Abstract
The recreational use of synthetic cannabinoids has recently increased. This increase is due, in part, to the recent availability of inexpensive compound sold legally online in bulk. In particular, JWH-018 (1-pentyl-3-(1-naphthoyl)indole) and JWH-073 (1-butyl-3-(1-naphthoyl)indole) have been found in herbal blends marketed as alternatives to cannabis. Although these particular compounds have recently been emergency scheduled in the United States, online suppliers have shifted sales to other, similar compounds that are not currently scheduled. However, the purity of the drugs obtained from online suppliers is not known. Relative purity of JWH-018 and JWH-073 from three different online suppliers was determined using high-performance liquid chromatography with ultraviolet detection and validated standards obtained from a traditional research chemical supplier. Our results show that JWH-018 and JWH-073 obtained from online vendors was of comparable purity to validated standards, even though the physical properties varied in color, texture, and odor. It is concluded that adverse events following consumption of synthetic cannabinoid preparations is unlikely to be due to impurities or residue from the manufacturing process, but rather to effects of the active drug or interactions with other psychoactive chemicals from herbs blended into products marketed as cannabis alternatives.
Introduction
The recreational use of synthetic cannabinoids has increased over the past several years. In particular, several aminoindoles have gained worldwide popularity due to the prevalence of online suppliers (1, 2). Until recently, these compounds were sold with no legal restrictions in the United States. Recently, however, the United States Drug Enforcement Agency (DEA) listed five such compounds as emergency scheduled substances (3, 4). Following that decision, the DEA was required to file a Notice of Intent to allow distributors at least 30 days to eliminate all remaining stocks of these substances. During that time, online retailers encouraged customers to stockpile the chemicals in anticipation of the end of legal sales. In the case of one supplier, 100 g of complimentary drug was added to randomly selected orders placed over December 15–17, 2010.
Two of the recently scheduled drugs are JWH-018 [1-pentyl-3-(1-naphthoyl)indole] and JWH-073 [1-butyl-3-(1-naphthoyl)indole]. These drugs have been identified in herbal blends that are sold as legal alternatives to marijuana (5). Furthermore, these drugs have been linked to side effects that required medical attention in recreational users (6, 7).
Suppliers purport to provide drugs with greater than 99% purity, yet provide no documentation in support of these claims, either online or included as a certificate of analysis with the shipment. Thus, it would be of interest to know the relative purity of the chemicals that are almost certainly being diverted to recreational street use. Impurities left from the manufacturing process could account for toxicities reported by some users, and here we address this possibility. We report the purity and some physical characteristics of JWH-018 and JWH-073 obtained from several different sources. Drug purchased from Cayman Chemical (Ann Arbor, MI), certified as being ≥ 98% pure, represented the standard against which samples obtained from three different online suppliers (one Chinese and two domestic sources) were compared. Samples were analyzed using high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection in an assay optimized for cannabinoids (8).
Methods
Drugs
JWH-018 and JWH-073 (≥ 98% purity) were purchased from Cayman Chemical Company and used as the standards against which the purity of JWH-018 or JWH-073 from the online sources was evaluated. JWH-018 was also obtained from three different, non-traditional, online sources: jwh018supplier.com [Newport Beach, CA, Source #1]; researchchemicalsupplier.com [Scottsdale, AZ, Source #2]; and IU Chem Holding Co., Ltd [Shanghai, China, Source #3]. Both jwh018supplier.com and researchchemicalsupplier.com purport to be domestic companies with products synthesized in the United States, though no domestic contact is currently listed on either website, and the domain registration for Research Chemical Supplier may be a proxy for an international company. Drug from each of the other sources was ordered online, with no minimum age requirement or limitations on amount purchased. Δ9-Tetrahydrocannabinol (Δ9-THC) was obtained from the National Institute on Drug Abuse (Rockville, MD) and used as an internal standard for the quantification of JWH-018 and JWH-073; it was also used as an aid for identification of the other compounds, which were identified by relative elution time. Also, RCS-4, JWH-250, JWH-081 pure standards were purchased from Cayman Chemical to test for the specificity of the chromatographic system for JWH-018 and JWH-073. Finally, JWH-018 was purchased from Sigma-Aldrich (St. Louis, MO) for observation of physical properties and chromatographic purposes.
