleischhacker WW, Kemmler G, et al. Various bilateral olfactory deficits in male patients with schizophrenia. Schizophr Bull. 2005;31:155–165. doi: 10.1093/schbul/sbi018. [DOI] [PubMed] [Google Scholar]
- 15.Moberg PJ, Arnold SE, Doty RL, et al. Impairment of odor hedonics in men with schizophrenia. Am J Psychiatry. 2003;160:1784–1789. doi: 10.1176/appi.ajp.160.10.1784. [DOI] [PubMed] [Google Scholar]
- 16.Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- 17.Docherty AR, Sponheim SR. Anhedonia as a phenotype for the Val158Met COMT polymorphism in relatives of patients with schizophrenia. J Abnorm Psychol. 2008;117:788–798. doi: 10.1037/a0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schurhoff F, Szoke A, Bellivier F, et al. Anhedonia in schizophrenia: a distinct familial subtype? Schizophr Res. 2003;61:59–66. doi: 10.1016/s0920-9964(02)00237-2. [DOI] [PubMed] [Google Scholar]
- 19.Kopala L, Good K, Morrison K, et al. Impaired olfactory identification in relatives of patients with familial schizophrenia. Am J Psychiatry. 2001;158:1286–1290. doi: 10.1176/appi.ajp.158.8.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopala L, Good K, Torrey E, Honer W. Olfactory function in monozygotic twins discordant for schizophrenia. Am J Psychiatry. 1998;155:134–136. doi: 10.1176/ajp.155.1.134. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Arlington, TX: American Psychiatric Publishing, Inc.; 1997. [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th., text revision ed. Washington, DC: Author; 2000. [Google Scholar]
- 23.Overall M, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 24.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- 25.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Washington, DC: American Psychiatry Press; 1997. [Google Scholar]
- 28.Kroken RA, Johnsen E, Ruud T, Wentzel-Larsen T, Jørgensen HA. Treatment of schizophrenia with antipsychotics in Norwegian emergency wards, a cross-sectional national study. BMC Psychiatry. 2009;9:24. doi: 10.1186/1471-244X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doty RL. The Smell Identification Test Administration Manual. Haddon Heights, NJ: Sensonics, Inc.; 1995. [Google Scholar]
- 30.Bromley SM, Doty RL. Odor recognition memory is better under bilateral than unilateral test conditions. Cortex. 1995;31:25–40. doi: 10.1016/s0010-9452(13)80103-7. [DOI] [PubMed] [Google Scholar]
- 31.Lang PJ. The Cognitive Psychophysiology of Emotion: Anxiety and the Anxiety Disorders. Hillsdale, NJ: Lawrence Erlbaum; 1985. [Google Scholar]
- 32.Good KP, Martzke JS, Daoud MA, Kopala LC. Unirhinal norms for the University of Pennsylvania Smell Identification Test. Clin Neuropsychol. 2003;17:226–234. doi: 10.1076/clin.17.2.226.16501. [DOI] [PubMed] [Google Scholar]
- 33.Cain WS. Bilateral interaction in olfaction. Nature. 1977;268:50–52. doi: 10.1038/268050a0. [DOI] [PubMed] [Google Scholar]
- 34.Franke P, Maier W, Hardt J, Hain C. Cognitive functioning and anhedonia in subjects at risk for schizophrenia. Schizophr Res. 1993;10:77–84. doi: 10.1016/0920-9964(93)90079-x. [DOI] [PubMed] [Google Scholar]
- 35.Finkel D, Pedersen NL, Larsson M. Olfactory functioning and cognitive abilities: a twin study. J Gerontol B Psychol Sci Soc Sci. 2001;56:P226–P233. doi: 10.1093/geronb/56.4.p226. [DOI] [PubMed] [Google Scholar]
- 36.Knaapila A, Tuorila H, Silventoinen K, et al. Environmental effects exceed genetic effects on perceived intensity and pleasantness of several odors: a three-population twin study. Behav Genet. 2008;38:484–492. doi: 10.1007/s10519-008-9211-6. [DOI] [PubMed] [Google Scholar]
- 37.Knaapila A, Keskitalo K, Kallela M, et al. Genetic component of identification, intensity and pleasantness of odours: a Finnish family study. Eur J Hum Genet. 2007;15:596–602. doi: 10.1038/sj.ejhg.5201804. [DOI] [PubMed] [Google Scholar]
- 38.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449:468–472. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- 39.Brewer WJ, Pantelis C, Anderson V, et al. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158:107–115. doi: 10.1176/appi.ajp.158.1.107. [DOI] [PubMed] [Google Scholar]
- 40.Malaspina D, Coleman E, Goetz RR, et al. Odor identification, eye tracking and deficit syndrome schizophrenia. Biol Psychiatry. 2002;51:809–815. doi: 10.1016/s0006-3223(01)01319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578–584. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- 42.Moberg PJ, Arnold SE, Doty RL, et al. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28:1444–1461. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- 43.Kim YK, Watanuki S. Characteristics of electroencephalographic responses induced by a pleasant and an unpleasant odor. J Physiol Anthropol Appl Human Sci. 2003;22:285–291. doi: 10.2114/jpa.22.285. [DOI] [PubMed] [Google Scholar]
- 44.Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- 47.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Favrod J, Giuliani F, Ernst F, Bonsack C. Anticipatory pleasure skills training: a new intervention to reduce anhedonia in schizophrenia. Perspect Psychiatr Care. 2010;46:171–181. doi: 10.1111/j.1744-6163.2010.00255.x. [DOI] [PubMed] [Google Scholar]