Abstract
When cyanide is introduced into the body, it quickly transforms through a variety of chemical reactions, normally involving sulfur donors, to form more stable chemical species. Depending on the nature of the sulfur donor, cyanide may be transformed into free thiocyanate, the major metabolite of cyanide transformation, 2-amino-2-thiazoline-4-carboxylic acid or protein-bound thiocyanate (PB-SCN) adducts. Because protein adducts are generally stable in biological systems, it has been suggested that PB-SCN may have distinct advantages as a marker of cyanide exposure. In this study, plasma was analyzed from 25 smokers (chronic low-level cyanide exposure group) and 25 non-smokers for PB-SCN. The amount of PB-SCN found in the plasma of smokers, 1.35 µM, was significantly elevated (p < 0.0001) when compared to non-smokers, 0.66 µM. Differences in sub-groups of smokers and non-smokers were also evaluated. The results of this study indicate the effectiveness of analyzing PB-SCN in determining instances of chronic cyanide exposure with possible extension to confirmation of acute cyanide exposure.
Introduction
Cyanide exposure through dietary sources, such as almonds and cassava root (1–2), inhalation of smoke from fires, work in the mining and metallurgy fields (3), and occupations in plastics manufacturing and chemical production all contribute to chronic exposure to cyanide. Another significant source of chronic cyanide exposure is the act of smoking (4–5), in which smokers are introduced to small doses of cyanide through cigarette smoke inhalation. Chronic exposure to cyanide, even at low levels, can promote the development of serious health conditions, including myelin degeneration and abnormal thyroid activity (6–7). A study by Philbrick et al. (8) determined that chronic cyanide exposure could cause significant detriment to a person's overall health, including irreversible damage of the nervous system. This study contributed to EPA regulated cyanide exposure limits of 30 mg kg−1/day−1.
When cyanide enters the body, it quickly transforms into less toxic metabolites (t1/2 = 0.34–1.0 h in biological fluids) (9), which makes direct analysis of cyanide unreliable under most circumstances. Therefore, a more stable marker of cyanide exposure could more dependably be used to confirm exposure (10–13). Free thiocyanate is the major metabolite of cyanide exposure and is produced from the reaction of cyanide with free sulfur donors (e.g., thiosulfate) in the presence of rhodanese (14–15). Although thiocyanate has a longer half-life than cyanide in biological fluids [t1/2 = 96–192 h (9–13)], low-dose cyanide exposure is difficult to positively confirm from the analysis of free thiocyanate because large, and highly variable, endogenous background concentrations in biological fluids (16) cannot be exclusively attributed to cyanide metabolism (17–19). For example, broccoli, cauliflower and cabbage contain substantial levels of thiocyanate (20–21) and thiocyanate is associated with antibacterial activity in saliva (22–23).
Another marker of cyanide exposure is produced when cyanide reacts with cystine to form 2-amino-2-thiazoline-4-carboxylic acid (ATCA) (24). Although recent studies have shed some light on its value as a marker of cyanide exposure (11, 24, 25), little is known about ATCA, including its half-life in biological systems. Alternatively, a recently suggested marker of cyanide exposure is produced when cyanide reacts with disulfide bonds in proteins to form protein-bound thiocyanate (PB-SCN) (26, 27, 28). Although the half-life of PB-SCN is unknown, if the protein adduct is stable, the half-life should be similar to that of the parent protein in blood [25 days for albumin (29–31) and 55 days for hemoglobin (32)]. The current study showed that chronic cyanide exposure, as modeled by exposure to cigarette smoke, could be determined through the analysis of plasma PB-SCN.
Experimental
Materials
Sodium thiocyanate, sodium cyanide, tetrabutylammonium sulfate (TBAS), concentrated HCl, ethyl acetate, acetone, sodium carbonate and stable-isotope labeled potassium thiocyanate (KS13C15N) were obtained from Sigma-Aldrich (St. Louis, MO). Pentafluorobenzyl bromide (PFBBr) was acquired through Thermo Scientific (Hanover Park, IL). All solvents used in this study were at least HPLC grade. All aqueous solutions were prepared with deionized, distilled water (18.2 MΩ cm) from a LabConco Water Pro PS system. A stock solution of approximately 100 µM thiocyanate was used to prepare all standard solutions.