Sample preparation
Each powdered drug sample was characterized in terms of color, texture, and odor. A primary stock solution of 1 mg/mL was prepared by dissolving drug in methanol. Aliquots of the primary stock solution were stored at –80°C. On the day of each experiment, an aliquot of the primary stock solution was diluted with mobile phase to attain working stock solutions that were injected into the HPLC system. Primary stock and working stock solutions were tested on at least two separate occasions.
HPLC system
The HPLC system consisted of a Waters model 515 pump, a Waters model 717 autosampler, a Waters model 2487 UV detector, and a Grace C18 column (5 µm, 4.6-mm i.d. × 150 mm). The mobile phase consisted of either 65% (v/v) or 95% (v/v) acetonitrile and 10 mM potassium phosphate buffer using Milli-Q water (pH 2.5). The flow rate of the mobile phase was 0.5 mL/min. UV detection was performed at a fixed wavelength of 220 nm.
Validation of the HPLC assay
The analytical range of calibrators for both JWH-018 and JWH-073 (Cayman standards) dissolved in mobile phase was 31.3 to 5000 ng/mL. For JWH-018 and JWH-073, the limits of detection (LOD) were 2.93 and 1.93 ng/mL, respectively, and the limits of quantification (LOQ) were 9.76 and 6.44 ng/mL, respectively. The LOD and LOQ were determined as the concentrations that could be detected at 3 and 10 times background noise, respectively. Precision and accuracy of the assay for JWH-018 at 1000 ng/mL were 3.4% and 99.6%, respectively. Precision and accuracy of the assay for JWH-073 at 1000 ng/mL were 2.0% and 98.3%, respectively.
Analysis and purity calculation
Working drug solutions for JWH-018 and JWH-073 from online sources were prepared at a concentration of 1000 ng/mL and quantified using analytical range calibrator curves of Cayman standards on two separate occasions. Purity of JWH-018 and JWH-073 from each non-traditional, online source was expressed as a percentage of the expected concentration.
Results
Characteristics of drug obtained from each source are shown in Table I. JWH-018 and JWH-073 from different sources varied substantially in color, texture, and odor. Color ranged from white (JWH-018 from Sigma) to dark brown (JWH-018 from Source #2). Texture ranged from a fine powder (JWH-018 from Source #3) to a chunky powder (JWH-018 from Sigma) to a plastic or resin (JWH-018 from Source #2) to a gel (JWH-018 from Source #1). The odor of naphthalene was present for JWH-018 samples from Source #2 and Source #3. Dissolving drug from each supplier in methanol, then evaporating the solvent resulted in drug that was similar in appearance (white), texture (chunky powder), and odor (none) across all suppliers.
Table I.
Drug Characteristics
| Drug | Source | Texture | Color | Odor | Purity |
|---|---|---|---|---|---|
| JWH-018 | jwh018supplier.com | resin/plastic | dark brown | naphthylene odor | 91.4% |
| researchchemicalsupplier.com | fine powder | light brown | naphthylene odor | 84.7% | |
| IU Chem Holding Co. | sticky gel | bright yellow | no odor | 75.6% | |
| Sigma-Aldrich | chunky powder | white | light chemical odor | ||
| Cayman Chemical | solution | N/A | N/A | >98.0% | |
| JWH-073 | researchchemicalsupplier.com | fine powder | light brown | naphthylene odor | 96.5% |
| Cayman Chemical | fine powder | white | no odor | >98.0% |
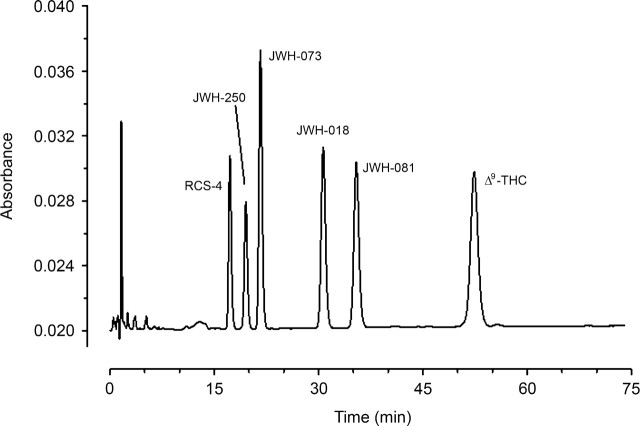
A sample chromatogram of six cannabinoids dissolved in mobile phase is shown in Figure 1. The elution times in minutes for the compounds were 17.5 (RCS-4), 19.8 (JWH-250), 21.9 (JWH-073), 31.0 (JWH-018), 35.7 (JWH-081), and 52.7 (Δ9-THC). Working drug solutions of individual drugs were also injected to test for contaminating substances using a mobile phase containing only 65% (v/v) acetonitrile. The chromatograms of these injections showed only a single major peak indicating that the preparations from all sources were essentially pure.
