Abstract
Introduction:
Aversive smoking has been investigated as a smoking cessation technique that involves rapid smoking in a clinic or laboratory and typically involves (a) puffing every 6–10 s or (b) smoking 3 or more cigarettes sequentially in 8–20 min. Rapid smoking usually results in dizziness, sore throat, nausea, and other unpleasant feelings.
Methods:
To explore rapid smoking, 161 smokers (75 with schizophrenia [SS]; 86 controls [CON]) were assessed in a single day (24 ± 2 hr), ad libitum smoking topography session using the Clinical Research Support System micro portable topography device.
Results:
SS smoked significantly more cigarettes in the 24-hr period versus CON and the time between puffs, or interpuff interval (IPI) was shorter in SS versus CON by an average of 6.5 s (p < .001). The median IPI was also significantly shorter in SS versus CON (9.3 vs.15.7 s; p < .001). SS were twice as likely to have IPIs ≤6 s than CON (OR = 2.32, 95% CI = 1.68, 3.20; p < .001). SS were also more likely to smoke 3 or more cigarettes in any 20 min during a 24-hr topography session (OR = 2.32, 95% CI = 1.03, 2.44; p < .001). Rapid smoking was associated with baseline characteristics of smoking more cigarettes per day, higher Fagerström score, and higher carbon monoxide level but not with serum cotinine values or trans-3′-hydroxycotinine/cotinine ratios.
Conclusions:
Using either definition, SS exhibit patterns of rapid smoking that they seemingly do not experience as aversive, since it reflects their naturalistic pattern of smoking, outside of the laboratory.
Introduction
Rapid smoking is an aversive counter-conditioning technique that has been investigated as a potential strategy to help smokers quit. It consists of smoking rapidly to produce an unpleasant syndrome that becomes a conditioned response to smoking and might help the individual stop smoking. Symptoms of rapid smoking can include transient nausea, dizziness, lightheadedness, clammy skin, burning throat, tingling sensation, headache, and heart racing (Lichtenstein, Harris, Birchler, Wahl, & Schmahl, 1973; Juliano, Houtsmuller, & Stitzer, 2006). Although unpleasant, rapid smoking seems to pose few serious safety problems (Hall, Sachs, Hall, & Benowitz, 1984; Russell, Raw, Taylor, Feyerabend, & Saloojee, 1978; Danaher 1977). Although initial studies suggested its usefulness for smoking cessation, most evidence is inconclusive due to methodological problems, and its practice has been mostly abandoned. (For more thorough discussion, see review by Hajek & Stead, 2004.) More recent studies of rapid smoking indicate that it is associated with short-term reductions in craving (Houtsmuller & Stitzer, 1999; Juliano et al., 2006) and longer latency, or time, to subsequent smoking (Dallery, Houtsmuller Pickworth, & Stitzer, 2003).
Rapid smoking protocols have typically included timed cigarette puffing that occurs every 6–10 s for 3 min or until the smoker is unable to continue (Lichtenstein et al., 1973). Paced smoking, in contrast, is a similar procedure, where the time between puffs (or interpuff interval [IPI]) is increased to 30 s. Paced smoking does not elicit aversive sensations and in some studies has been used as an inactive control (Hall et al., 1984). An alternate protocol for rapid smoking is smoking three or more cigarettes in brief time intervals, ranging from 8 to 20 min (Dallery et al., 2003; Hall et al., 1984; Juliano et al., 2006). As an aversive technique, smokers would not be expected to practice rapid smoking in their own environment. Studies of smoking topography in smokers with schizophrenia (SS) have found differences compared with community smokers who do not have mental illness. SS demonstrate more intense smoking characterized by more frequent puffs per cigarette and shorter time IPI (Williams et al., 2011; Tidey, Rohsenow, Kaplan, & Swift, 2005). Shorter IPI is associated with higher nicotine intake (Williams et al., 2011). Given the short time between puffs seen in other studies, we were interested to see if SS naturalistically practice rapid smoking using topography data gathered from a study of smoking behavior measured outside of the laboratory.
