Abstract
Introduction:
Electronic cigarettes (ECs) are marketed as nicotine delivery devices. Two studies with EC-naïve participants suggest that ECs deliver little or no nicotine. In those studies, standard-sized ECs were used, though experienced EC users often use larger devices that house higher voltage and/or longer lasting batteries. Whether user experience and device characteristics influence EC nicotine delivery is uncertain. The purpose of the present study was to examine the effects of ECs in experienced users who were using their preferred devices.
Methods:
Eight EC users (3 women) who had been using ECs for at least 3 months, completed one 5-hr session using devices they provided and the flavor/strength nicotine cartridges they selected. Sessions consisted of 4 phases: baseline, 10 puffs (30-s interpuff interval) from the device, 1-hr ad lib puffing period, and a 2-hr rest period (no puffing). Outcome measures in each phase included plasma nicotine concentration, heart rate, and subjective ratings of nicotine/product effects and abstinence symptoms.
Results:
Relative to baseline, plasma nicotine and heart rate increased significantly within 5 min of the first puff and remained elevated throughout the ad lib puffing period. Increases in ratings of direct effects of nicotine and product were observed as well as decreases in abstinence symptoms.
Conclusions:
User experience and/or device characteristics likely influence EC nicotine delivery and other effects. Systematic manipulation of these and other variables could elucidate conditions that produce intended effects.
Introduction
Electronic cigarettes (ECs) that consist of a battery, heater, and nicotine-containing liquid are marketed as devices that deliver nicotine to the user. ECs have become increasingly popular, and some reports suggest that they help users quit or reduce cigarette smoking (Ayers, Ribisi, & Brownstein, 2011; Etter, 2010; Etter and Bullen, 2011a; Heavner, Dunworth, Bergen, Nissen, & Phillips, 2009; McQueen, Tower, & Sumner, 2011; Polosa et al., 2011). Results of two clinical laboratory studies, conducted with EC-naïve cigarette smokers, suggest that ECs either deliver nicotine inefficiently or not at all (Bullen, McRobie, Thornley, Glover, & Laugesen, 2010; Vansickel, Cobb, Weaver, & Eissenberg, 2010). Those studies used standard-sized cigarette-like ECs, while experienced EC users (“vapers”) often use models that are larger than a cigarette and house higher voltage or longer life batteries (Foulds, Veldheer, & Berg, 2011). Furthermore, EC and tobacco cigarette puffing characteristics may differ, suggesting that inexperienced users may not use ECs in a manner that allows for sufficient nicotine delivery (Trtchounian et al., 2011). Whether user experience and/or device characteristics influence nicotine delivery is unknown. The purpose of the present study was to characterize the nicotine delivery profile, subjective, and cardiovascular effects of ECs in experienced ECs users who were using their preferred devices.
Methods
Eight EC users (3 women; 8 Caucasian) consented to and completed this single-session (5 hr) Institutional Review Board-approved protocol. Participants used their own fully charged EC, and a prefilled cartomizer (heater + liquid) of their chosen flavor/nicotine content was provided. At the start of the session, participants were surveyed regarding health, demographics, and EC use habits. Participants were included in the study if they reported using an EC for at least 3 months, used at least 2–3 ml of nicotine solution or two cartridges per day, used nicotine solution of at least 10 mg/ml nicotine, smoked fewer than five cigarettes per day, and were between the ages of 18 and 55. Participants were excluded if they reported any chronic health or psychiatric conditions, had a history of high or low blood pressure, or were unwilling to use a cartridge or cartomizer during the session.
Participants were required to abstain from all nicotine and tobacco product use, including ECs, for at least 12 hr prior to the session. Abstinence was verified upon arrival at the laboratory by a carbon monoxide level of 10 ppm or less and was further verified later by plasma nicotine levels at or below the limit of quantification (2 ng/ml). At session onset, a venous catheter was inserted into the participant’s forearm vein and continuous physiological monitoring commenced. Approximately 55 min later, blood was sampled, and 5 min after that, participants were provided with their EC and were instructed to take 10 puffs (30-s interpuff interval [IPI; as in Eissenberg, 2010; Vansickel et al., 2010]). Blood was sampled 5 and 15 min after the start of the 10-puff period. Then, a 60-min ad lib puffing period began, and blood was sampled every 15 min. Participants then entered a 2-hr rest period during which no puffing occurred, and blood was sampled every 30 min. Participants completed subjective questionnaires at baseline and again after the 10-puff, ad lib, and rest periods. At session’s end, the venous catheter was removed, physiological monitoring ceased, and participants were compensated.