Figure 1.
Sample chromatogram of various cannabinoids (see Methods for details). This chromatogram was obtained by loading 50 µL of a sample containing 1000 ng/mL of each compound, except Δ9THC, which was at a concentration of 2000 ng/mL.
Percent purity was similar for JWH-018 from all three online sources, ranging from 75.3% to 91.4%. Percent purity for JWH-073 from Source #2 was approximately 100%. Analytical results are shown in Table I.
Discussion
The major finding of this study was that JWH-018 and JWH-073 from several bulk online retailers are of high purity [range: 75%–100% of validated standards] despite substantially different physical characteristics among the samples from different sources. These non-traditional, online providers are likely among those supplying synthetic cannabinoids that are infused into herbal preparations such as “spice” or “K2” and marketed as alternatives to cannabis. Toxicity linked to the use of such products is unlikely to be due to impurities present in the raw synthetic cannabinoid preparation. Rather, such toxicity is more likely due to other constituents in the herbal preparations or to the effects of the cannabinoids themselves.
Samples of JWH-018 and JWH-073 from online sources were of comparable purity to validated preparations obtained from Sigma-Aldrich (data not shown) or Cayman Chemical. There was no evidence of other compounds in any of the preparations from any source as no other peaks were observed on the chromatograms. JWH-018, JWH-073, and Δ9-THC had different retention times, and when injected individually, none of the preparations showed any cross-contamination. Thus, chemicals obtained from online vendors were of comparable purity to those from traditional research chemical suppliers. Also, to demonstrate the specificity of the HPLC system for JWH-018 and JWH-073, several other spice compounds purchased as pure standards from Cayman (RCS-4, JWH-250, JWH-081) were co-injected with JWH-018, JWH-073, and Δ9THC. Baseline resolution of chromatographic peaks for all compounds was achieved when a mobile phase containing 65% (v/v) acetonitrile was used. This indicates that the online products were not cross-contaminated with these compounds.
Each of the samples had different physical characteristics. This appears to be due to minor trace residues remaining in the substance from the manufacturing process. For example, we observed the odor of naphthalene in JWH-018 from sources #2 and #3. Differences in color, texture, and odor among the samples were eliminated by dissolving each preparation in methanol and then drying the compounds to residue under a stream of nitrogen. After this process, individual compounds from all sources appeared to be identical in color, texture, and odor.
There have been reports of intoxication following consumption of herbal preparations containing JWH-018 and JWH-073 (6, 7, 9). Although descriptions of acute toxic effects of synthetic cannabinoid preparations are currently limited to case reports, some common findings are emerging. Intoxication with these products can result in anxiety and tachycardia, both known effects of cannabinoid receptor agonists (7). Other reported effects include paranoia, hallucinations, psychotomimetic effects, and a withdrawal syndrome (6, 7). Although details of the sources for these synthetic cannabinoid preparations is unknown, the results of the present study suggest that such toxicity is not due to impurities or adulterants. Rather, the toxicity could be due either to constituents of the herbal blends that are infused with the synthetic cannabinoids, to interactions between these psychoactive constituents in the herbal blend and the synthetic cannabinoids, or to the synthetic cannabinoids themselves.
