Abstract
Although schizophrenia affects a number of brain regions and produces a range of clinical symptoms, we believe its origins lie at the level of single neurons and simple networks. Owing to this, as well as to its high degree of heritability, we hypothesize that schizophrenia is amenable to cell-based studies in vitro. Using induced pluripotent stem cell–derived neurons and/or fibroblast-induced neurons, a limitless quantity of live human neurons can now be generated from patient skin biopsies. We predict that cell-based studies will ultimately contribute to our understanding of the molecular and cellular underpinnings of this debilitating disorder.
Key words: neurons, stem cells, genetics
Reprogramming of hiPSCs From Fibroblasts
The ability to reprogram patient somatic cells into human-induced pluripotent stem cells (hiPSCs) provides a limitless source of live human cells for modeling schizophrenia. Fibroblasts can be reprogrammed by overexpressing 4 critical genes, OCT4, SOX2, KLF4, and c-MYC.1,2 (c-MYC, a potent oncogene, can be eliminated from the reprogramming cocktail, although this results in a markedly lower efficiency.3) In addition, a second combination of genes, OCT4, SOX2, NANOG, and LIN28, is also capable of reprogramming.4 These reprogramming genes can be overexpressed through a variety of means to be discussed later in this review, including viral transduction or transfection of plasmid DNA or messenger RNA. hiPSCs are comparable to human embryonic stem cells in terms of gene expression, epigenetics, telomerase activity, proliferation, and pluripotency.1,2,4
Early methods of reprogramming relied upon constitutive retroviral or lentiviral expression systems, with 2 potential limitations: incomplete viral silencing of reprogramming factors and insertional mutagenesis upon viral integration. To address the first, a doxycycline-inducible lentiviral system was developed to repress viral genes after the reprogramming process was complete.5 Although these first-generation methods were ultimately sufficient to generate cell-based models for psychiatric disease,6,7 in the meanwhile, integration-free methods were developed. Adenovirus can generate integration-free iPSCs, but at poor efficiencies, owing to low expression of adeno-receptors in nonliver tissue.8 Plasmid vectors also yield iPSCs at low efficiencies and furthermore require repeated transfections.9,10 Synthetic modified mRNA reprogramming is efficient but requires daily transfections in addition to a proprietary media formulation during the reprogramming process.11 Most recently, 2 groups have demonstrated efficient reprogramming using nonintegrating Sendai virus.12,13 mRNA and Sendai viral reprogramming represent the best and the most robust methods available to date. Kits for both methods are commercially available at comparable costs through StemGent and Life Technologies, respectively. Integration-free reprogramming is now straightforward, although at present, only Sendai reprogramming is effective for both human fibroblast and blood cells (table 1).
Table 1.
Summary of Methods of Reprogramming to iPSCs
| Species | Factors Involved | Reprogramming Method | Integrating? | Efficient? | Reference |
|---|---|---|---|---|---|
| Mouse, human | OCT4, SOX2, KLF4, (c-MYC) or OCT4, SOX2, NANOG, LIN28 | Retroviral or lentiviral | Yes | Yes | Takahashi et al.,14 Yu et al.4 Park et al.15 |
| Mouse, human | OCT4, SOX2, KLF4, (c-MYC) | Inducible-lentiviral | Yes | Yes | Maherali et al.5 |
| Mouse | OCT4, SOX2, KLF4, c-MYC | Adenovirus | No | No | Stadfield et al. 8 |
| Mouse, human | OCT4, SOX2, KLF4, c-MYC | Plasmid transfection | No | No | Okita et al.9,16 |
| Human | OCT4, SOX2, KLF4, c-MYC | RNA transfection | No | Yes | Warren et al.11 |
| Mouse, human | OCT4, SOX2, KLF4, c-MYC | Sendai virus | No | Yes | Nishimura et al.12 Ban et al.13 |
Differentiation of hiPSCs to Neurons
Even state-of-the-art hiPSC neural differentiation protocols produce heterogeneous neural populations of mixed spatial and temporal identities. Although the relative frequency of a specific neuronal cell type might be favored, different iated populations are typically comprised of several types of neurons, as well as astrocytes, oligodendrocytes, neural precursors, and even non-neural cells. Published methods have demonstrated that stem cells can be biased to differentiate toward regional identities including forebrain,17–19, midbrain/hindbrain,20,21 and spinal cord.22,23 The precise temporal age of hiPSC-derived neurons, relative to fetal and adult human tissues, remains unresolved.