Physiological outcome measures included heart rate (measured every 20 s) and plasma nicotine concentration (blood centrifuged and plasma stored at −70 °C for analysis of nicotine concentration). Subjective and behavioral outcome measures included the Tiffany–Drobes Questionnaire of Smoking Urges Brief (QSU Brief; Cox, Tiffany, & Christen, 2001), 11 visual analogue scale (VAS) items that assessed nicotine/EC abstinence symptoms (Kleykamp et al., 2008), 10 VAS items that assessed the direct effects of nicotine (Blank, Sams, Weaver, & Eissenberg, 2008), 14 VAS items that assessed the direct effects of the EC (Vansickel et al., 2010) as well as the total number of puffs taken during the ad lib period.
Results
Participants were former smokers who had tried to quit smoking an average (SD) of 3.9 (1.8) times in their lifetime, had quit smoking for 11.4 (5.4) months, and had been using ECs for 11.5 (5.4) months. Their average age was 33.4 (8.6) years, and they had completed 15.3 (2) years of education. Seven participants used devices that did not resemble tobacco cigarettes and housed higher voltage and/or longer lasting batteries than in previous work (e.g., Vansickel et al., 2010). Six participants used 18 mg/ml nicotine solution, one used 9, and the other used 24 mg/ml solution.
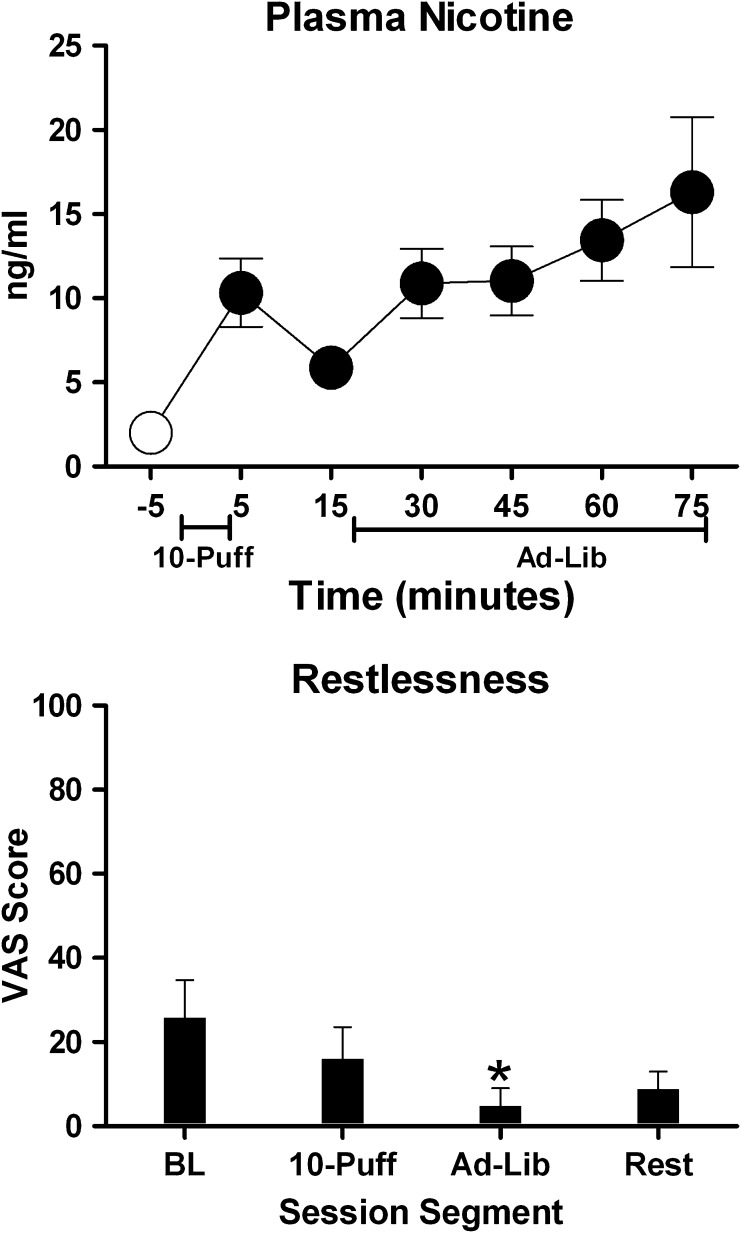
Plasma nicotine increased significantly from a baseline mean of 2 ng/ml (SEM = 0) to a mean of 10.3 ng/ml (SEM = 2) within 5 min of the first puff. Plasma nicotine reached a maximum mean concentration (M = 16.3 ng/ml, SEM = 4.5) by the end of the ad lib period (see Figure 1; relative to baseline all P < .05 by paired t test). Heart rate increased significantly from a baseline mean of 73.2 (SEM = 2.0) beats per minute (bpm) to 78 (SEM = 1.9) bpm within 5 min of the first puff and remained elevated during the ad lib period. The number of puffs during the 1-hr ad lib period ranged from 4 to 76 (M = 46.6, N = 7).
Figure 1.
