Abstract
Intracranial developmental venous anomalies (DVAs), also called venous angiomas, and Wilson’s disease are both considered rare disorders with varying degrees of neurologic and systemic manifestations; yet the coexistence of the two disorders is considered extremely rare, bearing in mind the low prevalence of each disorder. Epilepsy is a recognised presentation in these disorders and will be the focus of discussion in our report of a 21-year-old male patient who, based on a clinical examination and laboratory and neuroimaging results, was diagnosed with both Wilson’s disease and DVA. He presented initially at Sultan Qaboos University Hospital, Oman with tremors and writing difficulties in the right hand followed by the development of epilepsy, and was treated medically by de-coppering and antiepileptic medications. We also present a brief literature review of both disorders, their association with epilepsy, and treatment options. Family screening for patients with Wilson’s disease is pivotal in preventing unfavourable outcomes.
Keywords: Wilson’s disease, Intracranial venous angioma, Epilepsy, Case report, Oman
The terms developmental venous anomaly (DVA) and venous angioma are used synonymously in medical literature, although the former is currently more widely used as it is generally accepted that DVAs are formed in utero.1 DVAs are the most frequently recognised malformation among cerebral vascular malformations including capillary telangiectasias, cavernous malformations, and arteriovenous malformations, with an incidence of up to 2.6% in one brain autopsy series. DVAs are characterised by a cluster of venous radicles that converge into a collecting vein, resulting in the typical caput medusae appearance of the DVA.1
The aetiology of DVAs remains uncertain but may relate to arrested development of venous structures.2,3 Histologically, they consist of a number of abnormally thickened veins with normal feeding arteries and capillaries.3 The most common locations are frontoparietal region (36–64%) and the cerebellar hemisphere (14–27%); DVAs, however, can be seen anywhere, draining both superficially and deeply.4 The lesions are usually solitary (75%), except in blue rubber bleb naevus syndrome where they are multiple.2 In 8–33% of cases, they are associated with cavernous malformations and are referred to as mixed vascular malformations.2
On the other hand, Wilson’s disease, also known as hepatolenticular degeneration, is an autosomal recessive disorder produced by a gene mutation on chromosome 13. The gene encodes a transport protein, the mutation of which causes abnormal deposition of copper in the liver, brain (especially the basal ganglia), and corneas. Wilson’s disease typically begins in childhood but in some cases has its onset as late as the fifth or sixth decade. About one-third of patients present with psychiatric symptoms, one-third present with neurological features, and one-third present with hepatic disease. Neurological manifestations are largely extrapyramidal, including chorea, tremor, and dystonia. Other symptoms include dysphagia, dysarthria, ataxia, gait disturbance, and a fixed (sardonic) smile. Seizures may also occur in a minority of cases.5
In this particular patient, we are describing a unique coexistence of both Wilson’s disease and DVA with a rare presentation of epilepsy.
Case Report
A 21-year-old college student presented at Sultan Qaboos University Hospital, Oman, with an insidious onset tremor in the right hand and complained of difficulty in writing over a period of one year. He was a right-handed person with unremarkable past medical or surgical histories. His elder brother had died six years earlier at the age of 45 years because of acute fulminant hepatic failure while he was being prepared for liver transplantation; the underlying cause was found to be Wilson’s disease. However, no screening tests were performed on other family members. This patient’s neurological examination was significant for rigidity, and exaggerated deep tendon reflexes in the right upper limb with the presence of action tremor. There were no other significant neurologic or systemic findings.
A blood workup revealed low serum caeruloplasmin (0.04 g/L, reference range: 0.22–0.58 g/L) and thrombocytopenia of 86 × 109/L. Other investigations, including a liver function test, coagulation profile, thyroid function test, and a serum polymerase chain reaction (PCR) viral screening for Epstein–Barr virus (EBV) and cytomegalovirus, (CMV) were unremarkable. A 24-hour urinary copper was 2.4 umol/24 hour (reference range: 0.38 to 0.77 umol/24 hours). Ultrasonography of the abdomen showed splenomegaly with altered liver echoes suggestive of early fibrosis. A slit-lamp examination confirmed the presence of Kayser– Fleischer rings in both eyes, and the diagnosis of Wilson’s disease was established. He was started on de-coppering therapy, namely penicillamine and zinc sulfate.