Human plasma samples
In this study, the concentrations of PB-SCN from a total of 50 human (Homo sapiens) plasma samples were studied. Of these 50 subjects, 25 smoked regularly, and the remaining 25 subjects were classified as non-smokers based on a questionnaire that evaluated the number of cigarettes smoked daily, the type of cigarette and subjects' potential to be exposed to cyanide via other routes. Non-smokers were included to provide a baseline level of PB-SCN incurred from sources other than smoking, such as diet and environment. Blood samples from each of the subjects were collected, centrifuged to isolate the plasma, and mixed with an EDTA anti-coagulant to prevent protein coagulation. Samples were kept in a freezer at approximately –85°C until analysis.
Five males and 20 females comprised the group of 25 smokers, while seven males and 18 females made up the non-smokers group. None of the subjects were involved in an occupation in which chronic cyanide exposure was considered likely or were prescribed any medication that would expose them to low levels of cyanide (e.g., sodium nitroprusside). No individuals were pregnant at the time the plasma samples were collected and none of the non-smokers indicated significant exposure to second-hand cigarette smoke. Collection and analysis of human samples was approved prior to this study by the Human Subjects Advisory Committees at South Dakota State University, the Avera Medical Institute (Sioux Falls, SD), and the Human Research Protection Office of the U.S. Army Medical Research and Materiel Command (Ft. Detrick, MD).
Protein-bound thiocyanate analysis
Protein-bound SCN was analyzed from plasma samples according to the method previously described by Youso et al. (27), with minor modifications. Briefly, plasma proteins were precipitated, washed to remove free thiocyanate and dissolved in a carbonate buffer (pH 10) to release bound thiocyanate moieties from the protein. The extracted thiocyanate was then derivatized for analysis using PFBBr. The derivatized thiocyanate was extracted into ethyl acetate and analyzed using electron ionization gas chromatography–mass spectrometry (GC–MS parameters described by Youso et al. (27)). PB-SCN concentrations for each plasma sample tested were well above the detection limit of the analytical method (approximately 40 nM).
Quantification and data analysis
Quantification of thiocyanate was achieved by stable isotope dilution with the internal standard KS13C15N. Standard thiocyanate solutions with concentrations of 100–1,000 ng/mL were prepared by diluting the stock thiocyanate solution to achieve the desired concentration. The internal standard (100 µg/mL) was added to each standard and sample. Data are reported in the format mean ± standard deviation with the standard error of the mean (SEM) in parenthesis.
To statistically evaluate the plasma samples for significant differences in the PB-SCN concentrations of smokers compared with non-smokers, a two-tailed t-test was executed. Additional statistical analysis of different sub-groups of smokers and non-smokers (e.g., groups sorted by the average number of cigarettes smoked per day) was completed using analysis of variance (ANOVA) testing with Tukey's and Bonferroni's multiple range tests to determine significant differences between each sub-group.
