Abstract
Objectives:
This study evaluated the association between pus cells and semen parameters in infertile Pakistani males.
Methods:
A cross-sectional descriptive study was carried out in the Department of Reproductive Physiology/Health, National Institute of Health, Islamabad, Pakistan, from 2004 to 2009. A total of 1,521 subjects were analysed, along with 97 proven fathers as controls.
Results:
The mean of pus cells was 7.43 ± 0.43, 4.35 ± 0.34, and 4.26 ± 0.17 per high field in teratozoospermic, oligoasthenozoospermic, and asthenozoospermic groups, respectively, while it was 3.25 ± 0.26, 3.10 ± 0.19, and 2.98 ± 0.04 per high field in azoospermic, oligozoospermic and the proven father groups, respectively. The fewest pus cells were observed among proven fathers, which varied non-significantly (P >0.05) with all cases, except with teratozoospermic, oligozoospermic, and oligoasthenozoospermic cases. Pus cells showed an inverse relationship to sperm motility and count, except in azoospemia cases. Similarly, the fewest pus cells were observed among groups where normal forms where significantly more frequent (P <0.05). More pus cells were observed in cases where motility, and concentration or morphology was compromised. Similarly, low pus cell counts were seen in cases where sperm had the fewest head and neck defects. All kinds of sperm defects varied non-significantly (P >0.05) between proven fathers and normal concentration cases.
Conclusion:
High pus cell counts were observed in various subclasses of infertile patients. Ignorance of this pyospermic factor will make pyospermic patients to be misdiagnosed as normozoospermic. Therefore, the presence of pyospermia must be considered by physicians as a male infertility factor.
Keywords: Pyospermia, Male infertility, Pakistan
Advances in knowledge
- Sperm infection is a classic cause of infertility. Ignorance of the pyospermic factor would have rendered the “pyospermia only” patients as normozoospermic, and the rest azoo-, oligo-, astheno- or oligoasthenozoospermic. In such an instance, the pyospermic males would not be treated for pyospermia, and hence their state of infertility would persist. Therefore, it is suggested that presence of WBC in the semen should not be ignored by the treating physician and must be considered as a male fertility limiting factor.
Application to patient care
- This study will be helpful for clinicians, infertility specialists and andrologists in treating and managing patients with pyospermia.
High white blood cell (WBC) concentrations within semen are an indicator of infection; this condition, marked by pus in the semen, is termed pyospermia. Although small numbers of WBCs are a normal constituent of the semen, patients are only considered non-pyospermic as long as the WBC concentration remains below 5 per high power field (HPF).1 However, WBCs may have a valuable effect on sperm function when leukocyte levels range from 1–3 × 106/ml.2 The association of pyospermia with male infertility has been pointed out by many researchers.3–4
Infections may affect male fertility in different ways. Possible consequences are impairment of the spermatogenesis, the induction of an autoimmune mechanism, spematodysfunction, and inflammatory occlusion of the ejaculatory duct. Reduced motility of spermatozoa has been found in semen samples which contain high concentrations of bacteria.5 Similarly, 43% of pyospermic patients showed spontaneous downward variation in the absence of treatment.6
Antisperm antibodies play a significant role in male factor infertility. The prevalence of antisperm antibodies in the infertile population is approximately 10%. Earlier studies found that a higher prevalence of antisperm antibodies among infertile men with a history of bacterial prostitits or urethritis were pyospermic.7 Moreover, the sperm motile efficiency index (SMEI) was found to be significantly lower in pyospermic patients when compared with that of non-pyospermic men.1
In a 1997 study, the relationship between semen quality, pyospermia, and microbiology were studied in 201 semen isolates, which showed reduced fertility in 43% of patients who were pyospermic. The most common organisms detected were Enterobacteriaceae (2.8%), Gardnerella vaginalis (9.6%), Chlamydia trachomatis (1.6%), Mycoplasma genitalium (0.9%), and Ureaplasma urealyticum (11.85%).8
Male infertility is a serious global problem. Although the problem is mainly due to sperm defects, other factors are also responsible for male infertility, of which only one is pyospermia. With this in mind, the present study was designed to evaluate the association between pus cells and semen parameters in infertile Pakistani male patients.