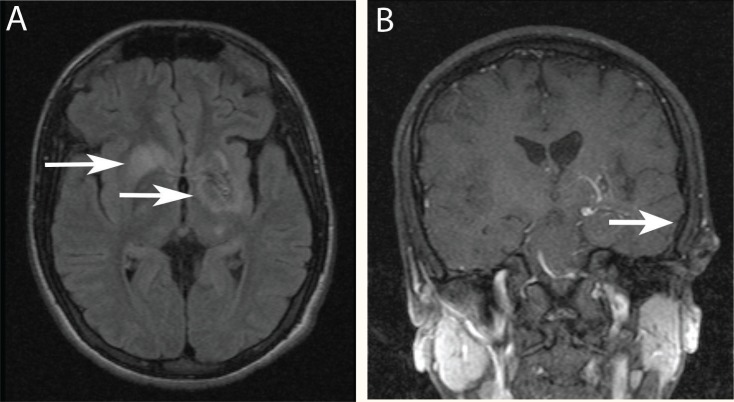
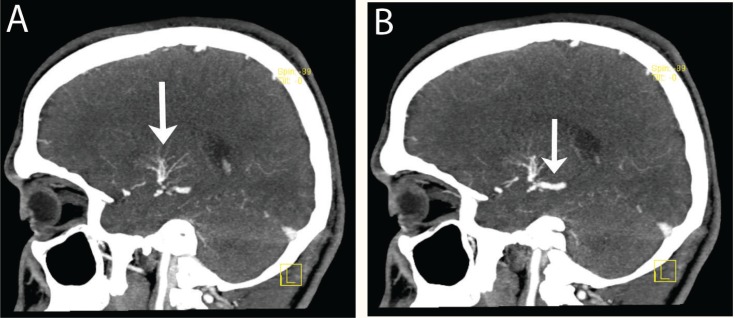
Contrast-enhanced brain magnetic resonance imaging (MRI) revealed hyperintense lesions seen on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences involving the right basal ganglia, left basal ganglia, left internal capsule, and left thalamus [Figure 1A] with abnormal vessel enhancement in the left basal ganglia [Figure 1B]. A computed tomography (CT) angiography of the brain showed small veins at the left basal ganglia draining into a large vein and thence into the vein of Galen with no evidence of a feeding artery or aneurysms [Figure 2A & B]. These radiological features were consistent with a diagnosis of DVA.
Figure 1A & B:
(A) Brain magnetic resonance imaging (MRI) scan of the patient, axial view, fluid-attenuated inversion recovery (FLAIR) sequence, showing hyper-intense lesions involving the right basal ganglia, left basal ganglia, left internal capsule and left thalamus. (B) Coronal view, gadolinium enhanced brain MRI scan of the patient showing abnormal vessels enhancement in the left basal ganglia.
Figure 2A & B:
Sequential sagittal views of brain computed tomography (CT) angiography of the patient showing a developmental venous anomaly (DVA) consisting of small veins like caput medusae, seen at the left basal ganglia (A), draining into a large vein and thence into the vein of Galen (B). There is no evidence of a feeding artery or aneurysms.
Two months later, the patient presented at the Emergency Department with an attack of generalised tonic-clonic convulsions and prolonged postictal state. Clinically, he was confused for two hours after which he recovered slowly with no new neurological deficit and his systemic examination was normal. A basic workup was remarkable for thrombocytopenia of 80 × 109/L, but other tests including electrolytes, bone profile, serum magnesium, renal function test, and liver function test were normal. An urgent brain CT scan did not show any new pathology. He was admitted to the high dependency unit for observation and after 6 hours he had a second witnessed generalised tonic-clonic convulsion for five minutes that was aborted by intravenous diazepam. Antiepileptic treatment (levetiracetam) was started and his electroencephalogram (EEG), done one day later, showed a normal background with occasional generalised slowing, but no definite epileptiform discharges were recorded.
