Abstract
Background
The objective of this study was to assess the impact of antimicrobial stewardship programs on the multidrug resistance patterns of bacterial isolates. The study comprised an initial retrospective analysis of multidrug resistance in bacterial isolates for one year (July 2007-June 2008) followed by prospective evaluation of the impact of Antimicrobial Stewardship programs on resistance for two years and nine months (July 2008-March 2011).
Setting
A 300-bed tertiary care private hospital in Gurgaon, Haryana (India)
Findings
Methods
Study Design
• July 2007 to June 2008: Resistance patterns of bacterial isolates were studied.
• July 2008: Phase I intervention programme Implementation of an antibiotic policy in the hospital.
• July 2008 to June 2010: Assessment of the impact of the Phase I intervention programme.
• July 2010 to March 2011: Phase II intervention programme: Formation and effective functioning of the antimicrobial stewardship committee. Statistical correlation of the Defined daily dose (DDD) for prescribed drugs with the antimicrobial resistance of Gram negatives.
Results
Phase I intervention programme (July 2008) resulted in a decrease of 4.47% in ESBLs (E.coli and Klebsiella) and a significant decrease of 40.8% in carbapenem-resistant Pseudomonas. Phase II intervention (July 2010) brought a significant reduction (24.7%) in carbapenem-resistant Pseudomonas. However, the resistance in the other Gram negatives (E.coli, Klebsiella, and Acinetobacter) rose and then stabilized. A positive correlation was observed in Pseudomonas and Acinetobacter with carbapenems and cefoperazone-sulbactam.
Piperacillin-tazobactam showed a positive correlation with Acinetobacter only. E.coli and Klebsiella showed positive correlation with cefoparazone-sulbactam and piperacillin-tazobactam.
Conclusion
An antimicrobial stewardship programme with sustained and multifaceted efforts is essential to promote the judicious use of antibiotics.
Keywords: Carbapenem resistance, Gram negatives, Antimicrobial stewardship program, DDD and Antimicrobial resistance
Findings
Introduction
Antimicrobial resistance is not a new phenomenon; however, the current magnitude and the speed with which it is developing is a cause for global concern including in India.
Multidrug resistant organisms (MDROs) are defined as microorganisms that are resistant to one or more classes of antimicrobial agents (e.g., ESBL, MRSA, VRE etc.) [1]. These highly resistant organisms deserve special attention in healthcare facilities as they are associated with increased lengths of stay, costs, and mortality [1]. They can also be transmitted between patients and healthcare workers and lead to the spread of antimicrobial resistance. In most instances, MDRO infections have clinical manifestations that are similar to infections caused by susceptible pathogens. However, options for treating these infections are often extremely limited.
The current need is to develop a robust antimicrobial stewardship programme which would enhance clinical outcomes, reduce non-judicious use of antibiotics and healthcare costs and minimize adverse effects of antimicrobial use (toxicity and resistance). Furthermore, an effective infection control program should be put in place for reducing the transmission of drug-resistant bacteria within the hospital. This should be developed at the local level in hospitals and a national level. A start has been made in India in terms of developing a National Policy for Containment of Antimicrobial Resistance by the Directorate General of Health Services in which a special task force has been created to fulfill the objectives above.
Materials and methods
The present study was conducted in a 300-bed tertiary care private hospital in Gurgaon, Haryana (India). The bacterial culture data of all samples was analyzed for a total period of 45 months (3 years and 9 months) (July 2007 to March 2011) in the Microbiology laboratory of the hospital. Standard culture methods were used (Practical Medical Microbiology 14th ed. by Colle and Fraser) and the isolates, both Gram positive and Gram negative were processed for identification and antibiotic susceptibility tests using VITEK® 2 Compact system (bioMérieux, Marcy l’Etoile, France), following CLSI guidelines [2-4]. The antibiogram of each confirmed isolate was studied and susceptibility results were compiled using the WHONET 5.4 programme.
Our study was divided into four stages:
July 2007 to June 2008: Resistance patterns of Gram-negative isolates- E.coli, Klebsiella, Pseudomonas and Acinetobacter were studied.
July 2008: Phase I intervention programme: Implementation of an antibiotic policy in the hospital. In addition, Infection control practices, such as hand hygiene, a clean environment, appropriate infection barriers and early identification and isolation of patients infected with a transmissible microorganism were also promoted through regular training sessions of the healthcare staff.
