Abstract
Introduction:
Understanding factors that render some individuals more vulnerable to smoking relapse during the early stages of a quit attempt is critical to tailoring treatment efforts. Development of laboratory models of relapse can provide a framework for identifying underlying mechanisms that may contribute to vulnerability. Here, we explored predictors of abstinence in a novel incentive-based model of relapse.
Methods:
Fifty-six nontreatment seeking daily smokers completed several nicotine dependence measures prior to participating in a 1-week abstinence incentive test. During the abstinence procedure, participants earned monetary reinforcement for each biochemically verified day of abstinence according to a descending schedule of reinforcement.
Results:
Compliance with the procedure was excellent. All but 3 participants were able to initiate abstinence; nearly 70% lapsed as incentives were reduced. Scores on the Fagerström Test for Nicotine Dependence (FTND), number of cigarettes smoked per day, and self-reported craving on the first day of abstinence each independently predicted time to lapse. The single item of time to first cigarette in the morning on the FTND significantly predicted time to lapse, even when controlling for other significant predictors just listed. The Nicotine Dependence Syndrome Scale (NDSS) and Wisconsin Inventory of Smoking Dependence Motives did not predict lapse, but the NDSS did predict reinitiation of abstinence among those experiencing an initial lapse.
Conclusions:
These findings partially replicate those of previous full-scale clinical trials and support the feasibility and validity of an incentive-based model of relapse. The time-limited and laboratory-based nature of this model has the potential to further investigations of underlying mechanisms contributing to relapse.
Introduction
Despite recent public health efforts, tobacco smoking remains a leading cause of preventable death. While the majority of smokers wish to quit, only a small percentage of those who attempt cessation each year are successful (Center for Disease Control and Prevention, 2002). As such, there is tremendous interest in identifying the factors that may lead some smokers to have greater difficulty quitting smoking than others.
Clinical smoking cessation trials have identified a multitude of variables predicting relapse among dependent smokers including, among other factors, severity of nicotine dependence (Japuntich et al., 2011; Piper et al., 2008), impulsivity (Powell, Dawkins, West, & Pickering, 2010; Yoon et al., 2007), beliefs and attitudes about smoking (Chassin, Presson, Sherman, Seo, & Macy, 2010; Rose, Chassin, Presson, & Sherman, 1996), self-efficacy (Cox et al., 2011; Schnoll, Subramanian, Martinez, & Engstrom, 2011), number of members within the social network providing social support (Japuntich et al., 2011), and psychiatric comorbidity (Breslau & Johnson, 2000; Kenney et al., 2009; Piper et al., 2010). Such diverse findings underscore the complexity of biological and contextual factors contributing to the persistence of smoking and highlight the need for research to elucidate potential mechanisms that may mediate risk for relapse. Indeed, while large scale clinical trials afford the greatest ecological validity and generalizability to real world quit attempts, human laboratory models of relapse are critical for providing a more nuanced understanding of the process of relapse and the pathways by which vulnerability is conferred. By conducting assessments in a highly controlled time-limited manner, laboratory models can dramatically reduce the overall expense and time commitment of clinical trials, target specific mechanisms of interest while minimizing unmeasured variance, and reduce attrition rates.
Several previous studies have explored smoking lapse and relapse using a variety of laboratory-based models (Chornock, Stitzer, Gross, & Leischow, 1992; Dallery & Raiff, 2007; Juliano, Donny, Houtsmuller, & Stitzer, 2006; Leeman, O’Malley, White, & McKee, 2010; McKee, Krishnan-Sarin, Shi, Mase, & O’Malley, 2006; Mueller et al., 2009; Shadel et al., 2011). These models typically involve offering an alternative incentive (e.g., monetary reward) for periods of abstinence from smoking, with increments of reinforced abstinence ranging from seconds (Dallery & Raiff, 2007) to minutes (McKee et al., 2006) to days (Juliano et al., 2006). This general approach allows for the assessment of the “relative” reinforcing value of smoking, which may go undetected in the absence of an alternative competing incentive (McKee, 2009). Within this framework, individual differences in sensitivity to both smoking reward and nondrug rewards may contribute to the decision to smoke, thereby providing a laboratory corollary to real-world smoking behavior, in which the choice to smoke involves weighing trade-offs between motivation to smoke and benefits of abstaining.