A recent report examined constituents of more than 140 samples of herbal blends sold as marijuana alternatives and found JWH-018 and JWH-073 in many of the samples. However, other consituents, including harmaline, caffeine, the opioid o-desmethyltramadol, nicotine, tocopheral, marrubin, and others, were also present in many samples (5). Interactions among these other constituents and the synthetic cannabinoids could be responsible for the adverse events described in clinical reports. Further, many of the plants included in the herbal blends have some psychoactive properties (5), and the interactions among these ingredients and the synthetic cannabinoids could also result in adverse events.
The adverse events reported in the literature that result from consumption of synthetic cannabinoid preparations bear the hallmarks of cannabinoid receptor agonist activity (10). Anxiety and tachycardia, as well as paranoia, perceptual distortions, and hallucinations are documented effects of CB1 receptor agonists. A long history of the potential toxicity and adverse event associated with use of Δ9-THC has been compiled (10). However, Δ9-THC is a weak partial agonist at CB1 receptors, and JWH-018 is a full-efficacy agonist in vitro (11). Until the advent of widely available synthetic cannabinoids, use of high-efficacy CB1 receptor agonists (either acutely or chronically) has not been documented in humans. It is possible that the relatively safe profile of Δ9-THC is not conferred to synthetic cannabinoids with far higher efficacy such as JWH-018.
In conclusion, synthetic cannabinoids JWH-018 and JWH-073 obtained from several online vendors were comparable in purity to validated preparations obtained from traditional research chemical suppliers. Differences in physical characteristics among the samples did not appear to be due to substantial impurities, but rather were likely due to the presence of very small amounts of chemicals used in the purification or synthesis process.
Acknowledgments
Supported by PHS grants DA19222 and DA26781. The authors wish to thank the National Institute on Drug Abuse (Rockville, MD) for the generous donation of Δ9-THC used in this study.
References
- 1.Huffman J.W., Zengin G., Wu M.-J., Lu J., Hynd G., Bushell K., Thompson A.L., Bushell S., Tartal C., Hurst D.P., Reggio P.H., Selley D.E., Cassidy M.P., Wiley J.L., Martin B.R. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg. Med. Chem. 2005;13:89–112. doi: 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 2.Gay M. Synthetic Marijuana Spurs State Bans. The New York Times. 2010 July 10. [Google Scholar]
- 3.McKinley J. “Synthetic Marijuana” Chemicals Ban. The New York Times. 2010 November 24. [Google Scholar]
- 4. Proposed Rules—2010—Schedules of Controlled Substances: Temporary Placement of Five Synthetic Cannabinoids Into Schedule I. http://www.deadiversion.usdoj.gov/fed_regs/rules/2010/fr1124.htm. (accessed November 2011) [Google Scholar]
- 5.Dresen S., Ferreirós N., Pütz M., Westphal F., Zimmermann R., Auwärter V. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J. Mass Spectrom. 2010;45:1186–1194. doi: 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann U.S., Winkelmann P.R., Pilhatsch M., Nees J.A., Spanagel R., Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.”. Dtsch Arztebl Int. 2009;106:464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneir A.B., Cullen J., Ly B.T. “Spice” Girls: Synthetic Cannabinoid Intoxication. J. Emerg. Med. 2011;40:296–299. doi: 10.1016/j.jemermed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Javors M.A., Sanchez J.J., McMahon L.R. Quantification of rimonabant (SR 141716A) in monkey plasma using HPLC with UV detection. J. Chromatogr. Sci. 2010;48:491–495. doi: 10.1093/chromsci/48.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vearrier D., Osterhoudt K.C. A Teenager With Agitation. Pediatr. Emerg. Care. 2010;26:462–465. doi: 10.1097/PEC.0b013e3181e4f416. [DOI] [PubMed] [Google Scholar]
- 10.Perrine D.M. The Chemistry of Mind-Altering Drugs: History, Pharmacology, and Cultural Context. Washington, D.C: American Chemical Society; 1996. Dissociatives and Cannabinoids: PCP, THC, ETCs; pp. 333–394. [Google Scholar]
- 11.Atwood B.K., Huffman J., Straiker A., Mackie K. JWH018, a common constituent of “Spice” herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br. J. Pharmacol. 2010;160:585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]