Results
Comparison of smokers and non-smokers
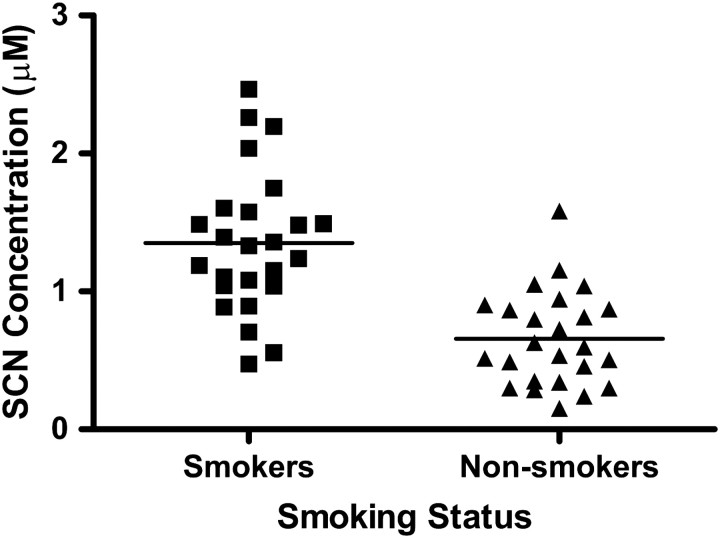
Figure 1 illustrates the individual concentrations of PB-SCN isolated from the plasma of each smoker and non-smoker, along with the mean concentration of PFB-SCN for each group (solid line in each group). The mean PB-SCN concentration in the plasma of smokers was found to be approximately twice that of non-smokers, 1.35 ± 0.51 µM (SEM = 0.10) and 0.66 ± 0.34 µM (SEM = 0.07), respectively, with concentration ranges of 2.26–0.47 µM for smokers and 1.58–0.15 µM for non-smokers. Although the ranges of PB-SCN concentrations overlap (Figure 1), the concentrations fall within a relatively narrow range, especially for non-smokers. The percent relative standard deviations for PB-SCN were 38 and 51% for smokers and nonsmokers, respectively. Statistical evaluation of the data revealed a significant difference (p < 0.0001) at a very high confidence level between the PB-SCN concentrations of smokers and non-smokers.
Figure 1.
The concentration of PB-SCN found in the plasma samples of smokers compared to non-smokers. The individual data points represent the concentration of PB-SCN in each sample. The solid line represents mean concentration of each group.
Statistically, PB-SCN compares favorably to other markers of cyanide exposure. For comparable studies, markers of cyanide exposure normally have significantly elevated concentrations in the biological fluids (i.e., urine, blood, and saliva) of smokers (i.e., p < 0.05 for a t-test). But for most studies, the significance is not as strong as that found for PB-SCN (16). This is, in part, due to the relatively low variability of plasma PB-SCN concentrations compared to other markers of cyanide exposure (16). Specifically, the percent relative standard deviations for other studies range from 7–100% for cyanide (33, 34), 10–140% for thiocyanate (35, 36), and 30–102% for ATCA (16–17). Significant concentrations of PB-SCN found in non-smoker plasma are indicative of endogenous cyanide or cyanide exposure from sources other than cigarette smoke, such as food or environmental exposure.
Comparison of gender differences
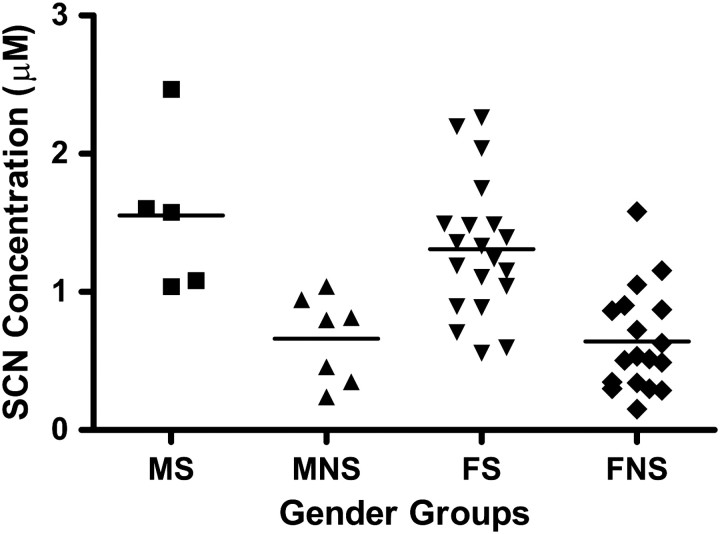
The smoker and non-smoker groups were separated into sub-groups (e.g., male smokers compared to female smokers) to determine if factors besides smoking status produced statistical differences in PB-SCN concentrations. Figure 2 shows the individual PB-SCN concentrations grouped by gender, with the number of participants and the mean concentration of PB-SCN reported in Table I.
Figure 2.
Individual concentrations of PB-SCN found in the plasma of male smokers (MS), male non-smokers (MNS), female smokers (FS) and female non-smokers (FNS). The solid line represents mean concentrations of the group.
Table I.