In the present study, we developed a laboratory abstinence incentive test to approximate a quit attempt among nontreatment seeking individuals lasting 1 week and using a descending payment schedule for reinforcement of abstinence. A 1-week time frame was chosen to capture the initial volatile period during which most smoking lapses occur (Brown et al., 2009; Garvey, Bliss, Hitchcock, Heinold, & Rosner, 1992) and to minimize the burden of daily laboratory assessments. Incentive amounts were selected to maximize intersubject variability in order to examine predictors of outcomes within the model. A high initial payment ($75) was used to encourage the initiation of abstinence among all participants and enable measurement of abstinence-induced craving and withdrawal, while a descending schedule was chosen in order to shorten the time to first lapse (Mueller et al., 2009) to facilitate assessment within a short-term laboratory model.
In adopting this approach, we sought to expand beyond the existing literature in two ways. Whereas several previous investigations have examined latency to smoke following a specific manipulation within a single laboratory session (Leeman et al., 2010; McKee et al., 2006), the current extension of the abstinence assessment across multiple days including only brief laboratory visits allows for a more naturalistic assessment of smoking behavior, thereby more closely approximating an actual quit attempt. Furthermore, in contrast to previous studies assessing reinstatement of smoking behavior following an initial “priming” exposure in a subset of individuals achieving initial abstinence over a period of several days (Chornock et al., 1992; Juliano et al., 2006), the present model sought to evaluate the early phases of a quit attempt in all participants, examining the ability to initiate abstinence and avoid a first lapse.
Within this framework, we explored whether known predictors of abstinence in full-scale clinical trials—namely, nicotine dependence, craving, and withdrawal—predicted variability in time to first lapse. We included the Wisconsin Inventory of Smoking Dependence Motives (WISDM), the Nicotine Dependence Syndrome Scale (NDSS), and the Fagerström Test for Nicotine Dependence (FTND) as measures of dependence, all of which have been previously shown to predict cessation success in clinical trials (Baker et al., 2007; Piper et al., 2008). Interestingly, the FTND and, in particular, the single item of the FTND assessing time to smoke the first cigarette (TTFC) in the morning has most consistently been shown to be associated with cessation success, with variability predicting abstinence above and beyond the WISDM or NDSS (Baker et al., 2007). Therefore, we predicted that both the FTND and the TTFC item would predict time to the first lapse within the abstinence incentive test. Furthermore, we predicted that smokers experiencing greater craving and withdrawal upon initiating abstinence would lapse sooner within the model.
Methods
Participants
Participants were smokers recruited from the community who participated in one of two different studies involving the ability to abstain from smoking when given an incentive to do so. Smokers qualified for participation if they were 18–65 years of age, self-reported smoking at least 5 cigarettes/day (CPD) for the past year, exhaled an expired-air carbon monoxide (CO) level of at least 8 ppm at the initial screening visit, and reported no intention to quit smoking in the next month. Exclusion criteria included self-reported significant medical or psychiatric illness in the past year, drug or alcohol dependence, use of nicotine replacement therapy, pharmacotherapy for smoking cessation, use of other tobacco products within the past 30 days, current use of any psychotropic medication, or pregnancy/lactation. Study assignment was based primarily on the timing of participant contact, given that the first study was nearly completed before the second study began. However, given that the second study required completion of a functional magnetic resonance imaging (fMRI) scan (described below), a few additional participants were routed into the first study when they had contraindications for completing the scan but were otherwise eligible for participation. All participants provided informed consent in accordance with approved protocol and guidelines of the University of Pittsburgh Institutional Review Board.
Fifty-eight participants were consented and participated in the abstinence incentive test; two were subsequently excluded due to procedural errors. The remaining 56 participants (55.4% female; mean age, 38.8 years ± 11.0 SD) were included in analyses. Of these, 46.4% were Caucasian, 48.2% were Black, and 5.4% identified with more than one race or preferred not to answer.
Study Design
All participants first completed an in-person screening and baseline assessment of several self-report and behavioral measures. Subsequent procedures differed according to which study participants were enrolled in: Twenty-seven were included in Study 1, during which ad libitum smoking behavior was assessed for 1 week; the remaining 29 participated in Study 2 and completed an fMRI session assessing blood oxygenation level dependent response to monetary reward following a period of overnight abstinence. Overnight abstinence was not explicitly reinforced with a monetary incentive but was required for continuation in the study. The results of the ad libitum smoking and fMRI assessments are not reported here. After a minimum of 2 days following completion of these procedures, all participants then participated in an abstinence incentive test during which abstinence from smoking was reinforced with money (see below for details).