Methods
This is a secondary data analysis from a study of nicotine intake and smoking topography on 161 participants (SS, n = 75 and control smokers [CON] without mental illness, n = 86; Williams et al., 2011). Procedures for subject recruiting and blood sampling have been described in the original report (Williams et al., 2011). Briefly, all subjects participated in a 24 ± 2-hr topography session that included smoking at home with a portable topography device. The data collection period started at approximately 3 p.m. and continued upon awakening the next day. The study also included blood draws for measurement of nicotine and cotinine. All subjects with schizophrenia were enrolled in mental health treatment, stable on antipsychotic medications and had their diagnosis confirmed with the Structured Clinical Interview for DSM-IV (Spitzer & Williams, 1985). CON had to be without any mental illness within the last year and could not be taking an antidepressant, mood stabilizer, or anxiolytic for any reason. The study was approved by the University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School Institutional Review Board.
Subjects completed an assessment battery including a smoking history, demographic and medication questionnaire, the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), and assessments of symptom severity (Positive and Negative Syndrome Scale; Kay, Opler & Lindenmayer, 1989, SS only). Subjects had a baseline expired carbon monoxide (CO) reading using an EC-50 Smokerlyzer (Bedfont Scientific, NJ). Subjects completed brief questionnaires assessing their urges to smoke (Questionnaire of Smoking Urges Brief Form [QSU]; Cox, Tiffany, & Christen, 2001), and mood states (Positive and Negative Affect Schedule, PANAS; Watson, Clark, & Tellegen, 1988) measured 1 hr after a morning cigarette. Subjects were required to bring their own cigarettes for all study procedures.
Participants were trained on the proper use of the Clinical Research Support System (CReSS) Micro Smoking Topography device (Plowshare/Borgwaldt-KC, Richmond, VA), a battery-operated portable device that measures a full complement of smoking behaviors. Following study procedures, the data are transferred from the handheld device to a desktop computer program. Nicotine and its metabolites, cotinine and trans-3′-hydroxycotinine (3HC), were quantified in serum by liquid chromatography–tandem mass spectrometry at the Clinical Pharmacology Laboratory at the University of California, San Francisco (Jacob et al., 2011).
Statistical Analysis
Two sample t tests and chi-square tests were used to compare the baseline differences in sociodemographic variables, symptom scores, and smoking characteristics between groups. We used a data cleaning procedure (Plowshare Technologies) to identify and delete erroneous puffs/cigarettes, which are described in greater detail in our prior work (Williams et al., 2011). Mean values of repeatedly measured topography variables including IPI and puff count were estimated and compared using a random effects nested linear model analysis (Littell, Milliken, Stroup, Wolfinger, & Schabenberger, 2006) in which the random effects component was used to model the different variation in the SS and CON subjects and in the double nested structure of data (puffs within cigarettes and cigarettes within subjects). Median IPI values were compared with the Wilcoxon two-sample test. Analyses were performed using SAS Proc Mixed procedure. p Values less than .05 were considered to be statistically significant. Bonferroni corrections were applied to adjust for Type I error rates resulting from multiple comparisons, as appropriate. Generalized estimating equations, using SAS Proc Genmod, were used to examine which group (SS vs. CON) was more likely to have IPIs ≤6 and ≤10 s. We used random effects logistic regression analyses to compare differences between rapid smoking and non-rapid smoking groups. All statistical analyses were performed using SAS v. 9.1.
Results
Characteristics of the Sample
Baseline clinical and sociodemographic data for the sample are described in Table 1. SS were older and more likely to be men compared with CON (both p < .01). Although both groups smoked the same average number of cigarettes per day, SS had higher baseline expired CO (23.1 vs. 19.5; p < .05). On other characteristics including FTND total score, age of first smoking, number of past quit attempts, race/ethnicity, and education, there were no differences between groups. Serum nicotine and cotinine levels were significantly higher in SS compared with CON (31.3 vs. 24.4 ng/ml and 450.9 vs. 303.9 ng/ml, respectively; both p < .001). Mean 3HC/cotinine ratios were not different between groups (mean 0.54 vs. 0.49; p = .49).