July 2008 to June 2010: Assessment of the impact of the Phase I intervention programme.
July 2010 to March 2011: Phase II intervention programme: This included the formation and effective functioning of the antimicrobial stewardship committee.
Defined daily dose (DDD) per 1,000 inpatient-days for each drug (Cefoparazone/Sulbactam, Piperacillin/Tazobactam and Carbapenems{imipenem and meropenem}) prescribed every month was calculated according to the World Health Organization (WHO) anatomical therapeutic chemical (ATC) classification system 2009 [5]. This was statistically correlated with the antimicrobial resistance of E.coli, Klebsiella, Pseudomonas and Acinetobacter expressed as incidence density/1000 inpatients to determine the significance of the analysis.
Results and discussion
Infectious diseases continue to be a leading cause of mortality the world over and more so in developing countries with low access to health services (World Health Report 2007) [6].
The results of the bacterial cultures performed over a period of 45 months (July 2007 to March 2011) in the Microbiology lab of a tertiary care private hospital in Gurgaon, Haryana, India are tabulated (Table 1).
Table 1.
Total Cultures from July 2007 - Mar 2011
| Period | Total Samples | Break-up | Total Positives | Gram Negatives | Gram Positives | |
|---|---|---|---|---|---|---|
|
July 2007 -March 2011 |
28971 |
Urine |
10970 (37.8%) |
2035 |
1785 |
250 |
| |
|
Blood |
9386 |
920 |
676 |
244 |
| |
|
Respiratory |
3865 |
1300 |
1136 |
164 |
| |
|
Pus |
1601 |
854 |
505 |
349 |
| |
|
Stool |
1368 |
357 |
354 |
3 |
| |
|
Fluids |
1781 |
149 |
83 |
66 |
| 28971 | 5615 | 4539 (80.8%) | 1076 (19.1%) | |||
A total of 28 971 samples were cultured. The break-up into the various sample types showed that urine cultures were the predominant sample (10 970 out of 28 971) representing 37.86% of the total number.
A total of 5615 isolates were obtained from 28 971 cultures, giving a 19.38% culture yield. Out of the total isolates, 4539 were Gram-negative showing a clear preponderance of Gram-negative pathogens in the hospital environment (80.8%). 19.9% comprised the Gram-positive load (1076 out of 5615).
Among the Gram-negative isolates, E.coli (43.9%), Klebsiella (19.7%), Pseudomonas (15.1%) and Acinetobacter (9.69%) were the predominant isolates overall. The antibiograms showed the combined extended spectrum β-lactamases (ESBL) prevalence of 55.3% which included both E.coli and Klebsiella with 54.2% and 57.9% respectively. The prevalence of carbapenem resistance in Pseudomonas and Acinetobacter was found to be 27.7% and 85% respectively (Table 2).
Table 2.
Antimicrobial Resistance of Gram-negative Organisms
| Period |
E.coli |
Klebsiella |
E.coli + Kleb |
Acinetobacter |
Pseudomonas |
||||
|---|---|---|---|---|---|---|---|---|---|
| Total Isolates | ESBL (%) | Total Isolates | ESBL (%) | ESBL (%) | Total Isolates | Carbapenem Resistant (%) | Total Isolates | Carbapenem Resistant (%) | |
| July 2007- June 2008 |
278 |
142 |
61 |
40 |
182 (53.6%) |
16 |
2 (12.5%) |
89 |
19 (21.3%) |
| (51%) |
(65.7%) |
||||||||
| July 2008- Dec 2008 |
253 |
154 |
108 |
40 |
185 (51.2%) |
27 |
17 (63%) |
79 |
10 (12.6%) |
| (57.3%) |
(37.0%) |
||||||||
| Jan 2009- Dec 2009 |
601 |
280 |
371 |
168 |
448 (46.0%) |
136 |
121 (88.9%) |
294 |
97 (32.9%) |
| (46.5%) |
(45.2%) |
||||||||
| Jan 2010- Jun 2010 |
320 |
184 |
122 |
93 |
277 (62.6%) |
102 |
89 (87.2%) |
86 |
30 (34.8%) |
| |
|
(57.5%) |
|
(76.2%) |
|
|
|
|
|
| July 2010- Dec 2010 |
378 |
225 |
125 |
96 |
321 (63.8%) |
116 |
104 (89.6%) |
99 |
24 (24.2%) |
| (59.5%) |
|
(76.8%) |
|
|
|
|
|
||
| Jan 2011 - Mar 2011 |
167 |
106 |
111 |
83 |
189 (67.9%) |
43 |
41 (95.3%) |
42 |
11 (26.2%) |
| |
|
(63.4%) |
|
(74.7%) |
|
|
|
|
|
| Total | 1997 | 1091 |
898 | 520 |
1602 (55.3%) | 440 | 374 (85%) | 689 | 191(27.7%) |
| (54.6%) | (57.9%) | ||||||||
In India, various studies have shown the prevalence of ESBL in E.coli and Klebsiella to range from 20% to 60% [7-9]. The carbapenem resistance range reported in Pseudomonas was 26-43% [10-12] and in Acinetobacter, 21-39% [13-15].