Procedures
Screening and Assessment
Participants were recruited via flyers and advertisements and completed an initial telephone screen to determine interest and eligibility. Participants were then invited to complete an in-person screening session during which informed consent was obtained and further eligibility was determined. During the in-person screen, breath and urine samples were used to assess blood alcohol level and illicit drug use, respectively. CO was assessed with two breath samples: one upon arrival and the other after participants were allowed to smoke a cigarette. In order to prevent exclusion of participants who may not have smoked recently prior to entering the laboratory, the minimum CO inclusion criterion was satisfied if either CO sample was greater than 8 ppm (All but four participants met criteria with the first breath sample). Participants then completed a battery of computer-administered questionnaires assessing demographic information, medical and psychiatric history, nicotine use history, and nicotine dependence. Following determination of eligibility, an assessment battery was administered, including additional measures of nicotine dependence, cigarette craving, and withdrawal. These scales have been thoroughly described elsewhere (FTND: Heatherton, Kozlowski, Frecker, & Fagerström, 1991; NDSS: Shiffman, Waters, & Hickcox, 2004; WISDM: Piper et al., 2004; Minnesota Nicotine Withdrawal Scale [MNWS]: Hughes & Hatsukami, 1986). A four-item version of the Questionnaire on Smoking Urges (QSU-4: Carter & Tiffany, 2001) was administered during assessment only for Study 1.
Abstinence Incentive Test
The abstinence incentive test involved a series of brief daily visits to the laboratory during which abstinence from smoking was biochemically verified and reinforced with money according to a descending payment schedule. All participants were instructed to initiate abstinence on the Sunday following completion of initial procedures. Participants then attended daily sessions lasting approximately 15 min each on Monday through Friday, plus an additional visit the following Monday. During each visit, participants reported the number of cigarettes smoked over the previous 24 hr (or 72 hr on the last day) and completed computerized questionnaires assessing craving (QSU-4) and withdrawal (MNWS). Breath CO samples were obtained daily to verify abstinence status. Participants were considered abstinent if they reported not smoking and had a CO reading of <6 ppm or a 50% reduction from the previous sample. In addition, salivary cotinine was measured beginning on the third day of reported abstinence using NicAlert test strips (cotinine level of ≤100 ng/mL was considered abstinent). Participants were paid immediately following verification of abstinence according to the following schedule: $75 on Monday, $55 on Tuesday, $40 on Wednesday, $25 on Thursday, $15 on Friday, and $15 on Monday for a total of $225 possible. Payments were made in cash or with bank cards from which cash could be immediately obtained. Participants were instructed that they could reinitiate abstinence following a slip, such that only the immediate incentive was lost.
Data Analyses
Cox regression was used to assess hazard ratios (HRs) for variables predicting the primary outcome of time to the first lapse in the abstinence incentive test. Significance testing for categorical variables (e.g., race) was determined by chi-square test. Furthermore, among those who lapsed prior to the last day of the abstinence incentive test, secondary analyses were conducted using binary logistic regression to identify factors predicting successful reinitiation of abstinence, defined as achieving at least one additional day of abstinence following the lapse. Statistical significance was defined as α < .05.
Results
Sample Smoking Characteristics
On average, participants were moderately dependent daily smokers, with a mean FTND of 5.3 (±2.0 SD) and a mean CPD of 16.4 (±5.6 SD). However, there was substantial variation across participants, with FTND scores ranging from 0 to 9 and CPD ranging from 6 to 30. Similarly, participants had extensive smoking histories as a group, reporting smoking daily for an average of 18.4 years (±10.7 SD), but ranging widely from 1.5 years to 40 years.
Compliance with the abstinence incentive test was excellent. Participants attended all in-person visits with the exception of two individuals who each missed one appointment and one individual who missed two appointments. Samples for these four missing data points were coded as nonabstinent. Furthermore, a high degree of convergence was observed between biochemical measures of abstinence. A total of 114 cotinine samples were analyzed from 42 participants. Of these samples, 107 (94%) were consistent with the classification based on the corresponding CO test. For the remaining seven samples, the cotinine reading was higher than the abstinence cutoff of 100 ng/mL or less despite CO readings of less than 6 ppm. On those occasions, participants were not reinforced for abstinence.