Table 1.
Characteristics of the Sample
| Smokers with schizophrenia (n = 75) | Control smokers (n = 86) | |
| M (SD) | M (SD) | |
| Baseline carbon monoxide (ppm)* | 23.1 (12.2) | 19.5 (7.6) |
| Cigarettes per day | 22.3 (11.5) | 20.0 (7.7) |
| Total Fagerström Test for Nicotine Dependence score | 5.9 (2.0) | 5.5 (1.9) |
| Age*** | 45.7 (10.5) | 38.0 (12.0) |
| Age of first smoking | 14.7 (5.2) | 14.7 (3.7) |
| Past quit attempts | 3.3 (4.1) | 4.2 (16.8) |
| Serum nicotine (ng/ml)*** | 31.3 (12.1) | 24.4 (10.6) |
| Serum cotinine (ng/ml)*** | 450.9 (199.1) | 303.9 (128.1) |
| Trans-3′-hydroxycotinine/cotinine ratio | 0.54 (0.38) | 0.49 (0.31) |
| Count (%) | Count (%) | |
| Male gender** | 55 (73.3) | 44 (51.2) |
| Smoking mentholated cigarette brand | 51 (68.0) | 61 (70.9) |
| Race/ethnicity | ||
| Black | 35 (46.7) | 25 (29.1) |
| Caucasian | 33 (44.0) | 45 (52.3) |
| Hispanic | 4 (5.3) | 13 (13.1) |
| Other | 3 (4.0) | 3 (3.5) |
Note. Independent sample t test, Mann–Whitney or chi-square test.
*p < .05. **p < .01. ***p < .001.
Rapid Smoking
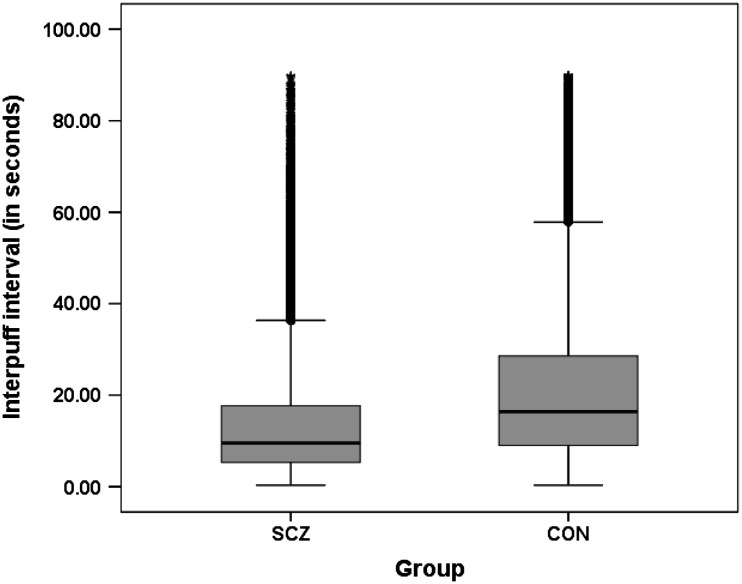
As reported in Williams et al. (2011), SS differed significantly from CON on measures of smoking topography. During the assessment period, data from 38,691 individual puffs (2,966 total cigarettes) were collected. SS smoked an average of 2.8 more puffs per cigarette than CON (both p < .001) in addition to smoking more cigarettes in the 24-hr testing session (mean 21.0, SS vs. 16.0, CON). The mean time between puffs or IPI was significantly shorter in SS (16.0 vs. 22.6 s; p < .001). Due to the skewed distribution of the data, we also examined the median IPI and found that it was shorter in SS than CON (9.3 vs.15.7 s; p < .001; Figure 1). Average total time to finish smoking a cigarette was shorter in SS compared with CON (4.5 vs. 5.5 min; p < .001).
Figure 1.
Distribution of interpuff interval scores.