Strong evidence now links schizophrenia to aberrant activity of 3 neural populations: cortical glutamatergic neurons, gamma-aminobutyric acid (GABA)ergic neurons, and midbrain dopaminergic (mDA) neurons. Pharmacological24,25 and animal studies 6,27 suggest that disease results, at least in part, due to reduced glutamatergic input onto GABAergic neurons. Conversely, antagonists of dopamine receptors reduce the positive symptoms of schizophrenia, which is also associated with increased dopamine synthesis, release, receptor numbers, and resting-state synaptic concentrations.28,29 Both cortical glutamatergic and GABAergic neuronal populations and mDA neuronal populations can now be efficiently differentiated in vitro from hiPSCs. Two groups have recently published methods to differentiate pluripotent stem cells to cortical neurons. One group reported that when neural induction occurs in the presence of 2 inhibitors of SMAD signaling, Noggin, and SB431542, the addition of vitamin A efficiently induces a cortical progenitor population at an efficiency that approaches 100%, which can be further expanded in the presence of FGF2 and differentiated to functional cortical neurons following an extended period of corticogenesis.19 Consistent with this, the other group showed that induction of forebrain fate can occur in the presence of FGF2 and inhibitors of the bone morphogenetic protein, Wnt/β-catenin, and TGF-β/activin/nodal pathways; further differentiation resulted in populations of glutamatergic and GABAergic neurons expressing cortical markers, albeit at lower efficiencies than the first report, perhaps owing to a lack of retinoid signaling.18 mDA neurons are particularly relevant to the study of schizophrenia, and efficient protocols have been developed to differentiate pluripotent stem cells to mDA neurons by recapitulating the well-understood program of specification that occurs during embryonic development. Yields have been systemically optimized for the past 4 years, from ~20% to >80% mDA neurons,30,31 using a 2-step process. First, neural induction occurs in the context of dual SMAD inhibition. Second, mDA specification occurs following strong activation of SHH signaling (in combination with purmorphamine, a small-molecule SHH agonist) and WNT signaling (via CHIR99021, a potent GSK3B inhibitor). Cell surface markers to determine the exact temporal and regional identity of any individual neuron in a heterogeneous hiPSC-derived neural population are critically lacking; in live cultures, one cannot distinguish whether a neuron is glutamatergic, GABAergic, or dopaminergic (table 2).
Table 2.
Summary of Methods of hiPSC Neural Differentiation Relevant to Schizophrenia
| Species | Intended Cell Type | Purity | Method | Neuronal Characterization | Reference |
|---|---|---|---|---|---|
| Human | Cortical glutamatergic and GABAergic | Approaches 100% mixed cortical cells | Dual SMAD inhibition in the context of retinoids, followed by FGF2 treatment | Immunohistochemistry, gene expression, electrophysiology, and transplantation | Shi et al.,19 Mariani et al., 201218 |
| Human | Midbrain dopaminergic neurons | >80% | Dual SMAD inhibition followed by SHH and WNT stimulation | Immunohistochemistry, gene expression, electrophysiology, and transplantation | Chambers et al., 200919; Kriks et al., 201130,31 |
Direct Induction of iNeurons From Fibroblasts
Following the demonstration that a terminally differentiated cell could be reprogrammed to a pluripotent state, the next question was whether fully mature cells could be directly converted from one fate to another. Mouse fibroblasts were converted into induced neurons (iNs) by overexpressing Brn2, Ascl1, and Mytl1. 32 This finding was replicated in human fibroblasts, using the same 3 factors—BRN2, ASCL1, and MYTL1—with the addition of NEUROD1. 33 The conversion takes as little as 6 days and persists after the silencing of viral expression, but is inefficient, occurring in just 2%–4% of the original fibroblasts. Notably, these iNs, although capable of induced action potentials, were relatively immature and unable to form synapses without the presence of mouse cortical neurons.