Plasma Protein-Bound Thiocyanate Concentrations for Each Group and Sub-Group of Participants
| Group | N | Sub-group | N | [SCN]bound (μM) |
|---|---|---|---|---|
| Male | 12 | Smokers | 5 | 1.55 ± 0.58 (SEM = 0.28) |
| Non-smokers | 7 | 0.66 ± 0.31 (SEM = 0.12) | ||
| Female | 38 | Smokers | 20 | 1.31 ± 0.49 (SEM = 0.11) |
| Non-smokers | 18 | 0.64 ± 0.37 (SEM = 0.09) |
Differences in gender did not significantly affect plasma PB-SCN concentrations in the study population (p > 0.05). Comparisons of smoker and non-smoker groups, no matter the gender, resulted in significant differences between each of the groups (p < 0.05). Although the population of males in the current study was low, the similarity in the concentrations of PB-SCN found for males and females indicates that gender does not affect the plasma PB-SCN concentrations. The similarity of PB-SCN concentrations across genders is a desirable characteristic of a marker of cyanide exposure.
Daily cigarette consumption
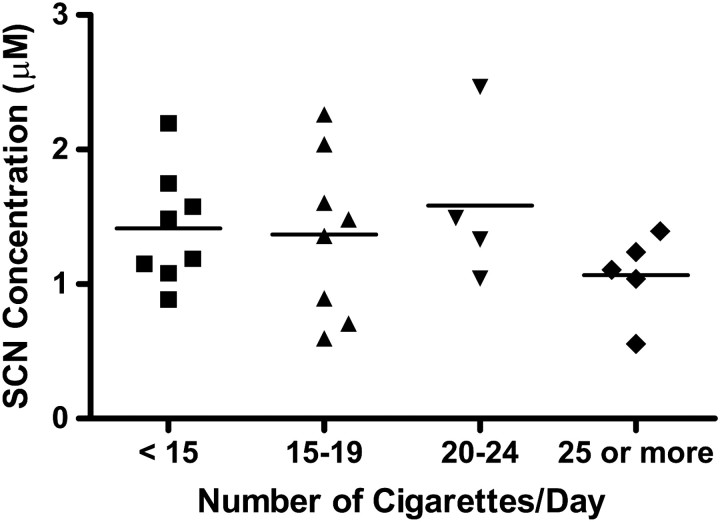
Smoking participants were also grouped according to daily cigarette consumption. The average plasma PB-SCN concentrations found for these groups are reported in Table II and the individual concentrations are displayed in Figure 3. Overall, there was a wide range of cigarettes smoked daily by the smoking participants (5 to 30) with an average of 16.2 cigarettes consumed per day. As visually evident in Figure 3 and confirmed by an ANOVA (with the multiple range tests described), no correlation could be made between the number of cigarettes smoked per day and the levels of PB-SCN found. Previous studies have also found no correlation between daily cigarette consumption and concentrations of cyanide and thiocyanate (37–39), although some studies have found a correlation (12, 36, 40–42).
Table II.
Plasma Protein-Bound Thiocyanate Concentrations Found for Each Group of Smokers (Grouped by Number of Cigarettes per Day)
| Number of cigarettes per day | N | [SCN]bound (μM) |
|---|---|---|
| ≥25 | 5 | 1.07 ± 0.35 (SEM = 0.14) |
| 20–24 | 4 | 1.58 ± 0.62 (SEM = 0.31) |
| 15–19 | 8 | 1.37 ± 0.61 (SEM = 0.21) |
| 5–14 | 8 | 1.41 ± 0.42 (SEM = 0.15) |
Figure 3.
Individual concentrations of protein-bound thiocyanate found in the plasma samples of male and female smoking sub-groups separated based on the number of cigarettes consumed daily. The solid line represents the mean concentrations of each sub-group.