Among the Gram positives, Staphylococcus aureus (36.8%), Coagulase negative Staphylococci (19.8%) and Enterococci (38.1%) were the predominant isolates (Additional file 1: Table S1). Of these isolates, the prevalence of Methicillin-resistant Staphylococcus aureus (MRSA) was 36.27%, Methicillin-resistant Coagulase-negative Staphylococci (MRSE) was 67.75% and Vancomycin-resistant Enterococci (VRE) was 6.58% overall (Table 3).
Table 3.
Antimicrobial Resistance of Gram-positive Organisms
| Period |
Staphylococcus aureus |
Coagulase Negative |
Enterococci |
|||
|---|---|---|---|---|---|---|
|
Staphylococci |
|
|
||||
|
Total |
(MRSA)* (%) |
Total |
(MRSE)**(%) |
Total |
(VRE)*** (%) | |
| Isolates | Isolates | Isolates | ||||
| July 2007-June 2008 |
53 |
15 (28.3%) |
22 |
16 (72.72%) |
102 |
4 (3.9%) |
| July 2008- Dec 2008 |
48 |
14 (29.2%) |
21 |
4 (19.04%) |
39 |
6 (15.3%) |
| Jan 2009- Dec 2009 |
135 |
51 (37.7%) |
57 |
49 (85.9%) |
107 |
6 (5.60%) |
| Jan 2010- Jun 2010 |
55 |
19 (34.5%) |
49 |
33 (67.3%) |
52 |
7 (13.4%) |
| July 2010- Dec 2010 |
74 |
26 (35.1%) |
41 |
28 (68.2%) |
89 |
4 (4.49%) |
| Jan 2011 - Mar 2011 |
32 |
19 (59.3%) |
24 |
14 (62.5%) |
21 |
3 (14.28%) |
| Total | 397 | 144 (36.2%) | 214 | 144 (67.2%) | 410 | 30 (7.3%) |
* - Methicillin-resistant Staphylococcus aureus.
** - Methicillin-resistant coagulase-negative Staphylococci.
*** - Vancomycin-resistant Enterococci.
According to our staged intervention plan, the resistance data of Gram-negative isolates (E.coli, Klebsiella, Pseudomonas and Acinetobacter) and Gram-positive isolates (Staphylococcus aureus, Coagulase-negative Staphylococci and Enterococci,) were retrospectively analyzed over a one-year period (July 2007 to June 2008). During this period, the overall ESBL prevalence in E.coli and Klebsiella was 53.6% and the percentage resistance to carbapenems in Pseudomonas and Acinetobacter was found to be 21.3% and 12.5%, respectively (Table 2). Among the Gram-positive isolates, MRSA showed a prevalence of 28.3%, MRSE 72.72% and VRE 3.9%. This data is shown in Table 3.
Phase I intervention programme
The Phase I intervention programme under which the antibiotic policy was introduced and implemented was initiated in July 2008. After assessing the impact of the program, it was observed that the resistance patterns of the isolates for the first 6 months (July 2008- Dec 2008) showed a minor decrease of 4.47% in combined ESBL prevalence in E.coli and Klebsiella and also a significant decrease of 40.8% in carbapenem resistance towards Pseudomonas. However, there was a significant increase in resistance in Acinetobacter. This could be because of a lowering of infection control standards or the entry of multidrug resistant Acinetobacter from other hospitals.