No further generalised tonic-clonic convulsions occurred after starting levetiracetam, but he had two episodes of staring and loss of awareness, suggestive of complex partial seizures. The levetiracetam dose was upgraded and after two days he was discharged home with follow-up appointments at the neurology and haematology outpatient clinics. Appointments also were arranged to screen for Wilson’s disease in his siblings.
Discussion
DVAs are often asymptomatic, follow a benign clinical course, and do not require follow-up imaging studies or specific medical management.1,6 However, DVAs may manifest clinically as headache, seizure, focal neurological deficits, and altered levels of consciousness due to thrombosis, haemorrhage, or mass effect of the DVA.6,7
Acute thrombosis of the draining vein of malformation was documented in 19 cases of symptomatic thrombosed DVAs leading to haemorrhagic or venous ischaemic infarctions.1,6 A symptomatic haemorrhage rate of 0.34% per year was observed, usually with minor manifestations, although fatal intracranial hemorrhages have been described.8 DVAs are associated with cavernous malformations in 13–40% of cases, and might be responsible for the majority of symptomatic cases previously attributed to DVAs. Mechanical compression of intracranial structures by a component of the DVA was also reported, and the most common associated symptoms were hydrocephalus, tinnitus, brainstem deficits, hemifacial spasms, and trigeminal neuralgia.7
Cerebral angiography is considered the gold standard for the diagnosis of DVAs, but they are usually identified with contrast-enhanced cross-sectional imaging modalities such as MRI, magnetic resonance angiography (MRA), and CT angiography.4,9 In our case, the diagnosis of DVA was based on enhancement of the abnormal vessels in the MRI [Figure 1B], as well as the classic caput medusa sign showed by CT angiography [Figure 2]. There was no other associated vascular malformation; neither bleeding nor thrombosis was observed in either study. DVAs should be treated conservatively in the vast majority of cases, with associated symptoms such as headaches and seizures managed medically.10 Surgery may be required in patients with haemorrhages associated with a DVA or with uncontrolled seizures.8 Thrombotic complications of DVAs require the same treatment and laboratory workup as cortical venous or sinus thrombosis (i.e. anticoagulation treatment with investigation of procoagulating factors or prothrombotic conditions).7
While focusing on epilepsy as a presentation in our patient, the prevalence of seizures in Wilson’s disease was reported to be 6.2–7.5%; these figures are 10 times higher than the prevalence in the general population.11–12 The diagnosis of epilepsy in our patient was established mainly on clinical bases, as his EEG showed occasional slowing with no epileptiform discharges. This can be explained by the fact that EEG has a relatively low sensitivity in the diagnosis of epilepsy, ranging from 25–56% accuracy. Its specificity is better, but again variable at 78–98%.13 The reported incidence of abnormal EEGs in Wilson’s disease varies considerably from 41–80%, and this was attributed to the methodological differences in each study group. In one large cohort of 282 patients with Wilson’s disease, the following EEG abnormalities were observed: a reduction in alpha frequency in six patients; an increase in beta frequency in five patients; an increase in theta activity in seven patients; an increase in delta activity in one patient; focal epileptiform discharges in four patients, and low voltage indeterminate activity in two patients. In our case, the MRI changes in the right basal ganglia, left basal ganglia, left internal capsule, and left thalamus [Figure 1A] are well-recognised radiological findings in Wilson’s disease with central nervous system involvement and are related to copper deposition in these sites.11
The exact pathophysiology of seizures in Wilson’s disease is not well understood; the following mechanisms were postulated: 1) direct toxicity of copper, probably by inhibition of membrane ATPase; 2) pyridoxine deficiency due to penicillamine, and 3) metabolic encephalopathy.11 Treatment of seizures in Wilson’s disease relies mainly on antiepileptic therapy, a low copper diet, and proper de-coppering medications including pyridoxine supplement if penicillamine is used. Pyridoxine is not required if the other copper chelating agents, trientine or tetrathiomolybdate, are used as an alternative to penicillamine due to intolerance or development of serious side effects.11,12
We used levetiracetam as an antiepileptic drug because of its pharmacokinetic advantages including rapid and almost complete absorption, absence of enzyme induction, absence of interactions with other drugs, and effectiveness in treatment of partial seizures with or without secondary generalisation.14,15
Conclusion
DVAs and Wilson’s disease are recognised as causes of broad spectrum central nervous system manifestations ranging from asymptomatic to life threatening. Epilepsy is a well-known complication of each disorder but it is unclear whether its incidence will increase if the disorders coexist; however, the management of coexisting cases may not differ from the management of each disorder individually. DVAs are treated conservatively in most cases, while Wilson’s disease is treated with de-coppering and epilepsy with appropriate antiepileptic therapy. Further management, including surgical intervention, will depend upon clinical progress and the appearance of further complications. Screening of asymptomatic family members of a Wilson’s disease patient would prevent unfavourable outcomes.