We examined the frequency of IPIs ≤6 s. SS were twice as likely to have IPIs ≤6 s than CON (OR = 2.32, 95% CI = 1.68, 3.20; p < .001). SS were also more likely to have IPIs ≤10 when compared with CON (OR = 2.69, 95% CI = 2.24, 3.22; p < .001). Using the alternative definition of rapid smoking (three or more cigarettes in a short period of time), we examined the frequency of smoking three or more cigarettes in any 20-min interval of the 24-hr smoking topography period. SS were twice as likely as CON to smoke three or more cigarettes in 20 min (OR = 2.32, 95%CI = 1.03, 2.44; p < .001). There were a total of six smokers in the dataset who smoked four or more cigarettes in any 20-min interval (5 SS and 1 CON) and one of these (SS) smoked seven cigarettes in a 20-min interval.
We then looked at individuals who exhibited rapid smoking behavior (N = 21/75 SS and 6/86 CON) using the definition of three or more cigarettes smoked in 20 min, to see if this group was associated with other demographic or smoking characteristics. Rapid smoking was associated with higher baseline cigarettes smoked per day (26.0 vs. 20.0, t = −2.84, df = 159, p < .01), total FTND (6.4 vs. 5.5, t = −2.35, df = 159, p < .05), and summary score and baseline CO (25.6 vs. 20.3, t = −2.46, df = 158, p < .05). There was no association between rapid smoking and serum cotinine values or 3HC/cotinine ratios.
Craving and Affect Scores
Items from the QSU were analyzed as two factors: “intention to smoke” (Factor 1) and “anticipation of relief from withdrawal” (Factor 2; Tiffany & Drobes, 1991). Rapid smokers from both groups (SS and CON) were collapsed into one group (N = 27) and adjusted for diagnosis group, since we previously found significant differences in SS compared with CON (Williams et al., 2011). Rapid smokers had higher subscale scores on Factor 2 (45.8 vs. 27.0, p < .01) and QSU general factor (i.e., average of both factors; 56.9 vs. 41.5; p < .05) but no differences for Factor 1 scores. Rapid smokers also differed significantly in PANAS scores; with significantly higher scores of negative affect (PANAS negative scores; 9.6 vs. 5.3, p < .05).
Discussion
Using either definition of rapid smoking, SS exhibit these behaviors. Although we did not directly measure aversive effects in this study, it is likely that SS do not experience this smoking as aversive, since it reflects their naturalistic pattern of smoking, outside of the laboratory. Since only twenty-seven smokers in the sample exhibited rapid smoking, we did not have the power to detect other differences in demographic or clinical characteristics, but this an interesting area for further study. Similarly it would be important to measure the presence or lack of aversive effects directly after rapid smoking in these subgroups of smokers. Lack of an aversive effect to nicotine is not likely due to rapid nicotine metabolism, since we have demonstrated no evidence of this in two prior studies of smokers comparing the 3HC/cotinine ratio (a noninvasive marker of the rate of nicotine metabolism; Dempsey et al., 2004) in SS versus controls and observed no association between rapid smoking and 3HC/cotinine ratios in the present study. Tolerance to high dose nicotine is also consistent with our other studies of SS that have documented higher nicotine levels than smokers without mental illness who smoked the same number of cigarettes per day (Williams et al., 2005; Williams et al., 2010). Our studies have also found higher levels of nicotine boost in SS (28 ng/ml) as well as a higher total dose of nicotine from smoking a single cigarette than CON (Williams et al., 2010).