The trend of decreasing resistance continued the following year (2009) for ESBLs (E.coli and Klebsiella) showing a significant decrease of 14.1% but the next six months (Jan 2010-June 2010) showed an increase of 36% bringing the ESBL prevalence up to 62.6%. The carbapenem resistance in Pseudomonas and Acinetobacter showed a substantial increase in the year 2009 followed by a minor decrease of 5.7% and 1.9% respectively over the next six months. This probably indicated an urgent need for further intervention as it was felt that the impact of the Phase I intervention was waning.
Phase II intervention programme
The Phase II intervention programme was initiated in July 2010 as the Antimicrobial Stewardship Programme based on IDSA (Infectious Diseases Society of America) guidelines [16]. This stage comprised the formation and effective functioning of the antimicrobial stewardship committee. The function of this committee was to optimize clinical outcome and minimize unintended consequences of antimicrobial use, namely toxicity and selection of drug-resistant pathogens, and to modify existing antibiotic guidelines as required depending on the antibiograms and discussion with physicians. It also included a prospective audit with intervention, feedback, formulary restriction and preauthorization which were implemented in combination with rigorous infection control policies and protocols to prevent the further spread of multi-resistant pathogens. Similar components of antimicrobial stewardship interventions were recommended by Patel et al. [17]
The impact of stewardship programs on antimicrobial use has been summarized in many study reviews [18,19]. In our study, a significant decrease of 24.7% was only observed in the case of carbapenem-resistant Pseudomonas in the nine-month period from July 2010 to March 2011. The rest of the resistance patterns remained fairly stable, possibly indicating that a more prolonged time period is required to have an impact. It may also indicate the natural evolution of antimicrobial resistance.
The results of Gram-positive organisms, namely MRSA, MRSE and VRE, did not show any significant correlation with the intervention programmes, and considering that they do not form a major part of the total isolates, their in-depth analysis is beyond the purpose of this paper. However, their detailed results are shown in Additional file 1: Table S1.
Correlation of antimicrobial consumption with resistance
ICU is a high antibiotic usage area; therefore, a correlation was simultaneously sought between antibiotic usage [antibiotic prescription (DDD/1000 in-patient days)] and resistance patterns in the phase II post-intervention period (July 2010 to March 2011) (Table 4). A positive correlation was observed with carbapenem (imipenem and meropenem) and cefoperazone-sulbactam usage and the development of resistance in Acinetobacter and Pseudomonas. However, Piperacillin-tazobactam showed a positive correlation with Acinetobacter but a negative correlation with Pseudomonas.
Table 4.
Correlation analysis between Antibiotic prescription and Antimicrobial resistance (July 2010-March 2011) in ICU
| Organism(s) and Drug resistance | r (Correlation Coefficient) | Interpretation of correlation |
|---|---|---|
|
E.coli + Kleb |
|
|
| Imepenem |
−0.29 |
Negative |
| Meropenem |
−0.39 |
Negative |
| CSL |
0.03 |
Positive |
| Pip/Tazo |
0.01 |
Positive |
|
Acinetobacter |
|
|
| Imepenem |
0.17 |
Positive |
| Meropenem |
0.29 |
Positive |
| CSL |
0.48 |
Positive |
| Pip/Tazo |
0.33 |
Positive |
|
Pseudomonas |
|
|
| Imepenem |
0.04 |
Positive |
| Meropenem |
−0.6 |
Negative |
| CSL |
0.62 |
Positive |
| Pip/Tazo | −0.39 | Negative |
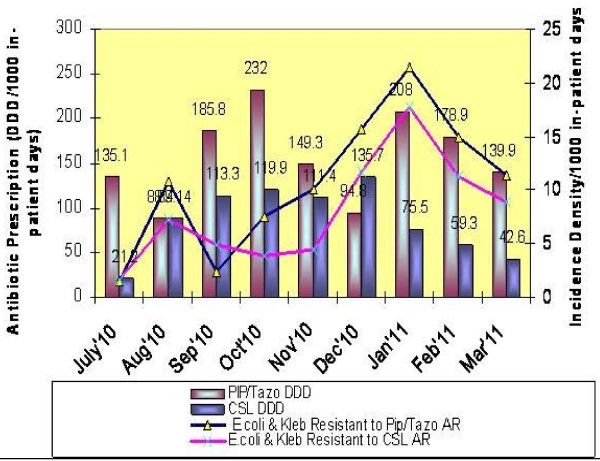
In E.coli and Klebsiella, a positive correlation was observed with the usage of cefoperazone-sulbactam and piperacillin-tazobactam, however a negative correlation was seen with the usage of carbapenems (imipenem and meropenem) and the development of resistance to these antibiotics (see Figures 1 and 2). There are several possible explanations for the lack of significant correlation between antibiotic prescription and resistance in our study. As may be pointed out, resistance selection pressure occurs at the individual level and calculating antibiotic prescription using DDD measurements does not measure individual exposure to antibiotics [20].