References
- 1.Ruíz DS, Yilmaz H, Gailloud P. Cerebral developmental venous anomalies: Current concepts. Ann Neurol. 2009;66:271–83. doi: 10.1002/ana.21754. [DOI] [PubMed] [Google Scholar]
- 2.Boukobza M, Enjolras O, Guichard JP, Gelbert F, Herbreteau D, Reizine D, et al. Cerebral developmental venous anomalies associated with head and neck venous malformations. AJNR Am J Neuroradiol. 1996;17:987–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Saba PR. The caput medusae sign. Radiology. 1998;207:599–600. doi: 10.1148/radiology.207.3.9609879. [DOI] [PubMed] [Google Scholar]
- 4.Lee C, Pennington MA, Kenney CM. MR evaluation of developmental venous anomalies: Medullary venous anatomy of venous angiomas. AJNR Am J Neuroradiol. 1996;17:61–70. [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley WG, Daroff RB, Fenichel GM, Jankovic J. Neurology in Clinical Practice. 5th ed. Vol. 1. London: Elsevier; 2007. p. 119. [Google Scholar]
- 6.Parker BJ, Sabb BJ. Developmental venous anomaly complicated by cerebral venous infarction. J Radiol Case Rep. 2007;2:48. doi: 10.2484/rcr.2007.v2i4.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira VM, Geibprasert S, Krings T, Aurboonyawat T, Ozanne A, Toulgoat F, et al. Pathomechanisms of symptomatic developmental venous anomalies. Stroke. 2008;39:3201–15. doi: 10.1161/STROKEAHA.108.521799. [DOI] [PubMed] [Google Scholar]
- 8.Malik GM, Morgan JK, Boulos RS, Ausman JI. Venous angiomas: An underestimated cause of intracranial hemorrhage. Surg Neurol. 1988;30:350–8. doi: 10.1016/0090-3019(88)90197-8. [DOI] [PubMed] [Google Scholar]
- 9.Peebles TR, Vieco PT. Intracranial developmental venous anomalies: Diagnosis using CT angiography. J Comput Assist Tomogr. 1997;21:582–6. doi: 10.1097/00004728-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Garner TB, Del Curling O, Jr, Kelly DL, Jr, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg. 1991;75:715–22. doi: 10.3171/jns.1991.75.5.0715. [DOI] [PubMed] [Google Scholar]
- 11.Taly AB, Meenakshi-Sundaram S, Sinha S, Swamy HS, Arunodaya GR. Wilson disease: Description of 282 patients evaluated over 3 decades. Medicine (Baltimore) 2007;86:112–21. doi: 10.1097/MD.0b013e318045a00e. [DOI] [PubMed] [Google Scholar]
- 12.Dening TR, Berrios GE, Walshe JM. Wilson’s disease and epilepsy. Brain. 1988;111:1139–55. doi: 10.1093/brain/111.5.1139. [DOI] [PubMed] [Google Scholar]
- 13.Smith S. EEG in the diagnosis, classification, and management of patients with epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:ii2–7. doi: 10.1136/jnnp.2005.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2008;4:507–23. doi: 10.2147/ndt.s2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyseng-Williamson KA. Levetiracetam: A review of its use in epilepsy. Drugs. 2011;71:489–514. doi: 10.2165/11204490-000000000-00000. [DOI] [PubMed] [Google Scholar]