Methods
This cross-sectional descriptive study of a non-probability sampling was carried out in the Department of Reproductive Physiology/Health of the Public Health Laboratories Division of the National Institute of Health in Islamabad, Pakistan, over a 5-year period, from 2004 to 2009. The participants included 1,521 infertile males and 97 proven fathers. Semen and seminal pus cell analysis and an assessment of various seminal parameters were carried out to determine pus levels. After obtaining approval from the ethics committee of the institute and taking informed consent from the patients, semen examinations of the patient and control groups were carried out according to the standardised method of the World Health Organization.9
Patients who had never received infertility treatments and who had no cause of infertility were included in the study. Semen samples were obtained through masturbation. Subjects who had undergone pelvic surgery or hernia repair, had diseases such as diabetes mellitus or thyroid disease, or were recreational drug users were not included in this study.
After fulfilling the protocols in standard operation procedures (SOPs) of the department, well mixed and homogenised semen samples were thoroughly checked by two trained senior technicians for concentration, activity, and morphology. Samples were rechecked by a pathologist/laboratory scientist and then documented. Finally, a report was issued. Pus cells were confirmed by the Giemsa staining technique.
Participants were divided into six groups on the basis of sperm concentration, motility, and morphology: azoospermic, oligozoospermic, polyzoospermic, or normozoospermic on the basis of their sperm concentrations, and asthenozoospermic or teratozoospermic on the basis of sperm motility and morphology. Men who had successfully impregnated their wives without medical intervention during the last six months were placed in the proven fathers’ seventh group.
Persons having no spermatozoa in their semen were classified as azoospermic and those having less than 15 million/ml were categorised as oligozoospermic. In cases where sperm concentrations were more than 250 million/ml, the subjects were placed in the polyzoospermic group, while those with counts ranging between 20–250 million/ml represented the normozoospermic group. Similarly, persons having an overall impaired sperm motility of less than 50% or less than 25% of active motility were categorised as asthenozoospermic. Sperm with a disturbed morphology of more than 70% of normal were considered teratozoospermic.10
The results obtained were subjected to statistical analysis by using Statistical Package for the Social Sciences (SPSS), Version 14, (IBM, Chicago, IL, USA). The results of the various pyospermic groups were compared with the control group by applying a t-test and the level of significance was determined. Values less than 0.05 were considered significant.
Results
The mean number (%) of pus cells was 7.43 ± 0.43, 4.35 ± 0.34, and 4.26 ± 0.17 per HPF in the teratozoospermic, oligoasthenozoospermic and asthenozoospermic groups, while it was 3.25 ± 0.26, 3.10 ± 0.19 and 2.98 ± 0.04 per HPF in azoospermic, oligozoospermic, and proven father groups. The fewest pus cells were observed among proven fathers, which varied non-significantly (P >0.05) in all cases, except with teratozoospermic, oligozoospermic, and oligoasthenozoospermic cases [Tables 1 & 2, Figure 1].
Table 1:
Pus cells with seminal morphology, including abnormal forms, and head, neck and tail defects in various groups. Means sharing a common letter do not differ significantly. Others differ significantly (P <0.05).
| Group | N | Pus Cells/HPF | Abnormal Forms (%) | Head Defects (%) | Neck Defects (%) | Tail Defects (%) |
|---|---|---|---|---|---|---|
| Az* | 203 | 3.25 ± 0.26 a | 0.00 (0.00) a | 0.00 (0.00) a | 0.00 (0.00) a | 0.00 (0.00) a |
| Ol† | 353 | 3.10 ± 0.19 ab | 38.54 (1.22) b | 26.42 (0.97) b | 6.64 (0.41) b | 6.60 (0.35) b |
| As‡ | 535 | 4.26 ± 0.17 c | 36.46 (1.00) bc | 23.40 (0.86) c | 4.86 (0.29) c | 8.89 (0.49) c |
| Oas§ | 159 | 4.35 ± 0.34 cd | 47.79 (1.76) d | 31.69 (1.47) d | 10.82 (0.73) d | 8.49 (0.62) cd |
| T¦ | 37 | 7.43 ± 0.43 e | 89.84 (1.29) e | 75.43 (2.99) e | 4.32 (0.89) ce | 10.08 (1.39) cde |
| N** | 221 | 3.29 ± 0.28 abf | 20.92 (1.04) f | 12.93 (0.68) f | 2.90 (0.27) ef | 5.28 (0.53) f |
| P†† | 13 | 3.85 ± 1.44 abcdfg | 14.46 (2.58) g | 6.31 (0.88) g | 0.77 (0.30) g | 7.38 (2.51) bcdefg |
| F‡‡ | 97 | 2.98 ± 0.04 abfg | 17.46 (0.00) h | 11.32 (0.87) f | 1.92 (0.23) h | 4.24 (0.62) fg |
HPF = high power field;
Az* = azoospermic;
Ol† = oligozoospermic;
As‡ = asthenozoospermic;
Oas§ = oligoasthenozoospermic;
T¦ = teratozoospermic;
N** = normozoospermic;
P†† = polyzoospermic;
F‡‡ = proven fathers
Table 2:
Seminal parameters (i.e. concentrations, active motility, sluggish, immotile, or containing pus cells) in various groups. Means sharing a common letter do not differ significantly. Others differ significantly (P <0.05).