Top panel: M (±1 SEM) plasma nicotine (assay’s limit of quantitation = 2 ng/ml; Breland, Kleykamp, & Eissenberg, 2006) levels at baseline (−5) and during the 10-puff and 1-hr ad lib puffing periods. Filled symbols indicate a significant difference from baseline. Bottom panel: M (+1 SEM) response to a visual analogue scale item assessing “restlessness” (0–100 scale) at baseline and the end of the 10-puff, ad lib, and rest periods. An asterisk indicates a significant difference from baseline. Data are from eight electronic cigarettes (EC) using participants who abstained from ECs for at least 12 hr before session. Paired t tests were used to compare means, p ≤ .05.
VAS ratings of “Anxious” were elevated at baseline (M = 13.9, SEM = 4.5), were significantly reduced following the 10-puff (M = 2.8, SEM = 2.5) and ad lib (M = 0.9, SEM = 0.6) periods, and returned to baseline levels after the rest period (M = 12.4, SEM = 4). VAS ratings of “Restlessness” were elevated at baseline (M = 26, SEM = 9), were significantly reduced following the ad lib period (M = 4.8, SEM = 4), and increased following the rest period (M = 8.8, SEM = 4) (see Figure 1). Similarly QSU Factor 1 (intention to smoke) scores were elevated at baseline (M = 21, SEM = 2.7), were significantly reduced following the ad lib puffing period (M = 5.6, SEM = 1.9), and returned to baseline levels after the rest period (M = 18.3, SEM = 2.2). Positive direct effects of EC administration were also observed. VAS ratings of Did the EC help you “Feel awake,” “Calm you down,” and “Concentrate” as well as Was the EC “Pleasant” and “Satisfying” and Did the EC “Reduce your hunger for food” and “Taste Good” increased significantly following the 10-puff period, peaked following the ad lib period, and decreased after the rest period. For example, ratings of “Satisfying” increased from 0.125 (SEM = 0.125) to 83.9 (SEM = 7) after the 10-puff bout and further increased to 92.6 (SEM = 3.3) following the ad lib period before dropping to 36.9 (SEM = 13.5) after the rest period. EC use did not significantly affect ratings on any other measure.
Discussion
Previous reports have described conditions of EC use that support minimal or no nicotine delivery (Bullen et al., 2010; Eissenberg, 2010; Vansickel et al., 2010). In those studies, current cigarette smokers, who were not experienced with ECs, engaged in brief periods of EC use. In addition, the ECs used in those studies were cigarette-sized EC models. In the current study, experienced EC users who provided their preferred ECs loaded with their selected flavor/nicotine concentration completed a 10-puff (30-s IPI) bout and a 1-hr ad lib puffing period. Under these conditions, EC use resulted in reliable nicotine delivery. These results are the first to demonstrate unequivocally that ECs alone are capable of increasing plasma nicotine concentrations to levels seen during cigarette smoking. In support of this conclusion, the results of a recent study in which the saliva cotinine concentration of self-reported EC users was measured are also consistent with nicotine exposure that approximated tobacco cigarette use (e.g., Etter & Bullen, 2011b). However, unlike the current laboratory study, results of this previous work may have been influenced by the concurrent use of other nicotine-containing products.
Interestingly, abstinence symptoms were observed prior to EC use, and these symptoms were suppressed after EC use, a potential indicator of nicotine dependence (Carter and Griffiths, 2009). Of course, these participants were former cigarette smokers and thus may have been nicotine dependent prior to initiating EC use. The extent to which EC use leads to the development of nicotine dependence in nicotine-naïve users is unknown.
Some reports suggest that ECs have the potential to be safe and efficacious replacements for tobacco cigarettes due to nicotine’s effects (Etter & Bullen, 2011a; Siegel, Tanwar, & Wood, 2011). That potential is undermined by ineffective devices and a requirement that users learn specific but as-yet-undetermined behaviors in order to maximize nicotine delivery. Thus, an important area for future research is the parametric manipulation of device characteristics and user behavior (i.e., puff topography) as well as other factors (e.g., nicotine concentration) that might contribute to safety and efficacy. In addition, the potential deleterious health effects of chronic inhalation of vaporized nicotine solutions are unknown (Etter, Bullen, Fouris, Laugesen, & Eissenberg, 2011). Clinical laboratory evaluation of lung and cardiovascular function, measurement of biomarkers of harm (e.g., inflammatory biomarkers such as C-reactive protein), as well as population level surveillance of adverse events associated with EC use will be necessary for ensuring safety and proper regulation. Taken together, results of the current and previous studies demonstrate considerable variability in EC nicotine delivery, device performance, and/or cartridge and vapor nicotine content (Bullen et al., 2010; Eissenberg, 2010; U.S. Food and Drug Administration, 2009; Vansickel et al., 2010; Williams & Talbot, 2011). One important potential benefit of EC regulation may be more consistent nicotine delivery, device performance, and cartridge and vapor content.