Independently, 2 groups demonstrated that the addition of key neuronal microRNAs to the induction yielded iNs with functional synapses in pure cultures. The addition of microRNA-124 to the factors MYTL1 and BRN2, 34 or the addition of microRNA-124 and microRNA-9/9 to a cocktail of NEUROD2, ASCL1, and MYTL1, 35 generated mature human iNs capable of forming functional synapses with each other in the absence of mouse cortical neurons. The first method produced predominantly excitatory neurons, whereas the latter generated a heterogeneous mixture of excitatory and inhibitory neurons; the molecular mechanisms contributing to these different neuronal identities remain unclear. Both methods showed efficiencies of approximately 10%, with substantial variability between fibroblast lines. iN populations remain extremely heterogeneous, consisting of nearly 90% non-neuronal cells, and the temporal and spatial identity of iNs, relative to the human brain, is unresolved.
The ability to generate neuronal populations of a specific subtype would be ideal for cell-based studies. Using pools of mDA-specific transcription factors, 2 groups have directly reprogrammed human fibroblasts into neuronal populations consisting predominantly of mDA neurons. Using the factors ASCL1, NURR1, LMX1A, and MASH1, induced mDA (iDA) neurons were generated from mouse and human fibroblasts36; these iDA released dopamine and were functionally integrated when transplanted into embryonic mouse brains. By enlarging the cocktail to ASCL1, NURR1, LMX1A, PITX3, FOXA2, and EN1, mouse iDAs were produced with gene expression profiles more closely resembling mDA neurons.37 Approximately 10% of the original fibroblasts were induced to iDAs; furthermore, upon transplantation of iDAs into the striatum of lesioned mice, striatum DA levels increased and function in a mouse model of PD improved. We anticipate that new protocols will soon be reported for the generation of additional neuronal subtypes.
Faster than hiPSC reprogramming and differentiation, iN production is now a robust method, but technical limitations remain when considering using this platform for cell-based studies of schizophrenia. First, iN generation bypasses neuronal development, eliminating the ability to test cellular phenotypes such as neural migration, specification, or maturation, which may be critical to disease progression. Second, this method results in terminally differentiated neurons, limiting the cellular material available for studies. To combine the strengths of hiPSC and iN systems, many groups sought a method to directly generate induced neural progenitor cells (iNPCs) from fibroblasts, in order to produce a substantial supply of neurons for study (table 3).
Table 3.
Summary of Methods of iNeuron Generation
| Species | Factors Involved | Neurotransmitter Identity | Regional Patterning | Other Validations | Reference |
|---|---|---|---|---|---|
| Mouse | Brn2, Ascl1, Mytl1 | Majority glutamatergic; many GABAergic; undetectable dopaminergic, cholinergic or serotinergic | Majority cortical | Immunostaining, electrophysiology | Vierbuchen et al.32 |
| Human | BRN2, ASCL1, MYTL1, NEUROD1 | Majority glutamatergic; few GABAergic; few dopaminergic; undetectable cholinergic or serotinergic | 17% Forebrain; undetectable midbrain; 21% PNS | Immunostaining, qPCR, electrophysiology | Pang et al.33 |
| Human | miR-124, MYTL1, BRN2 | 44% glutamatergic; 8% GABAergic; rare dopaminergic; undetectable cholinergic or serotinergic | Central nervous system | Immunostaining, electrophysiology | Ambasudhan et al.34 |
| Human | miR-124, miR-9/9, NEUROD2, ASCL1, MYT1L | Mixture of glutamatergic and GABAergic; few serotonergic; undetectable dopaminergic | Majority cortical; few striatum or cerebellum; undetectable PNS | Immunostaining, qPCR, electrophysiology, stimulation-dependent calcium imaging, exit from cell cycle (EdU) | Yoo et al.35 |
| Mouse, human | MASH1, ASCL1, NURR1, LMX1A | 85% dopaminergic; undetectable adrenergic or serotonergic | Midbrain | Immunostaining, qPCR, gene expression profiling, electrophysiology, dopaminergic release (HPLC), transplantation | Caiazzo36 |
| Mouse | Ascl1, Pitx3, (Lmx1a, Nurr1, Foxa2, EN1) | Midbrain dopaminergic neurons | Midbrain | Immunostaining, qPCR, gene expression profiling, dopaminergic release (HPLC), transplantation | Kim et al.37 |
Note: GABA, gamma-aminobutyric acid; HPLC, high-performance liquid chromatography; PNS, peripheral nervous system; qPCR, quantitative PCR.