| Group | N | Pus cells/HPF | Sperm Concentration | Active Motility (%) | Sluggish Motility (%) | Immotile (%) |
|---|---|---|---|---|---|---|
| Az* | 203 | 3.25 (0.26) a | 0.00 (0.00) a | 0.00 (0.00) a | 0.00 (0.00) a | 0.00 (0.00) a |
| Ol† | 353 | 3.10 (0.19) ab | 6.99 (0.35) b | 29.16 (0.96) b | 15.25 (0.51) b | 55.59 (1.07) b |
| As‡ | 535 | 4.26 (0.17) c | 50.11 (2.12) c | 17.77 (0.49) c | 11.67 (0.29) c | 70.57 (0.61) c |
| Oas§ | 159 | 4.35 (0.34) cd | 4.45 (0.42) d | 14.50 (0.86) d | 12.14 (0.56) cd | 73.36 (1.18) d |
| T¦ | 37 | 7.43 (0.43) e | 5.64 (1.15) bde | 17.73 (3.22) cde | 10.73 (1.27) cde | 71.54 (4.08) cde |
| N** | 221 | 3.29 (0.28) abf | 87.49 (3.51) f | 46.61 (1.27) f | 11.29 (0.41) cdef | 42.10 (1.28) f |
| P†† | 13 | 3.85 (1.44) abcdfg | 402.23 (39.70) g | 55.92 (4.19) g | 8.85 (1.62) cdefg | 35.23 (3.07) g |
| F‡‡ | 97 | 2.98 (0.04) abfg | 102.12 (1.34) h | 60.01 (0.58) g | 10.94 (1.38) cdefg | 28.85 (0.28) h |
HPF = high power field;
Az*= azoospermic;
Ol†= oligozoospermic;
As‡= asthenozoospermic;
Oas§= oligoasthenozoospermic;
T¦= teratozoospermic;
N**= normozoospermic;
P††= polyzoospermic;
F‡‡= proven fathers.
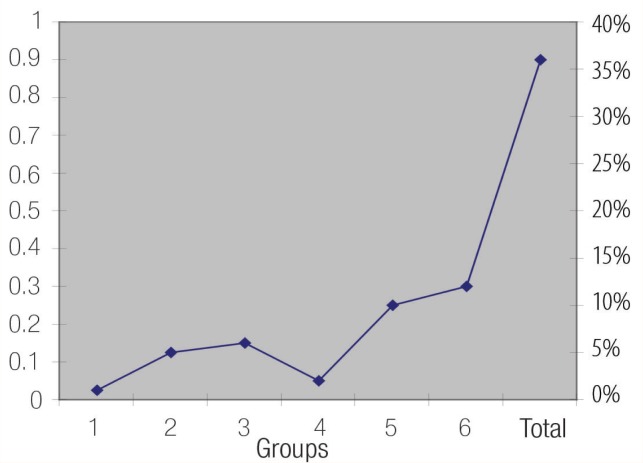
Figure 1:
Individual and combined incidence of categories of pyospermia in 1,521 male partners of infertile couples. 1 = oligopyospermia; 2 = oligoasthenopyospermia; 3 = asthenopyospermia; 4 = azoopyospermia; 5 = pyospermia; 6 = teratopyospermia.
Table 1 shows that fewer pus cells were observed in cases where the sperm displayed the fewest head and neck defects, such as in polyzoospermic subjects, followed by normal count cases and proven fathers, while tail defects were fewest in proven fathers. All kinds of defects varied non-significantly (P >0.05) between proven fathers and normal concentration cases.
Similarly, Table 2 shows an inverse relationship between pus cells sperm motility and count, except in azoospemic patients. The results show that pus cells are in decline when motility and count increases in the semen ejaculates. Similarly, the fewest pus cells were observed among groups (i.e. proven fathers and polyzoospermic cases) where normal forms where significantly higher (P <0.05). More pus cells were observed in cases where motility and concentration or morphology was compromised.