Since individuals with schizophrenia experience more than twenty years of reduced life expectancy largely due to the effects of smoking (Kelly et al., 2011; Miller, Paschall, & Svendsen, 2006), it is a priority to improve on methods to help these patients quit. A barrier to achieving this goal is that it is not yet clear why people with schizophrenia smoke differently (e.g., rapid puffing and higher nicotine intake). One hypothesis is that SS smoke more due to alterations in brain dopaminergic systems that increase the sensitivity to positive reinforcement from addicting substances (Chambers, Krystal, & Self, 2001). This may contribute to their excessive use of not only nicotine but also caffeine and other drugs (Gandhi, Williams, Menza, Galazyn, & Benowitz, 2010). Animal models of schizophrenia demonstrate addiction vulnerability (Chambers & Taylor 2004), and it has been argued that substance use is a core symptom of schizophrenia (Chambers et al., 2001). Higher nicotine levels from rapid puffing and shorter time between puffs may increase addictive potential and reinforcement value, possibly explaining why SS have reduced success in smoking cessation.
SS report higher levels of negative affect (NA), less positive affect, a greater anticipation that smoking will relieve NA (QSU Factor 2; Williams et al., 2011). QSU Factor 2 (anticipation that smoking will relieve negative affect) and PANAS negative scores in this study were associated with rapid smoking. This means that smoking more intensely (i.e., more frequent puffing and reduced IPI) may be in response to having less ability to tolerate NA whether from withdrawal or other reasons. In combination, our findings of higher nicotine intake and rapid puffing in SS may reflect an “urgency” to smoke in these individuals that is still poorly understood.
Lack of aversive effects is interesting as it relates to a newly described acetylcholine receptor alpha5 subunit single nucleotide polymorphism (SNP). This SNP is associated with smoking quantity and severity (Berrettini et al., 2008) including a lack of aversive effects to high dose nicotine (Fowler, Lu, Johnson, Marks, & Kenny, 2011). Animal models similarly show that deletion of alpha5 subunits enhances nicotine self-administration, supporting their role in nicotine intake mechanisms (Fowler, Arends, & Kenny, 2008). Alpha5 receptors are heavily concentrated in the medial habenula, which is being investigated as a major regulatory center for nicotine consumption, reward, and aversion (Frahm et al., 2011), in addition to its potential role in psychosis and psychiatric illness. Schizophrenia can be conceptualized as the most severe example of the disease of nicotine dependence by virtue of the repetitive smoking behavior, high nicotine intake, and reduced cessation, despite evidence of disease and early death (Tidey, 2011). Clinical similarities can be observed between nicotine intake behavior from studies of alpha5 nicotinic systems and observational studies of schizophrenia. These warrant further studies to explore possible mechanisms of high nicotine intake in SS.
Funding
This work was supported by a grant from the National Institute of Mental Health (MH076672-01A1 to JMW) and from the National Institute on Drug Abuse (DA12393, NLB).
Declaration of Interests
JMW receives research support from Pfizer and has been an Advisory Board Member for Pfizer. N LB has been a paid consultant to pharmaceutical companies that market or are developing smoking cessation medications, including Pfizer, GlaxoSmithKline, Novartis, Sanofi-Aventis, Accrux and Aradigm. MLS receives research support from Pfizer. The other authors have no significant relationships to disclose.