Substantial variability was observed in abstinence outcomes during the abstinence incentive test (Figure 1). The vast majority of participants (95%) were able to initiate abstinence on the first day of the test, earning the $75 incentive (only three participants did not initiate abstinence). However, as incentives decreased, participants began to lapse so that by the last day of the study nearly 70% (n = 39) had lapsed. Percentage of participants lapsing for the first time on each day of the test were as follows: 23% on Day 2, 11% on Day 3, 7% on Day 4, 16% on Day 5, and 7% on Day 8. Seventeen participants (30%) remained abstinent throughout the entire procedure and earned the full $225 (percentages total 99 due to rounding). Among those who lapsed prior to the last day of the study (n = 35), 11 (31%) reinitiated abstinence on a subsequent day and earned further incentives, while 24 (69%) continued to smoke throughout the remainder of the procedure.
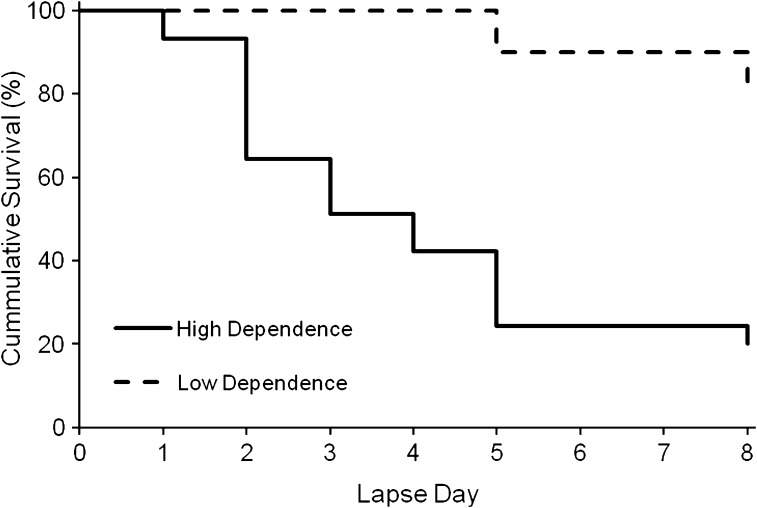
Figure 1.
Survival curves for time to smoking lapse plotted separately for individuals high and low in nicotine dependence as defined by time to first cigarette in the morning of less than, or greater than, 30 min, respectively.
Predictors of Time to First Lapse
Hazard ratios for individual predictors of time to first lapse in the abstinence incentive test are presented in Table 1. None of the demographic variables, including age, sex, race, income, or education level were significant predictors of lapse. Among smoking use variables, higher CPD was associated with greater likelihood of lapse (HR = 1.069, p < .05), while baseline CO (taken after smoking), years smoking daily, and age of first puff of a cigarette all failed to reach significance. Among nicotine dependence measures, higher scores on the FTND predicted a greater likelihood of lapse (HR = 1.188, p < .05). Furthermore, smoking within 30 min of waking in the morning—as indicated by the single item TTFC—was strongly associated with greater likelihood of lapse (Figure 1; HR = 6.974, p < .01). Indeed, TTFC appeared to explain the association between FTND and lapse likelihood, as the remaining FTND items failed to significantly predict lapse when the TTFC item was removed (HR = 1.175, p > .10). To determine the extent to which TTFC predicted outcomes beyond smoking intensity (i.e., CPD), we tested the association between TTFC and lapse with CPD included as a covariate. Smoking within 30 min of waking continued to independently predict a greater likelihood of lapse when controlling for CPD (HR = 6.245, p < .05).
Table 1.
Hazard Ratios and CIs for Demographic Variables, Smoking Use Variables, and Nicotine Dependence Measures Predicting Time to First Lapse
| Predictor variable | Hazard ratio | CI |
| Age | 1.004 | (0.974–1.035) |
| Sex | 0.649 | (0.345–1.220) |
| Race | X2 = .966 | |
| Income | 0.980 | (0.785–1.225) |
| Education level | 0.954 | (0.564–1.614) |
| Cigarettes per day | 1.069* | (1.002–1.140) |
| Carbon monoxide | 1.016 | (0.979–1.054) |
| Years of daily smoking | 1.018 | (0.987–1.050) |
| Age first puff | 0.989 | (0.895–1.093) |
| FTND | 1.188* | (1.016–1.390) |
| Time to first cigarette | 6.974** | (1.670–29.119) |
| NDSS | 1.004 | (0.975–1.035) |
| WISDM | 1.013 | (0.991–1.035) |
| Craving on Day 1 of abstinence | 1.016* | (1.004–1.029) |
| Withdrawal on Day 1 of abstinence | 1.015* | (1.000–1.030) |
Note. FTND = Fagerström Test for Nicotine Dependence; NDSS = Nicotine dependence syndrome scale; WISDM = Wisconsin inventory of smoking dependence motives.