Discussion
High pus cell counts were observed in various subclasses of infertile patients. The fewest pus cells were observed among proven fathers. Pus cells showed an inverse relationship to sperm motility and count, except in azoospemia cases. Similarly, the fewest pus cells were observed among groups where normal forms where significantly more frequent. More pus cells were observed in cases where motility, and concentration or morphology was compromised. Similarly, low pus cell counts were seen in cases where sperm had the fewest head and neck defects. All kinds of sperm defects varied non-significantly between proven fathers and normal concentration cases.
Pyospermia is one of the most important causes of male infertility, but the distribution, origin, and role of pus cells in semen is still controversial. Researchers have reported pyospermia’s negative effects on semen parameters and even on in vitro fertilisation (IVF). This negative effect stems from the fact that pyospermia increases reactive oxygen species (ROS) which may cause sperm damage, leading to significantly increased male infertility.11
Aspects of factors affecting male infertility need to be addressed specifically in order to treat the patient effectively. In the present study, although 36% of the examined patients exhibited pyospermia, only 10% had isolated pyospermia; while 26% were pyospermic in addition to having concentration and motility disturbances [Figure 1]. In a similar study, pyospermia was found in the semen samples of up to 23% of infertile men.12 Pyospermia has been reported to affect negatively sperm penetration assays, count, and motility.13 The highest incidence reported in the present investigation was that in teratopyospermic and oligoasthenopyospermic patients [Table 2]. The probable cause of impaired semen motility appears to be that the leukocytes have a negative effect on semen parameters.1,13–14 The presence of significant numbers of WBC in the semen correlates with altered sperm parameters and diminished fertility. However, it is not known with certainty whether these changes are because of increased leukocyte concentrations or due to an underlying cause that may cause both pyospermia and altered sperm function.12 Leukocytes represent the main source of ROS both in seminal plasma and in sperm suspensions, and have a negative influence on sperm function and fertilisation rates.15
The sperm deformity index (SDI) is calculated by dividing the total number of deformities observed by the number of sperm selected. The score is a novel expression of the quality of sperm morphology, which has been shown to be a more powerful predictor of male fertility and of in vitro fertilisation outcomes as opposed to an assessment of the proportion of sperm with normal morphology.16 It also has been reported that a significant positive correlation exists between pyospermia and compromised sperm morphology, including tail defects, acrosomal damage, and high SDI scores.17 In another study, the findings suggest that leukocytes had a positive association with normal morphology and progressive motility.18 The same finding was also observed in our study. Table 1 shows that pus cells were found high in teratozoospermic cases where morphology was compromised (89.84%). The altered morphology exhibited is mainly that of head defects (75.43%), which was in agreement with the previous study cited.
Some studies have shown a disassociation between bacteria growing in semen and pyospermia.12 This disassociation was noted in our study. Of all of the patients who were found to be pyospermic, only a few samples yielded bacterial growths in semen cultures. Other studies in the West, have observed that pyospermia may not necessarily be linked to microbial activity.4,13 The low percentage of positive bacterial cultures in our population may be due to our sociocultural practices, along with the frequent inappropriate and indiscriminate use of antibiotics.
Finally, in the present study, pyospermia was detected in 36% of males. Only 10% of these were pyospermic, while 26% combined with it other semen disturbances. By ignoring pyospermia, the 10% of purely pyospermic cases would otherwise be classified together with patients free of male factor infertility, causing a decrease in male factor contribution towards infertility. Similarly, and in other conditions as well, the remaining factors alone could be considered the cause of infertility for therapeutic purposes. Many studies have shown that pyospermia reduces male fertility, and unless the state of pyospermia is corrected, the fertility of these patients will remain affected.3,4,12
Conclusion
High pus cell counts were observed in various subclasses of infertile patients, confirming that semen infection is a classic cause of male infertility. Our study results showed that pyospermia is associated either with reduced sperm motility or altered normal morphology. If not properly treated, infertility will persist in such patients. Therefore, it is suggested that the presence of pus cells in the semen should not be ignored by the treating physician and must be considered as a factor limiting male fertility.