Direct Induction of iNPCs From Fibroblasts
The induction of iNPCs from fibroblasts provides, in a manner that better recapitulates neural development, a limitless supply of neurons for models of psychiatric disease. This year, 5 groups reported the generation of iNPCs from fibroblasts. The reports fell loosely into 2 different strategies: (1) incomplete hiPSC reprogramming in conjunction with growth in neural conditions and (2) overexpression of neural transcription factors.
Partial sets of reprogramming factors can generate iNPCs. iNPCs generated by inducing constitutive expression of SOX2, KLF4, and c-MYC in the presence of transient OCT4 38 differentiated into populations of up to 30% neurons or nearly 100% astrocytes, whereas iNPCs obtained by overexpressing OCT4, SOX2, and KLF4 in the presence of another pluripotency gene, ZIC3, 39 differentiated into astrocytes, oligodendrocytes, and motor neurons but not central nervous system neurons. Notably, both these methods result in iNPCs that ultimately downregulate pluripotency genes and upregulate NPC markers.
Several combinations of neural transcription factors appear to be sufficient to generate iNPCs, although at present all reports have required persistent long-term expression of induction factors to maintain iNPC identity. Three factors—SOX2, BRN2, and FOXG1—can induce direct conversion of mouse fibroblasts into tripotent iNPCs capable of generating neurons, astrocytes, and oligodendrocytes; however, expression of just SOX2 and FOXG1 produced mainly neuron-restricted progenitors, and expression of just FOXG1 and BRN2 generated progenitors of oligodendrocytes and immature neurons incapable of action potentials.40 A larger combination of factors—SOX2, BRN2, NR2E1, BMI1, HES1, HES5 and c-MYC—could also convert mouse fibroblasts into iNPCs, although mature neurons from these iNPCs did not subject to electrophysiological validation.41 Most recently, a third group used just 1 factor, SOX2, to generate tripotent iNPCs that could differentiate to mature neurons capable of action potentials, from both mouse and human fibroblasts.42 It should be noted that SOX2-iNPC generation is markedly less efficient, requiring selection via three rounds of neurosphere suspensions. Generating iNPCs with just SOX2, however, opens the possibility of patterning iNPCs to specific identities by inducing via SOX2 in conjunction with other subtype-specific transcription factors.
iNPC technology provides a fast and robust protocol to obtain proliferative neural precursors and generates more homogeneous populations than current iN methods, all while bypassing time-consuming iPSC generation. Most iNPC protocols have been validated in vivo by functional integration of iNPC-derived neural cells into mouse brains.38–40,42 (None of the studies reported any cases of tumor formation by iNPCs.) At the moment, the major technical limitation to be addressed in iNPC generation is the adoption of integration-free induction techniques. New methods to permit patterning of specific regional identities are also critical. As the efficiency, purity, and patterning of iNPC methods advance, the adoption of these methods will provide a limitless source of neurons for the in vitro study of schizophrenia (table 4).
Table 4.
Summary of Methods of iNPC Generation
| Species | Transcription Factors | Patterning | NPC/Neuron Characterization | Reference |
|---|---|---|---|---|
| Mouse | Sox2, Klf4, c-Myc, transient Oct4 | Heterogeneous mixture of ventral fore, mid, and hindbrain fates | Immunostaining, gene expression profiling, qPCR, electrophysiology | Thier et al.38 |
| Human | ZIC3, OCT4, NANOG, SOX2 | Heterogeneous differentiation into motor neurons, astrocytes, and oligodendrocytes | Immunostaining, global gene expression profile, qPCR | Kumar et al.39 |
| Mouse | Sox2, FoxG1, Brn2 | Mainly forebrain | Immunostaining, qPCR, FACS, electrophysiology | Lujan et al.40 |
| Mouse | Six2, Brn2, Nr2e1, Bmi1, Hes1, Hes5, c-Myc | Heterogeneous differentiation to neurons, astrocytes, and midbrain dopaminergic neurons | Immunostaining, qPCR | Tian et al.41 |
| Mouse, human | SOX2 | No specific neuronal fate | Immunostaining, qPCR, gene expression profiling, methylation patterns, electrophysiology | Ring et al.42 |
Note: NPC, neural progenitor cell; qPCR, quantitative PCR; FACS, fluorescence-activated cell sorting.