*p < .05. **p < .01.
We then evaluated whether craving and withdrawal during abstinence predicted time to first lapse in the abstinence incentive test (Table 1). Excluding the three participants who were unable to initiate abstinence on the first day of the test, scores on Day 1 of abstinence for the QSU-4 and MNWS were used as predictors within the Cox regression models. When entered separately, higher levels of craving and withdrawal when initiating abstinence were each associated with a greater likelihood of lapsing during the abstinence test (HR = 1.016 and 1.015, respectively, both p < .05). The significant effect of craving on abstinence outcomes persisted when controlling for CPD (HR = 1.015, p < .05) or for the effects of withdrawal (1.014, p < .05). Furthermore, when TTFC and craving during abstinence were entered together into the same model, TTFC continued to independently predict lapse (HR = 4.971, p < .05), although craving during abstinence did not (HR = 1.009, p > .10). Finally, we evaluated whether the abstinence-induced changes in craving and withdrawal from baseline levels similarly predicted abstinence outcomes (Table 2). Because the QSU-4 was only available at baseline for Study 1, analyses of abstinence-induced changes in craving were restricted to Study 1 participants. As a group, participants showed a significant increase in both craving, t(24) = 6.139, p < .001, and withdrawal, t(52) = 4.516, p < .001, as a function of abstinence. When using the difference scores between baseline and Day 1 of abstinence for both craving and withdrawal, results described above were unchanged with the exception that abstinence-induced increases in withdrawal did not significantly predict lapse outcomes (HR = 1.008, p > 0.10).
Table 2.
Hazard Ratios and CIs for Abstinence-Induced Changes in Craving and Withdrawal Predicting Time to First Lapse
| Variable | Baseline measure | Day 1 abstinence measure | T difference | Hazard ratio | CI |
| Questionnaire of Smoking Urges-4a | 23.14 | 72.22 | −6.139** | 1.014* | (1.001–1.027) |
| Minnesota Withdrawal Scale | 22.42 | 38.16 | 4.516** | 1.008 | (0.996–1.021) |
Note. aIncludes only participants from Study 1 (n = 25).
*p < .05. **p < .001.
Predictors of Reinitiating Abstinence After Lapse
Of the 39 participants who lapsed during the abstinence incentive test, 35 of them did so prior to the last day of the test, thereby allowing them the possibility of reinitiating abstinence. While most participants continued to smoke after their first lapse, 11 (31%) successfully achieved abstinence on at least one day following their initial lapse. We therefore explored possible factors contributing to the reinitiation of abstinence. No demographic or smoking use variables reached significance, including CPD. Furthermore, contrary to time to first lapse, FTND and TTFC were not associated with the ability to reinitiate abstinence. However, higher total score on the NDSS was associated with significantly reduced likelihood of abstinence following a lapse (OR = 0.909, p < .05), while total score on the WISDM exhibited a nearly significant trend in the same direction (OR = .946, p = .055).
Discussion
This study examined predictors of smoking lapse in a brief incentive-based laboratory model of smoking abstinence. Although participants were required to make daily laboratory visits to verify abstinence, compliance was excellent, supporting the feasibility of the procedure. In addition, a wide range of interindividual variability in time to first lapse was observed, indicating that the model was sensitive to individual differences in smoking behavior. When examining predictors of abstinence within the model, FTND and TTFC were both significant predictors of time to the first lapse. These findings are consistent with results from full-scale clinical trials (Baker et al., 2007; Japuntich et al., 2011; Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994; Piper et al., 2008), supporting the validity of the model as an index of the ability to successfully initiate a quit attempt.
Given the substantial time and resources required for conducting large clinical trials, the laboratory model described here has the potential to provide a cost-effective alternative for testing both individual differences and experimental factors that might influence the ability to quit smoking. Furthermore, the inclusion of daily laboratory visits provides an opportunity for conducting detailed assessment of the processes and state-based changes occurring within individual participants that may contribute to lapse and relapse. In the present study, higher levels of craving and withdrawal upon initiating abstinence were associated with earlier lapse. However, craving did not appear to explain the association between TTFC and smoking lapse, as TTFC continued to significantly predict abstinence outcomes when both variables were included in the same model. These findings illustrate the potential utility of this model in exploring how state-based changes may explain or add to trait level predictors, thus providing a framework for elucidating mechanisms by which nicotine dependence or other factors may contribute to relapse. Although the assessment of craving and withdrawal in the present study represents a small initial step, exploration of other state changes in mood, affect, or behavior remains an important future direction.