Discussion
The major goal of this study was to more firmly establish or to disprove the correlation between chronic low-level cyanide exposure (i.e., smoking) and concentrations of plasma PB-SCN found by Youso et al. (27). A secondary goal was to determine baseline concentrations and variability of PB-SCN for smokers and non-smokers, which must be known to use this marker to determine involuntary cyanide exposure. Statistically, the concentrations of PB-SCN are clearly significantly different when comparing smokers and non-smokers (p < 0.0001). However, the strong significant difference does not necessarily offer insight into the type of correlation between the marker and the toxic agent exposure. To help support the relationship of a proposed marker with a parent agent, we have previously suggested using the ratio of a high-level exposure group (e.g., smokers) to a low-level exposure group (e.g., non-smokers) within the same study (16, 25), henceforth referred to as a biomarker concentration ratio (BCR). Comparing BCR values of the parent agent and a proposed marker can help quantify the relationship of the proposed marker to the parent agent independent of variability in sampling, storage, or analysis techniques. Similar BCRs for the parent agent and the proposed marker indicate a strong relationship. BCRs are particularly important for the evaluation of cyanide markers because of the extreme effects of sampling, storage and analysis methods on certain markers of cyanide exposure (16). For this study, the PB-SCN BCR is 2.0, which compares favorably to intra-study blood cyanide BCRs of 1.2–3.1 (16). The similar BCR between cyanide and PB-SCN suggests a strong correlation of PB-SCN with cyanide exposure. For the other markers of cyanide exposure, the BCR of free thiocyanate in blood ranges from 1.7–7.1 (16) and the only study of plasma ATCA concentrations produced a BCR of 1.5 (25). This indicates that processes besides cyanide exposure may contribute to free thiocyanate [which is also supported by other studies (18, 19)], but that PB-SCN and ATCA are correlated well to cyanide exposure.
The concentration of PB-SCN was independent of gender (i.e., p > 0.05 for sub-groups of males and females) and did not correlate significantly with reported daily cigarette consumption. The lack of correlation with apparent cyanide dose (i.e., number of cigarettes smoked daily) may be due to a number of factors. One potential factor in the consistency of plasma PB-SCN of smokers (Figure 3) is long-term accumulation of thiocyanate in plasma proteins. Long-term accumulation of PB-SCN would dilute the relationship between cyanide dose and PB-SCN considering that there is a finite number of available sites in the protein for reaction with cyanide (27). Another key issue is variability in smoking behavior related to an individual's ability to consume a desired dose of nicotine from a cigarette (43, 44). Other factors, including smoking history, frequency (i.e., the consistency of an individual in smoking a certain number of cigarettes per day), differences in cigarette brands smoked, and inaccurate estimation of daily cigarette consumption by the participants, may also contribute to the lack of correlation of PB-SCN concentrations with apparent daily cigarette consumption. The relationship between PB-SCN and cyanide dose must be further evaluated.
Future studies will include the analysis of PB-SCN after acute cyanide exposure, the determination of the half-life of PB-SCN, and a similar study to the present one, but with an expanded study population (with greater control over the number of people admitted within each smoking group desired) to verify endogenous levels of the proposed marker of cyanide exposure.
Acknowledgments
The research was supported by the CounterACT Program, National Institutes Of Health Office of the Director and the National Institute of Allergy and Infectious Diseases, Inter Agency Agreement Number Y1-OD-0690-01/A-120-B.P2010-01 and the USAMRICD under the auspices of the US Army Research Office of Scientific Services Program Contract No. W911NF-11-D-0001 administered by Battelle (Delivery order 0079, Contract No TCN 11077). We gratefully acknowledge funding from the National Institutes of Health and the Department of Defense through the Cyanide Medical Countermeasures and the Medical Diagnostics research areas. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the National Institutes of Health, Department of the Army or the Department of Defense.