hiPSC-, iNPC-, and iN-based Models of Schizophrenia
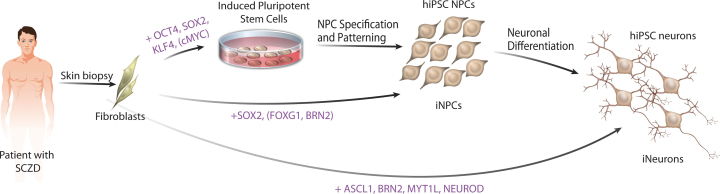
Although no reported studies have yet characterized iNPCs or iNs from patients with schizophrenia, a number of groups, including ours, have now published studies of schizophrenia-hiPSC neurons (figure 1). The first-generated schizophrenia-hiPSCs were from patients with a DISC1 mutation,43 but patient-derived neurons were not characterized. We reported neuronal phenotypes of hiPSC neurons from four patients with complex genetic forms of schizophrenia and showed that schizophrenia-hiPSC neurons had reduced neuronal connectivity and altered gene expression profiles.7 Although nearly 25% of the genes with altered expression had been previously implicated in schizophrenia, we also identified a number of new pathways that may contribute to schizophrenia. A third group observed a 2-fold increase in extra-mitochondrial oxygen consumption, as well as elevated levels of reactive oxygen species in NPCs derived from hiPSCs from one schizophrenia patient.44 Together, these studies offer an excellent proof of concept that reprogramming-based disease models can be used to study schizophrenia, although given the inherent variability between schizophrenia patients, more scalable methods to enable studies of larger numbers of patients need to be developed. Findings from all 3 studies await replication across larger patient cohorts.
Fig. 1.
Cell-based modeling of psychiatric disorders. Fibroblast cells obtained from patients can be used to generate live human neurons with a genetic background known to produce the disease state. First, fibroblasts can be reprogrammed to hiPSCs by transient expression of OCT4, SOX2, KLF4, and c-MYC and then subsequently differentiated into NPCs and mature neurons. Second, fibroblasts can be directly converted to iNPCs by transient expression of SOX2 and then subsequently differentiated to neurons. Third, fibroblasts can be directly converted into a neuronal fate by transient expression of ASCL1, BRN2, MYT1L, and NEUROD.
At present, these cell-based studies only recapitulate molecular and cellular phenomena related to schizophrenia resulting from an underlying genetic defect; therefore, patient cohorts are typically selected on the basis of a known genetic defect or strong family history of schizophrenia. Technical constraints currently limit the patient cohort size for which neurons can be generated and characterized in vitro; cell-based studies remain necessarily small for the time being. Because of the clinical and genetic heterogeneity of this disorder, these methods may currently be most appropriate for the study of patients with a particular genetic or clinical commonality. For example, cell-based studies might be particularly useful in testing the effect of a rare copy number variant such as 22q11.2 or the genetic basis of clozapine responsiveness. The first strategy parallels traditional mouse-based studies of schizophrenia that investigate the effects of rare loci, whereas the second takes full advantage of the ability of these cell-based methods to investigate complex genetic disorders without full knowledge of all the genes involved. Both are valid approaches; the first studies the effect of a single gene, the second studies the effect of genetic background.
When considering using induced cells to study schizophrenia, it is important to keep in mind the limitations of hiPSC-derived and induced neurons. Cell-based models presume that hiPSC-derived or fibroblast-induced neurons are “similar enough” to those in the human brain to be informative. Populations are typically comprised of neurons of mixed spatial identities and maturity and also may be incompletely patterned. Despite these important caveats, we believe that these new methods of modeling will result in insights into the initiation, progression, and treatment of schizophrenia.