Figure 1.
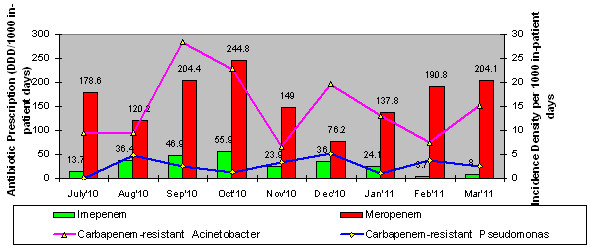
Antimicrobial Resistant A cinetobacter & Pseudonomas isolates and respective Carbapenem prescription volumes form Jul'10 to Mar'11 in ICU.
Figure 2.
Antimicrobial Resistant E, coli & Kleb isolates (%) and respective Piperacillin/Tazobactam & Cefoparazone/Sulbactam Antibiotic prescription volume form Jul'10 to Mar'11 in ICU.
In conclusion, our data demonstrated correlation between antibiotic prescription and Gram-negative bacterial resistance to several, but not all, key antimicrobial agents in the hospital. In areas where Gram-negative bacterial resistance is endemic and prescription of broad-spectrum antimicrobial agents is high, factors other than antimicrobial usage may be equally important in maintaining high resistance rates [21].
The natural march of resistance and the entry of more drug-resistant organisms in the tertiary care centre also play a role in increasing overall resistance percentages.
Our study highlights the increasing resistance in Gram-negative bacteria towards antibiotics in our hospital. As this study was limited to the antibiotic-resistant bacteria isolated from patients in the tertiary care hospital, the true extent of resistance to these agents among bacterial isolates from community-acquired infections is not clear. Also an attempt should be made to risk-stratify the patients into three types; type 1 being patients who have had no prior antibiotic treatment or contact with the healthcare system, young patients with few co-morbid conditions, Type 2 as recent admission with short antibiotic therapy and older patients and Type 3 as long hospitalization, multiple antibiotic therapies and immunocompromised state.
Antimicrobial stewardship programs are clearly required in hospitals to promote and emphasize the rational use of antimicrobials. Although resistance is a worldwide concern, it is first and foremost a local problem: selection and amplification of resistant members of a species are occurring in individual hospitals (and communities), which can then spread worldwide [22]. An effective infection control program can make a significant contribution to limiting the spread of resistance. However, in the present study, the gaps in the infection control program due to the higher turnover rate of the nursing staff, regular admission of patients infected with resistant strains into the hospital and the high endemicity of drug-resistant pathogens in the region could explain the limited impact of the antimicrobial stewardship actions undertaken in this hospital.
Competing interests
No potential conflict of interest in terms of financial and other relationships exists for any of the three authors (Namita Jaggi, Pushpa Sissodia and Lalit Sharma).
Authors’ contributions
NJ conceived the study, and participated in its design and coordination and helped to draft the manuscript. PS participated in the design of the study, data collection and provided writing assistance and statistical analysis. LS provided technical assistance for the specimen processing, identification and susceptibility testing of bacterial isolates. All authors read and approved the final manuscript.
Supplementary Material
Table S1. Gram Positive Culture data of various samples from July 2007 to March 2011.
Contributor Information
Namita Jaggi, Email: namitajaggi24@gmail.com.
Pushpa Sissodia, Email: pushpasissodia@gmail.com.
Lalit Sharma, Email: lalit.srl01@rediffmail.com.
Acknowledgement
We owe great thanks to Artemis Health Institute for extending their kind support as a funding source and encouraging us in writing this paper.