References
- 1.Eminedoki D.G., Monanu M.O., Anosike E.O. Thiocyanate levels of mainly dietary origin in serum and urine from a human population sample in Port Harcourt, Nigeria. Plant Foods for Human Nutrition. 1994;46:277–285. doi: 10.1007/BF01088426. [DOI] [PubMed] [Google Scholar]
- 2.Murray S., Lake B.G., Lake S., Edwards A.J., Springall C., Bowey E.A., et al. Effect of cruciferous vegetable consumption on heterocyclic aromatic amine metabolism. Carcinogenesis. 2001;22:1413–1420. doi: 10.1093/carcin/22.9.1413. [DOI] [PubMed] [Google Scholar]
- 3.Baud F.J., Barriot P., Toffis V., Riou B., Vicaut E., Lecarpentier Y., et al. Elevated blood cyanide concentrations in victims of smoke inhalation. New England Journal of Medicine. 1991;325:1761–1766. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 4.Scherer G. Carboxyhemoglobin and thiocyanate as biomarkers of exposure to carbon monoxide and hydrogen cyanide in tobacco smoke. Experimental and Toxicolologic Pathology. 2006;58:101–124. doi: 10.1016/j.etp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Forsyth J.C., Mueller P.D., Becker C.E., Osterloh J., Benowitz N.L., Rumack B.H., et al. Hydroxycobalamin as a cyanide antidote: Safety, efficacy, and pharmacokinetics in heavily smoking normal volunteers. Clinical Toxicology. 1993;31:277–294. doi: 10.3109/15563659309000395. [DOI] [PubMed] [Google Scholar]
- 6.Ghawabi S.H.E., Gaafar M.A., El-Saharti A.A., Ahmed S.H., Malash K.K. Chronic cyanide exposure: A clinical, radioisotope, and laboratory study. Occupational and Environmental Medicine. 1975;32:215–219. doi: 10.1136/oem.32.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manzano H., Benedito de Sousa A., Soto-Blanco B., Guerra J.L., Maiorka P.C., Gorniac S.L. Effects of long term cyanide ingestion by pigs. Veterinary Research Communications. 2007;31:93–104. doi: 10.1007/s11259-006-3361-x. [DOI] [PubMed] [Google Scholar]
- 8.Philbrick D.J., Hopkins J.B., Hill D.C., Alexander J.C., Thomson R.G. Effects of prolonged cyanide and thiocyanate feeding in rats. Toxicology and Environmental Health. 1979;5:579–592. doi: 10.1080/15287397909529770. [DOI] [PubMed] [Google Scholar]
- 9.Baskin S.I., Kelly J.B., Maliner B.I., Rockwood G.A., Zoltani C.K. Cyanide poisoning. In: Tuorinsky S.D., editor. Textbook of military medicine, medical aspects of chemical and biological warfare. Washington, DC: Borden Institute; 2008. pp. 371–410. [Google Scholar]
- 10.Ishii A., Seno H., Suzuki K.W., Suzuki O. Determination of cyanide in whole blood by capillary gas chromatography with cryogenic oven trapping. Analytical Chemistry. 1998;70:4873–4876. doi: 10.1021/ac980498b. [DOI] [PubMed] [Google Scholar]
- 11.Lundquist P., Rosling H., Sorbo B., Tibbling L. Determination of cyanide in whole blood, erythrocytes, and plasma. Clinical Chemistry. 1997;33:1228–1230. [Google Scholar]
- 12.Tsuge K., Kataoka M., Seto Y. Cyanide and thiocyanate levels on blood and saliva of healthy adult volunteers. Journal of Health Science. 2000;46:343–350. [Google Scholar]
- 13.Baskin S.I., Petrikovics I., Kurche J.S., Nicholson J.D., Logue B.A., Maliner B.J., et al. Insights on cyanide toxicity and methods of treatment. In: Flora S.J.S., Romano J.A. Jr., Baskin S.I., Sekhar K., editors. Pharmacological perspectives if toxic chemicals and their antidotes. New Delhi, India: Narosa Publishing; 2004. pp. 105–146. [Google Scholar]
- 14.Baskin S.I., Porter D.W., Rockwood G.A., Romano J.A., Patel H.C., Kiser R.C., et al. In vitro and in vivo comparison of sulfur donors as antidotes to acute cyanide intoxication. Journal of Applied Toxicology. 1999;19:173–183. doi: 10.1002/(sici)1099-1263(199905/06)19:3<173::aid-jat556>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Frankenberg L. Enzyme therapy in cyanide poisoning: Effect of rhodanese and sulfur compounds. Archives of Toxicology. 1980;45:315–323. doi: 10.1007/BF00293812. [DOI] [PubMed] [Google Scholar]