Funding
This report was supported partially by the National Institutes of Health (R01 CA120142 to TE and T32 DA007027 to ARV).
Declaration of Interests
The authors have no conflicts of interest to declare. The sponsor had no role in the design and conduct of the study; or in the preparation, review, or approval of the manuscript. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
The authors acknowledge, with gratitude, the hard work and dedication of the staff and students of VCU’s Clinical Behavioral Pharmacology Laboratory (Ms. Barbara Kilgalen, Ms Janet Austin, and Ms. Caroline Cobb) as well as the staff of VCU’s Bioanalytical Core Laboratory Service Center.
References
- Ayers JW, Ribisi KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. American Journal of Preventive Medicine. 2011;40:448–453. doi: 10.1016/j.amepre.2010.12.007. doi:10.1016/j.amepre.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Blank MD, Sams C, Weaver MF, Eissenberg T. Nicotine delivery, cardiovascular profile, and subjective effects of an oral tobacco product for smokers. Nicotine & Tobacco Research. 2008;10:417–421. doi: 10.1080/14622200801901880. doi:10.1080/14622200801901880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8:727–738. doi: 10.1080/14622200600789585. doi:10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Bullen C, McRobie H, Thornley S, Glover M, Laugesen M. Effect of an electronic cigarette on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomized cross-over trial. Tobacco Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. doi:10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug and Alcohol Dependence. 2009;105S:14–25. doi: 10.1016/j.drugalcdep.2009.04.003. doi:10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. doi:10.1080/14622200124218. [DOI] [PubMed] [Google Scholar]
- Eissenberg T. Electronic nicotine delivery devices: Ineffective nicotine delivery and craving suppression after acute administration. Tobacco Control. 2010;19:87–88. doi: 10.1136/tc.2009.033498. doi:10.1136/tc.2009.033498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF. Electronic cigarettes: A survey of users. BMC Public Health. 2010;10:231. doi: 10.1186/1471-2458-10-231. doi:10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Bullen C. Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011a;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. doi:10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. European Respiratory Journal. 2011b;38:1219–1220. doi: 10.1183/09031936.00066011. doi:10.1183/09031936.00066011. [DOI] [PubMed] [Google Scholar]
- Etter JF, Bullen C, Fouris AD, Laugesen M, Eissenberg T. Electronic nicotine delivery systems: A research agenda. Tobacco Control. 2011;20:243–248. doi: 10.1136/tc.2010.042168. doi:10.1136/tc.2010.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): Views of aficionados and clinical/public health perspectives. International Journal of Clinical Practice. 2011;65:1037–1042. doi: 10.1111/j.1742-1241.2011.02751.x. doi:10.1111/j.1742-1241.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- Heavner K, Dunworth J, Bergen P, Nissen C, Phillips CV. Electronic cigarettes (e-cigarettes) as potential tobacco harm reduction products: Results of an online survey of e-cigarette users. Tobacco Harm Reduction. 2009 [Internet]. Nov [cited 2010 Feb 9]; working paper 011: [about 15 pp.]. Retrieved from http://www.tobaccoharmreduction.org/wpapers/011.htm. [Google Scholar]
- Kleykamp BA, Jennings JM, Sams C, Weaver MF, Eissenberg T. The influence of transdermal nicotine on tobacco/nicotine abstinence and the effects of a concurrently administered cigarette in women and men. Experimental and Clinical Psychopharmacology, 2008;16:99–112. doi: 10.1037/1064-1297.16.2.99. [DOI] [PubMed] [Google Scholar]
- McQueen A, Tower S, Sumner W. Interviews with “vapers”: Implications for future research with electronic cigarettes. Nicotine & Tobacco Research. 2011;13:860–867. doi: 10.1093/ntr/ntr088. doi:10.1093/ntr/ntr088. [DOI] [PubMed] [Google Scholar]
- Polosa R, Paquale C, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-cigarette) on smoking reduction and cessation: A prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. doi:10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking cessation tool: Results from an online survey. American Journal of Preventive Medicine. 2011;40:472–475. doi: 10.1016/j.amepre.2010.12.006. doi:10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine & Tobacco Research. 2011;12:905–912. doi: 10.1093/ntr/ntq114. doi: 10.1093/ntr/ntq114. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. “Evaluation of e-cigarettes”. 2009. Retrieved from http://www.fda.gov/downloads/Drugs/ScienceResearch/UCM173250.pdf. [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology, Biomarkers, and Prevention. 2010;19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. doi:10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Talbot P. Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production. Nicotine & Tobacco Research. 2011;13:1276–1283. doi: 10.1093/ntr/ntr164. doi:10.1093/ntr/ntr164. [DOI] [PubMed] [Google Scholar]