References
- Siegal DJ, Rhinehart E, Jackson M, Chiarello. Multidrug-Resistant organisms in Healthcare Settings. Healthcare Infection Control Practices Advisory Committee (HICPAC); 2006. pp. 1–74. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. Wayne: Clinical and Laboratory Standards Institute; 2007. (Document M100-S17, Volume 27,no. 1). [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Nineteenth Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. (Document M100-S19, Volume 29, no. 3). [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-first Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. (Document M100-S21, Volume 31, no. 1). [Google Scholar]
- World Health Organization. WHO Collaborating Centre for Drug Statistics Methodology. 2009. (ATC/DDD index). http://www.whocc.no/atcddd/
- World Health Report. A safer future. Geneva: World Health Organization; 2007. [ http://www.who.int/whr/2003/en/] [Google Scholar]
- Rajini E, Sherwal BL. Anuradha Original Research. Detection of Extended-Spectrum β-lactamases in AmpC β-lactamase-Producing Nosocomial Gram-negative Clinical Isolates from a Tertiary Care Hospital in Delhi. Ind Medica. 2007;4(6):2008–01–2008–02. [Google Scholar]
- Kumar MS, Lakshmi V, Rajagopalan R. Occurrence of Extended spectrum beta lactamases among Enterobacteriaceae spp isolated at a tertiary care institute. Indian J Med Microbiol. 2006;24(3):208–211. [PubMed] [Google Scholar]
- Menon T, Bindu D, Kumar CPG, Nalini S, Thirunarayan MA. Comparision of double disc and three dimensional methods to screen for ESBL producers in a tertiary care hospital. Indian J Med Microbiol. 2006;24(2):117–120. doi: 10.4103/0255-0857.25192. [DOI] [PubMed] [Google Scholar]
- Gladstone P, Rajendran P, Brahmadathan KN. Incidence of carbapenem resistant non-fermenting Gram-negative bacilli from patients with respiratory infections in the intensive care unit. Indian J Med Microbiol. 2005;23:189–191. doi: 10.4103/0255-0857.16593. [DOI] [PubMed] [Google Scholar]
- Taneja N, Aharwal SM, Sharma M. Imipenem resistance in nonfermenters causing nosocomial urinary tract infections. Indian J Med Sci. 2003;57:294–299. [PubMed] [Google Scholar]
- Varaiya A, Kulkarni M, Bhaleker P, Dogra J. Incidence of carbapenem- resistant Pseudomonas aeruginosa in diabetes and cancer patients. Indian J Med Microbiol. 2008;26(3):238–240. doi: 10.4103/0255-0857.42033. [DOI] [PubMed] [Google Scholar]
- Corbella X, Montero A, Pujol M, Dominguez MA, Ayats J, Argerish MJ, Garrigosa F, Ariza J, Gudiol F. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of Multidrug resistant Acinetobacter baumannii. J Clin Microbiol. 2008;38(11):4086–4095. doi: 10.1128/jcm.38.11.4086-4095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Basu S, Dasgupta S, Singh AK, Viswanathan R. Carbapenem resistance in Acinetobacter baumannii isolated from blood of neonates with sepsis. Indian J Med Microbiol. 2010;28:416–417. doi: 10.4103/0255-0857.71814. [DOI] [PubMed] [Google Scholar]
- Wu Tak-Chiu. Carbapenem-resistant or Multidrug-resistant Acinetobacter baumannii- a Clinician’s Perspective. Medical Bulletin. 2011;16(4):6–9. [Google Scholar]
- Dellit. et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- Patel D, Lawson W, Guglielmo BJ. Antimicrobial stewardship programs: interventions and associated outcomes. Expert Rev Anti Infect Ther. 2008;6(2):209–222. doi: 10.1586/14787210.6.2.209. [DOI] [PubMed] [Google Scholar]
- MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman N. Antimicrobial stewardship. Am J Med. 2006;119(6A):S53–S61. doi: 10.1016/j.amjmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Turnidge J, Christiansen K. Antibiotic use and resistance— proving the obvious. Lancet. 2005;365:548–549. doi: 10.1016/S0140-6736(05)17920-3. [DOI] [PubMed] [Google Scholar]
- Hsu Li-Yang, Tan T-Y, Tam VH, Kwa A, Fisher Dale Andrew, Koh Tse-Hsien. Surveillance and Correlation of Antibiotic Prescription and Resistance of Gram-Negative Bacteria in Singaporean Hospitals. Antimicrob Agents Chemother. 2010;54(3):1173–1178. doi: 10.1128/AAC.01076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien TF. Emergence spread, and environmental effect of antimicrobial resistance: how use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clin Infect Dis. 2002;34(3):S78–S84. doi: 10.1086/340244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Gram Positive Culture data of various samples from July 2007 to March 2011.