- 16.Logue B.A., Hinkens D.M., Baskin S.I., Rockwood G.A. The analysis of cyanide and its breakdown products in biological samples. Critical Reviews in Analytical Chemistry. 2010;40:122–147. [Google Scholar]
- 17.Isom G.E., Baskin S.I. Enzymes involved in cyanide metabolism. In: Sipes I.G., McQueen C.A., Gandolfi A.J., editors. Comprehensive toxicology. New York, NY: Elsevier Science; 1997. pp. 477–488. [Google Scholar]
- 18.Wood J.L., Williams E.F.J., Kingsland N. The conversion of thiocyanate sulfur to sulfate in the white rat. Journal of Biological Chemistry. 1947;170:251–259. [Google Scholar]
- 19.Wood J.L., Williams E.F.J. The metabolism of thiocyanate in the rat and its inhibition by propylthiouracil. Journal of Biological Chemistry. 1949;177:59–67. [PubMed] [Google Scholar]
- 20.Sanchez C.A., Blount B.C., Valentin-Blasini L., Krieger R.I. Perchlorate, thiocyanate, and nitrate in edible coal crops (Brassica sp.) produced in the lower Colorado River region. Bulletin of Environmental Contamination and Toxicology. 2007;79:655–659. doi: 10.1007/s00128-007-9292-6. [DOI] [PubMed] [Google Scholar]
- 21.Heaney R.K., Fenwick G.R. The analysis of glucosinolates in Brassica species using gas chromatography: Direct determination of the thiocyanate ion precursors, glucobrassicin and neoglucobrassicin. Journal of the Science of Food and Agriculture. 1980;31:593–599. [Google Scholar]
- 22.Thomas E.L., Milligan T.W., Joyner R.E., Jefferson M.M. Antibacterial activity of hydrogen peroxide and the lactoperoxidase-hydrogen peroxide-thiocyanate system against oral streptococci. Infection and Immunity. 1994;62:529–535. doi: 10.1128/iai.62.2.529-535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clem W.H., Klebanoff S.J. Inhibitory effect of saliva on glutamic acid accumulation by lactobacillus acidophilus and the role of the lactoperoxidase-thiocyanate system. Journal of Bacteriology. 1966;91:1848–1853. doi: 10.1128/jb.91.5.1848-1853.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logue B.A., Kirschten N.P., Petrikovics I., Moser M.A., Rockwood G.A., Baskin S.I. Determination of the cyanide metabolite 2-aminothiazoline-4-carboxylic acid in urine and plasma by gas chromatography-mass spectrometry. Journal of Chromatography B. 2005;819:237–244. doi: 10.1016/j.jchromb.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 25.Logue B.A., Maserek W.K., Rockwood G.A., Keebaugh M., Baskin S.I. Analysis of the cyanide metabolite, 2-amino-2-thiazoline-4-carboxylic acid, in plasma and its feasibility as a retrospective marker of cyanide exposure. Toxicology Mechanisms and Methods. 2009;19:202–208. doi: 10.1080/15376510802488165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fasco M.J., Hauer C.R., Stack R.F., O'Hehir C., Barr J.R., Eadon G.A. Cyanide adducts with human plasma proteins: Albumin as a potential exposure surrogate. Chemical Research in Toxicology. 2007;20:677–684. doi: 10.1021/tx6003425. [DOI] [PubMed] [Google Scholar]
- 27.Youso S.L., Rockwood G.A., Lee J.P., Logue B.A. Determination of cyanide exposure by gas-chromatography mass-spectrometry analysis of cyanide-exposed plasma proteins. Analytica Chimica Acta. 2010;677:24–28. doi: 10.1016/j.aca.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Fasco M.J., Stack R.F., Lu S., Hauer C.R., III, Schneider E., Dailey M., et al. Unique cyanide adduct in human serum albumin: potential as a surrogate exposure marker. Chemical Research in Toxicology. 2011;24:505–514. doi: 10.1021/tx100344e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterling K. The turnover rate of serum albumin in man as measured by I131-tagged albumin. Journal of Clinical Investigation. 1951;30:1238–1242. doi: 10.1172/JCI102542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noort D., Benschop H.P., Black R.M. Biomonitoring of exposure to chemical warfare agents: A review. Toxicology and Applied Pharmacology. 2002;184:116–126. [PubMed] [Google Scholar]