Although FTND and TTFC were significant predictors of abstinence outcomes, no association was found between NDSS or WISDM and time to first lapse. This is surprising, given that both NDSS and WISDM have been shown to predict smoking cessation outcomes in other studies (Courvoisier & Etter, 2010; Piper et al., 2008; Shiffman et al., 2004). However, in one study comparing all three measures of nicotine dependence, the FTND was found to be the single best predictor of smoking cessation outcomes across all time points, from abstinence initiation to 6-month follow-up (Piper et al., 2008). By contrast, specific subscales of the WISDM and NDSS improved prediction of outcomes beyond the FTND only at the end of treatment. Thus, it is possible that the lack of association between abstinence and the NDSS and WISDM in the present study is a function of (a) the FTND as a better index of smoking cessation success, (b) the focus on initiation rather than maintenance of abstinence in the present study, and (c) analysis of the global measures rather than evaluation of specific subscales.
Furthermore, each of these dependence measures was derived from distinct theoretical backgrounds and may be assessing different aspects of dependence. The FTND was designed to emphasize physical dependence and withdrawal (Fagerström, 1978; Heatherton et al., 1991), although it has also been argued to primarily assess the motivational impact of abstinence on smoking behavior (Piper, McCarthy, & Baker, 2006). In this regard, it is not surprising that the FTND (and TTFC) was the strongest predictor of lapse within the abstinence incentive test. By contrast, the NDSS and WISDM are both multidimensional scales, attempting to capture underlying processes or motives inherent in nicotine addiction. For example, the WISDM includes subscales such as “tolerance,” which is conceptually similar to the FTND, as well as those capturing such diverse constructs as response to environmental smoking cues and a sense of emotional attachment to smoking. Thus, it is possible that some subscales of these measures—particularly those that are conceptually relevant to the present procedure, like “tolerance” or “craving”—may be predictive of lapse, while other subscales may be sensitive to factors that have less impact in the present test. We restricted the present analyses to total scores to minimize the number of comparisons; however, exploration of subscale scores would be of interest in future studies aimed at determining whether specific dependence-related processes are associated with risk for a smoking lapse.
It is important to note that the abstinence incentive test employed here provides an index of the relative reinforcing value of smoking. Thus, failure to sustain abstinence may reflect heightened reinforcing value of smoking per se or a decrease in the reinforcing value of the alternative monetary reward. Several studies have demonstrated that abstinent smokers experience diminished capacity for reward relative to both satiated smokers and nonsmokers, including less enjoyment from ordinarily pleasurable events and reduced response to financial reward (Dawkins, Powell, West, Powell, & Pickering, 2006; Powell, Dawkins, & Davis, 2002; Powell, Pickering, Dawkins, West, & Powell, 2004). Thus, individual differences in nondrug reward processing during abstinence may predict lapse behavior above and beyond a simple drive for smoking reinforcement. Furthermore, some smokers, such as those with a history of depression, could be particularly vulnerable to deficits in reward processing contributing to smoking relapse—a hypothesis that could be explored within this model. The potential for nondrug reward processing to influence smoking behavior also has direct implications for contingency management procedures, to which all smokers are not equally responsive (Dallery, Glenn, & Raiff, 2007; Glenn & Dallery, 2007). The present model provides a framework for exploring who may most benefit from incentives for abstinence and the processes contributing to their success.
Higher scores on the NDSS and, marginally, the WISDM predicted reduced likelihood of reinitiating abstinence following a lapse. However, the FTND was not associated with abstinence reinitiation. Although at first glance this seems puzzling, the FTND was itself a strong predictor of lapse likelihood. By restricting the sample to those who lapsed during the procedure, we also restricted the range of FTND scores, so that only the most highly dependent remained. By contrast, variation in the NDSS and WISDM—which were not predictive of lapse likelihood—remained among those who lapsed and was shown to be predictive of reinitiation. This suggests that even among those who are heavily dependent on the FTND, the WISDM and NDSS may tap into additional factors contributing to resumption of smoking after an initial lapse. Of course, the short duration of the procedure and the limited sample size leave it unclear as to what aspect of smoking behavior is actually being examined here. However, it is tempting to speculate that the ability to resume abstinence after a slip within the present model may be relevant for preventing the transition from lapse to relapse among those making a quit attempt (Shiffman et al., 2006). Future work would be needed to formally test this possibility.