Funding
Brain and Behavior Young Investigator Grant.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006. 126 663–676 [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc 2007. 2 3081–3089 [DOI] [PubMed] [Google Scholar]
- 3. Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 2008. 26 101–106 [DOI] [PubMed] [Google Scholar]
- 4. Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007. 318 1917–1920 [DOI] [PubMed] [Google Scholar]
- 5. Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C, Hochedlinger K. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell 2008. 3 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchetto MC, Carromeu C, Acab A, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 2010. 143 527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennand KJ, Simone A, Jou J, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011 doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science 2008. 322 945–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods 2011. 8 409–412 [DOI] [PubMed] [Google Scholar]
- 10. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 2007. 448 313–317 [DOI] [PubMed] [Google Scholar]
- 11. Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010. 7 618–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishimura K, Sano M, Ohtaka M, et al. Development of defective and persistent Sendai virus vector: a unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem 2011. 286 4760–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ban H, Nishishita N, Fusaki N, et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci USA 2011. 108 14234–14239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007. 131 861–872 [DOI] [PubMed] [Google Scholar]
- 15. Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell 2008. 134 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science 2008. 322 949–953 [DOI] [PubMed] [Google Scholar]
- 17. Watanabe K, Kamiya D, Nishiyama A, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 2005. 8 288–296 [DOI] [PubMed] [Google Scholar]
- 18. Mariani J, Simonini MV, Palejev D, et al. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci USA 2012. 109 12770–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses Nat Neurosci. 2012. 15 477–486, S471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawasaki H, Mizuseki K, Nishikawa S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 2000. 28 31–40 [DOI] [PubMed] [Google Scholar]
- 21. Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA 2004. 101 12543–12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li XJ, Du ZW, Zarnowska ED, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol 2005. 23 215–221 [DOI] [PubMed] [Google Scholar]
- 23. Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell 2002. 110 385–397 [DOI] [PubMed] [Google Scholar]
- 24. Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuro endocrine responses. Arch Gen Psychiatry 1994. 51 199–214 [DOI] [PubMed] [Google Scholar]
- 25. Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 2007. 13 1102–1107 [DOI] [PubMed] [Google Scholar]
- 26. Wen L, Lu YS, Zhu XH, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci USA 2010. 107 1211–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ting AK, Chen Y, Wen L, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci 2011. 31 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987. 44 660–669 [DOI] [PubMed] [Google Scholar]
- 29. Kessler RM, Woodward ND, Riccardi P, et al. Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry 2009. 65 1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009. 27 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kriks S, Shim JW, Piao J, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011. 480 547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010. 463 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pang ZP, Yang N, Vierbuchen T, et al. Induction of human neuronal cells by defined transcription factors. Nature 2011. 476 220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ambasudhan R, Talantova M, Coleman R, et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 2011. 9 113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoo AS, Sun AX, Li L, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011. 476 228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caiazzo M, Dell’Anno MT, Dvoretskova E, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011. 476 224–227 [DOI] [PubMed] [Google Scholar]
- 37. Kim J, Su SC, Wang H, et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts Cell Stem Cell 2011. 9 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thier M, Wörsdörfer P, Lakes YB, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 2012. 10 473–479 [DOI] [PubMed] [Google Scholar]
- 39. Kumar A, Declercq J, Eggermont K, Agirre X, Prosper F, Verfaillie CM. Zic3 induces conversion of human fibroblasts to stable neural progenitor-like cells. J Mol Cell Biol 2012. 4 252–255 [DOI] [PubMed] [Google Scholar]
- 40. Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA 2012. 109 2527–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tian C, Ambroz RJ, Sun L, et al. Direct conversion of dermal fibroblasts into neural progenitor cells by a novel cocktail of defined factors. Curr Mol Med 2012. 12 126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ring KL, Tong LM, Balestra ME, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 2012. 11 100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chiang CH, Su Y, Wen Z, et al. Integration-free induced pluripotent stem cells derived from schizophrenia patients with a DISC1 mutation. Mol Psychiatry 2011. 16 358–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulsen BD, Maciel RD, Galina A, et al. Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transplant. In press doi: 10.3727/096368911X600957. [DOI] [PubMed] [Google Scholar]