References
- 1.Satoh S, Satoh K, Orikasa S, Maehar I, Takahashi M, Hiramatsu M. Studies on pyospermia in male infertility. Nippon Hinyokika Gakkai Zasshi. 1990;81:170–2. doi: 10.5980/jpnjurol1989.81.170. [DOI] [PubMed] [Google Scholar]
- 2.Kaleli S, Ocer F, Irez T, Budak E, Aksu MF. Does leukocytospermia associate with poor semen parameters and sperm functions in male infertility? The role of different seminal leukocyte concentrations. Eur J Obstet Gynecol Reprod Biol. 2000;89:185–91. doi: 10.1016/s0301-2115(99)00204-3. [DOI] [PubMed] [Google Scholar]
- 3.Maruyama H. Studies on the usefulness of a long-term high dose of methylcobalamin in-patients with oligozoospermia. Hin Kiyo. 1985;33:151–6. [PubMed] [Google Scholar]
- 4.Matthews GJ, Goldstein M, Henry JM, Schlegel PN. Non-bacterial pyospermia: a consequence of clomiphene citrate therapy. Int J Fertil Menopausal Stud. 1995;40:187–91. [PubMed] [Google Scholar]
- 5.Krause W, Weidner W. Sexually transmitted diseases as causes of disorders of male fertility. Z Hautkr. 1989;64:596, 599–601. [PubMed] [Google Scholar]
- 6.Lackner JE, Lakovic E, Waldhor T, Schatzl G, Marberger M. Spontaneous variation of leukocytospermia in asymptomatic infertile males. Fertil Steril. 2008;90:1757–60. doi: 10.1016/j.fertnstert.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Jarow JP, Kirkland JA, Jr, Assimos DG. Association of antisperm antibodies with chronic non-bacterial prostates. Urology. 1990;36:154–6. doi: 10.1016/0090-4295(90)80215-9. [DOI] [PubMed] [Google Scholar]
- 8.Kjaergaard N, Kristensen B, Hansen ES, Farholt S, Schonheyder HC, Uldbjerg N, et al. Microbiology of semen specimens from males attending a fertility clinic. APMIS. 1997;105:566–70. doi: 10.1111/j.1699-0463.1997.tb05054.x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO Laboratory Biosafety Manual. 3rd ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 10.Schirren C. Textbook of Practical Andrology. Hamburg: Schireng AG.; 1983. pp. 17–31. [Google Scholar]
- 11.Li J, Liu RZ. Progress in leukocytospermia research. Zhonghusa Nan Ke Xue. 2006;12:730–2. 736. [PubMed] [Google Scholar]
- 12.Jarvi K, Noss MB. Pyospermia and male infertility. Can J Urol. 1994;1:25–30. [PubMed] [Google Scholar]
- 13.Berger RE, Karp LE, Williamson RA, Koehler J, Moore DE, Holmes KK. The relationship of pyospermia and seminal fluid bacteriology to sperm function as reflected in the sperm penetration assay. Fertil Steril. 1982;37:557–64. doi: 10.1016/s0015-0282(16)46166-2. [DOI] [PubMed] [Google Scholar]
- 14.Corradi G, Jaszovszky I. A pyospermia hatasa a fertilitasra. Vizsgalatok szelektiv feherversejt festessel, elasztaz meghatarozassal es hagyomanyos ondoparameterekkel. (Effect of pyospermia on fertility. Studies by selective leukocyte staining, elastase determination and routine sperm parameters) Orv Hetil. 1994;135:573–76. [PubMed] [Google Scholar]
- 15.Ricci G, Perticarari S, Boscolo R, Simeone R, Martinelli M, Fischer-Tamaro L, et al. Leukocytospermia and sperm preparation: A flow cytometric study. Reprod Biol Endocrinol. 2009;19:128. doi: 10.1186/1477-7827-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aziz N, Buchan I, Taylor C, Kingsland CR, Lewis-Jones I. The sperm deformity index: A reliable predictor of the outcome of oocyte fertilization in vitro. Fertil Steril. 1996;66:1000–8. doi: 10.1016/s0015-0282(16)58697-x. [DOI] [PubMed] [Google Scholar]
- 17.Aziz N, Agarwal A, Lewis-Jones I, Sharma RK, Thomas AJ., Jr Novel associations between specific sperm morphological defects and leukocytospermia. Fertil Steril. 2004;82:621–7. doi: 10.1016/j.fertnstert.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 18.Lackner JE, Agarwal A, Mahfouz R, du Plessis SS, Schatzl G. The association between leukocytes and sperm quality is concentration dependent. Reprod Biol Endocrinol. 2010;5:12. doi: 10.1186/1477-7827-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]