A potential barrier to interpretation is the likely covariance between time-dependent changes in the processes underlying risk for a lapse (e.g., withdrawal) and decreasing monetary incentives. Although withdrawal symptoms reported during the first day of abstinence did predict greater likelihood of lapse, the sample size limitations noted above prevented assessment of whether increases in withdrawal beyond the first day may have further contributed to lapse. Withdrawal typically increases and peaks within the first week of abstinence (Hughes, 2007), so that increases in symptoms may have covaried with the decreasing schedule of abstinence incentives, leaving it unclear as to which factor was more influential. Furthermore, withdrawal in the present study was not a significant predictor of abstinence outcomes when analyzing change in symptoms from baseline. Without accounting for baseline levels, self-reported withdrawal during abstinence could have been confounded by other mood or anxiety related symptoms not specifically evoked by abstinence from smoking. The lack of an effect of abstinence-induced changes in withdrawal on abstinence outcomes may be due to limited sample size and prevents drawing any clear conclusions about the role of withdrawal in the present study. Future work with larger samples and other schedules of reinforcement for abstinence could help to determine the role of withdrawal and parse out the relative importance of withdrawal versus the value of abstinence incentives.
The present sample was restricted to smokers who were nontreatment seekers with no comorbid psychopathology, thereby limiting generalizability (Perkins, Stitzer, & Lerman, 2006). For example, it is unclear to what extent the participants’ expectations of returning to smoking at the conclusion of the study may have influenced their behavior. However, it is particularly striking that the same measures shown to predict cessation in clinical trials also predicted outcomes here, given the sharp contrast in motives for abstaining. It would be of interest to explicitly evaluate the role of treatment seeking status, as well as other variables including psychiatric comorbidity or pharmacotherapy, within this model in future studies.
Overall, this study provides initial support for the feasibility and validity of a short-term laboratory-based model of abstinence that may be useful for evaluating individual differences in vulnerability to lapse and relapse.
Funding
This research was supported by Pfizer Global Research Award on Nicotine Dependence and National Institutes of Health grant DA023459 to ECD. Ms. Sweitzer was supported by the Behavioral Brain Research Training Grant (T32GM081760) and by the National Science Foundation Integrative Graduate Education and Research Training grant (DGE0549352).
Declaration of Interests
The authors declare no conflicts of interest.
Acknowledgments
The authors would like to thank Gina Sparacino for her assistance with laboratory procedures and data collection.
References
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, et al. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research. 2007;9(Suppl. 4):S555–S570. doi: 10.1080/14622200701673480. doi:10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. American Journal of Public Health. 2000;90:1122–1127. doi: 10.2105/ajph.90.7.1122. doi:10.2105/AJPH.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, et al. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11:493–502. doi: 10.1093/ntr/ntp041. doi:10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. doi:10.1037/1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and economic costs United States, 1995–1999. Morbidity & Mortality Weekly Report. 2002;51:300–303. Retrieved from http://www.cdc.gov/mmwr/ [PubMed] [Google Scholar]
- Chassin L, Presson CC, Sherman SJ, Seo DC, Macy JT. Implicit and explicit attitudes predict smoking cessation: Moderating effects of experienced failure to control smoking and plans to quit. Psychology of Addictive Behaviors. 2010;24:670–679. doi: 10.1037/a0021722. doi:10.1037/a0021722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chornock WM, Stitzer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: Effects on relapse. Psychopharmacology (Berlin) 1992;108:495–500. doi: 10.1007/BF02247427. doi:10.1007/BF02247427. [DOI] [PubMed] [Google Scholar]
- Courvoisier DS, Etter JF. Comparing the predictive validity of five cigarette dependence questionnaires. Drug and Alcohol Dependence. 2010;107:128–133. doi: 10.1016/j.drugalcdep.2009.09.011. doi:10.1016/j.drugalcdep.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Cox LS, Wick JA, Nazir N, Cupertino AP, Mussulman LM, Ahluwalia JS, et al. Predictors of early versus late smoking abstinence within a 24-month disease management program. Nicotine & Tobacco Research. 2011;13:215–220. doi: 10.1093/ntr/ntq227. doi:10.1093/ntr/ntq227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Glenn IM, Raiff BR. An internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence. 2007;86:230–238. doi: 10.1016/j.drugalcdep.2006.06.013. doi:10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Dallery J, Raiff BR. Delay discounting predicts cigarette smoking in a laboratory model of abstinence reinforcement. Psychopharmacology (Berlin) 2007;190:485–496. doi: 10.1007/s00213-006-0627-5. doi:10.1007/s00213-006-0627-5. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I–effects on incentive motivation. Psychopharmacology (Berlin) 2006;189:355–367. doi: 10.1007/s00213-006-0588-8. doi:10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Fagerström K.-O. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. doi:10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: A report from the Normative Aging Study. Addictive Behaviors. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. doi:10.1016/0306-4603(92)90042-T. [DOI] [PubMed] [Google Scholar]