References
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Molecular Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. doi:10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. doi:10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: Sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biological Psychiatry. 2004;56:308–316. doi: 10.1016/j.biopsych.2004.05.019. doi:10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. doi:10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dallery J, Houtsmuller EJ, Pickworth WB, Stitzer ML. Effects of cigarette nicotine content and smoking pace on subsequent craving and smoking. Psychopharmacology (Berlin) 2003;165:172–180. doi: 10.1007/s00213-002-1242-8. doi:10.1007/s00213-002-1242-8. [DOI] [PubMed] [Google Scholar]
- Danaher BG. Research on rapid smoking: Interim summary and recommendations. Addictive Behaviors. 1977;2:151–166. doi: 10.1016/0306-4603(77)90013-2. doi:10.1016/0306-4603(77)90013-2. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, III, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical Pharmacology and Therapeutics. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. doi:10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behavioural Pharmacology. 2008;19:461–484. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;47:597–601. doi: 10.1038/nature09797. doi:10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, et al. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. doi:10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Gandhi KK, Williams JM, Menza M, Galazyn M, Benowitz NL. Higher serum caffeine in smokers with schizophrenia compared to smoking controls. Drug and Alcohol Dependence. 2010;110:1–2. doi: 10.1016/j.drugalcdep.2010.01.021. doi:10.1016/j.drugalcdep.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Stead LF. Aversive smoking for smoking cessation. Cochrane Database of Systematic Reviews. 2004;(4) doi: 10.1002/14651858.CD000546.pub2. CD000546. doi:10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RG, Sachs DP, Hall SM, Benowitz NL. Two-year efficacy and safety of rapid smoking therapy in patients with cardiac and pulmonary disease. Journal of Consulting and Clinical Psychology. 1984;52:574–581. doi: 10.1037//0022-006x.52.4.574. doi:10.1037/0022-006X.52.4.574. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K.-O. The Fagerström test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Stitzer ML. Manipulation of cigarette craving through rapid smoking: Efficacy and effects on smoking behavior. Psychopharmacology (Berlin) 1999;142:149–157. doi: 10.1007/s002130050874. doi:10.1007/s002130050874. [DOI] [PubMed] [Google Scholar]
- Jacob P, III, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3’-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. doi:10.1016/j.chromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Houtsmuller EJ, Stitzer ML. A preliminary investigation of rapid smoking as a lapse-responsive treatment for tobacco dependence. Experimental and Clinical Psychopharmacology. 2006;14:429–438. doi: 10.1037/1064-1297.14.4.429. doi:10.1037/1064-1297.14.4.429. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): Rationale and standardisation. British Journal of Psychiatry Supplement. 1989;7:59–67. [PubMed] [Google Scholar]
- Kelly DL, McMahon RP, Wehring HJ, Liu F, Mackowick KM, Boggs DL, et al. Cigarette smoking and mortality risk in people with schizophrenia. Schizophrenia Bulletin. 2011;37:832–838. doi: 10.1093/schbul/sbp152. doi:10.1093/schbul/sbp152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein E, Harris DE, Birchler GR, Wahl JM, Schmahl DP. Comparison of rapid smoking, warm, smoky air, and attention placebo in the modification of smoking behavior. Journal of Consulting and Clinical Psychology. 1973;40:92–98. doi: 10.1037/h0034039. doi:10.1037/h0034039. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd edn. Cary NC: SAS Institute Inc; 2006. [Google Scholar]
- Miller BJ, Paschall CB, III, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatric Services. 2006;57:1482–1487. doi: 10.1176/ps.2006.57.10.1482. doi:10.1176/appi.ps.57.10.1482. [DOI] [PubMed] [Google Scholar]
- Russell MAH, Raw M, Taylor C, Feyerabend C, Saloojee Y. Blood nicotine and carboxyhemoglobin levels after rapid-smoking aversion therapy. Journal of Consulting and Clinical Psychology. 1978;46:1423–1431. doi: 10.1037//0022-006x.46.6.1423. doi:10.1037/0022-006X.46.6.1423. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB. Structured clinical interview for DSM-III-R. New York: Biometrics Research Department, New York State Psychiatric Institute; 1985. [Google Scholar]
- Tidey J. Smoking is associated with an increased risk of death in people aged 35–54 with schizophrenia. Evidence Based Mental Health. 2011;14:99. doi: 10.1136/ebmh.2011.100179. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug and Alcohol Dependence. 2005;80:259–265. doi: 10.1016/j.drugalcdep.2005.04.002. doi:10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. doi:10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. doi:10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Lu SE, Kumar S, Shen J, Foulds J, et al. Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine & Tobacco Research. 2010;12:855–859. doi: 10.1093/ntr/ntq102. doi:10.1093/ntr/ntq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Lu SE, Kumar S, Steinberg ML, Cottler B, et al. Shorter interpuff interval associated with higher nicotine intake in smokers with schizophrenia. Drug and Alcohol Dependence. 2011;118:313–319. doi: 10.1016/j.drugalcdep.2011.04.009. doi:10.1016/j.drugalcdep.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophrenia Research. 2005;79:323–335. doi: 10.1016/j.schres.2005.04.016. doi:10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]