- 31.Turner P.C., Dingley K.H., Coxhead J., Russell S., Garner C.R. Detectable levels of serum aflatoxin B1-adducts in the United Kingdom population: Implications for aflatoxin-B1 exposure in the United Kingdom. Cancer Epidemiology, Biomarkers & Prevention. 1998;7:441–447. [PubMed] [Google Scholar]
- 32.Coghlin J., Gann P.H., Hammond S.K., Skipper P.L., Taghizadeh K., Paul M., et al. 4-Aminobipheny1 hemoglobin adducts in fetuses exposed to the tobacco smoke carcinogen in utero. Journal of the National Cancer Institute. 1991;83:274–280. doi: 10.1093/jnci/83.4.274. [DOI] [PubMed] [Google Scholar]
- 33.Sano A., Takezawa M., Takitani S. Spectrofluorometric determination of cyanide in blood and urine with naphthalene-2,3-dialdehyde and taurine. Analytica Chimica Acta. 1989;225:351–358. [Google Scholar]
- 34.Lundquist P., Rosling H., Sörbo B. Determination of cyanide in whole blood, erythrocytes, and plasma. Clinical Chemistry. 1985;31:591–595. [PubMed] [Google Scholar]
- 35.Hasuike Y., Nakanishi T., Moriguchi R., Otaki Y., Nanami M., Hama Y., et al. Accumulation of cyanide and thiocyanate in haemodialysis patients. Nephrology Dialysis Transplantation. 2004;19:1474–1479. doi: 10.1093/ndt/gfh076. [DOI] [PubMed] [Google Scholar]
- 36.Hassan S.S.M., Badr I.H.A., Kamel A.H., Mohamed M.S. A novel poly(vinyl chloride) matrix membrane sensor for batch and flow-injection determinations of thiocyanate, cyanide and some metal ions. Analytical Sciences. 2009;25:911–917. doi: 10.2116/analsci.25.911. [DOI] [PubMed] [Google Scholar]
- 37.Demkowska I., Polkowska Z., Namiesnik J. Application of ion chromatography for the determination of inorganic ions, especially thiocyanates in human saliva samples as biomarkers of environmental tobacco smoke exposure. Journal of Chromatography B. 2008;875:419–29. doi: 10.1016/j.jchromb.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Levine M.S., Radford E.P. Occupational exposures to cyanide in Baltimore fire fighters. Journal of Occupational Medicine. 1978;20:53–56. doi: 10.1097/00043764-197801000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Pre J., Vassy R. Plasma thiocyanate and cigarette-smoking status. Medical Science Research. 1992;20:671–672. [Google Scholar]
- 40.Connolly D., Barron L., Paull B. Determination of urinary thiocyanate and nitrate using fast ion-interaction chromatography. Journal of Chromatography B. 2002;767:175–180. doi: 10.1016/s0378-4347(01)00557-6. [DOI] [PubMed] [Google Scholar]
- 41.Hassan S.S.M., Mohmoud W.H., Elgazwy A.S.H., Badawy N.M. A novel potentiometric membrane sensor based on aryl palladium complex for selective determination of thiocyanate in the saliva and urine of cigarette smokers. Electroanalysis. 2006;18:2070–2078. [Google Scholar]
- 42.Yamanaka S., Takaku S., Takaesu Y., Nishimura M. Validity of salivary thiocyanate as an indicator of cyanide exposure from smoking. Bulletin of Tokyo Dental College. 1991;32:157–163. [PubMed] [Google Scholar]
- 43.Benowitz N.L., Hall S.M., Herning R.I., Jacob P., III, Jones R.T., Osman A.-L. Smokers of low yield cigarettes do not consume less nicotine. New England Journal of Medicine. 1983;309:139–142. doi: 10.1056/NEJM198307213090303. [DOI] [PubMed] [Google Scholar]
- 44.Benowitz N.L., Jacob P., III, Kozlowski L., Yu L. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide exposure. New England Journal of Medicine. 1986;314:1310–1313. doi: 10.1056/NEJM198611203152102. [DOI] [PubMed] [Google Scholar]