- Glenn IM, Dallery J. Effects of internet-based voucher reinforcement and a transdermal nicotine patch on cigarette smoking. Journal of Applied Behavior Analysis. 2007;40:1–13. doi: 10.1901/jaba.2007.40-1. doi:10.1901/jaba.2007.40-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K.-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research. 2007;9:315–327. doi: 10.1080/14622200701188919. doi:10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, Fiore MC, et al. Smoker characteristics and smoking-cessation milestones. American Journal of Preventive Medicine. 2011;40:286–294. doi: 10.1016/j.amepre.2010.11.016. doi:10.1016/j.amepre.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. Journal of Abnormal Psychology. 2006;115:166–173. doi: 10.1037/0021-843X.115.1.166. doi:10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Kenney BA, Holahan CJ, Holahan CK, Brennan PL, Schutte KK, Moos RH. Depressive symptoms, drinking problems, and smoking cessation in older smokers. Addictive Behaviors. 2009;34:548–553. doi: 10.1016/j.addbeh.2009.03.020. doi:10.1016/j.addbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug and Alcohol Dependence. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. doi:10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Leeman RF, O’Malley SS, White MA, McKee SA. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology (Berlin) 2010;212:25–32. doi: 10.1007/s00213-010-1902-z. doi:10.1007/s00213-010-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. doi:10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berlin) 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. doi:10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller ET, Landes RD, Kowal BP, Yi R, Stitzer ML, Burnett CA, et al. Delay of smoking gratification as a laboratory model of relapse: Effects of incentives for not smoking, and relationship with measures of executive function. Behavioral Pharmacology. 2009;20:461–473. doi: 10.1097/FBP.0b013e3283305ec7. doi:10.1097/FBP.0b013e3283305ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: A proposal for new methodologies. Psychopharmacology (Berlin) 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. doi:10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Piper ME, McCarthy DE, Baker TB. Assessing tobacco dependence: A guide to measure evaluation and selection. Nicotine & Tobacco Research. 2006;8:339–351. doi: 10.1080/14622200600672765. doi:10.1080/14622200600672765. [DOI] [PubMed] [Google Scholar]
- Piper ME, McCarthy DE, Bolt DM, Smith SS, Lerman C, Benowitz N, et al. Assessing dimensions of nicotine dependence: An evaluation of the Nicotine Dependence Syndrome Scale (NDSS) and the Wisconsin Inventory of Smoking Dependence Motives (WISDM) Nicotine & Tobacco Research. 2008;10:1009–1020. doi: 10.1080/14622200802097563. doi:10.1080/14622200802097563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, et al. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. doi:10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, et al. Psychiatric disorders in smokers seeking treatment for tobacco dependence: Relations with tobacco dependence and cessation. Journal of Consulting and Clinical Psychology. 2010;78:13–23. doi: 10.1037/a0018065. doi:10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: Tests of an incentive motivational model. Biological Psychiatry. 2002;51:151–163. doi: 10.1016/s0006-3223(01)01208-2. doi:10.1016/S0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Pickering A. Relapse to smoking during unaided cessation: Clinical, cognitive and motivational predictors. Psychopharmacology (Berlin) 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. doi:10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addictive Behaviors. 2004;29:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. doi:10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Rose JS, Chassin L, Presson CC, Sherman SJ. Prospective predictors of quit attempts and smoking cessation in young adults. Health Psychology. 1996;15:261–268. doi: 10.1037//0278-6133.15.4.261. doi:10.1037/0278-6133.15.4.261. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Subramanian S, Martinez E, Engstrom PF. Correlates of continued tobacco use and intention to quit smoking among Russian cancer patients. International Journal of Behavioral Medicine. 2011;18:325–332. doi: 10.1007/s12529-010-9131-8. doi:10.1007/s12529-010-9131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Martino SC, Setodji C, Cervone D, Witkiewitz K, Beckjord EB, et al. Lapse-induced surges in craving influence relapse in adult smokers: An experimental investigation. Health Psychology. 2011;30:588–596. doi: 10.1037/a0023445. doi:10.1037/a0023445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, et al. Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. doi:10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. doi:10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology. 2007;15:176–186. doi: 10.1037/1064-1297.15.2.186. doi:10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]