Abstract
Introduction:
The initiation and maintenance of tobacco use are influenced by several factors, but of equal and often overlooked importance, until recently, is the palatability of the product. Because of the role that flavor may play in the initiation and maintenance of tobacco use, the Food and Drug Administration has decided to ban the use of flavorings, other than menthol, from cigarettes. To date, little attention has been paid to the impact of flavoring in smokeless tobacco (ST) products.
Methods:
This study combined the data from 5 previously completed treatment or switching studies to examine the choice of brand flavor in the course of ST use, from initiation to regular use.
Results:
The analyses revealed that a majority of subjects’ first and current choice of product was flavored, specifically mint or wintergreen, and that a significant number of ST users switched to a flavored brand after already initiating ST use with a regular nonflavored product. In this population, however, flavored products did not appear to lead to greater dependence or increased exposure to nicotine or carcinogens.
Conclusions:
More treatment seeking ST users began by using mint-flavored product and switched to and were current users of mint-flavored products. It is possible that mint products play a role in the initiation and maintenance of ST use.
Introduction
Initiation and maintenance of tobacco use are influenced by several factors including nicotine yield, pH of the product and the speed of delivery (U.S. Department of Health & Human Services, 2010). Of equal importance is the palatability of the product or product appeal (Henningfield, Hatsukami, Zeller, & Peters, 2011). Palatability of a product can be enhanced by a number of factors including the flavors that are added to it. Flavor in cigarettes, which contributes to the cigarettes taste, smell, and sensory effects, have been considered to facilitate initiation (World Health Organization, 2007) and contribute to abuse liability or maintenance of its use (Henningfield et al., 2011). As a result of this concern, the Family Smoking Prevention and Control Act in the United States banned flavored cigarettes with the exception of menthol cigarettes. With menthol cigarettes, the Act specified that the Tobacco Product Scientific Advisory Committee (TPSAC) was to review the science to determine the impact of menthol cigarettes on public health, considering the effects of menthol on the uptake of and cessation from smoking. Based on this scientific review, the TPSAC’s report (TPSAC, 2011) concluded that:
Menthol cannot be considered merely a flavoring additive to tobacco. Its pharmacological actions reduce the harshness of smoke and the irritation from nicotine, and may increase the likelihood of nicotine addiction in adolescents and young adults who experiment with smoking. Furthermore, the distinct sensory characteristics of menthol may enhance the addictiveness of menthol cigarettes, which appears to be the case among youth. TPSAC has found that the availability of menthol cigarettes has adverse impact on public health by increasing the numbers of smokers with resulting premature death and avoidable morbidity.
Consequently, TPSAC made the following overall recommendation to the U.S. Food and Drug Administration: “Removal of menthol cigarettes from the marketplace would benefit public health in the United States.”
To date, no one has examined the impact of flavor in smokeless tobacco (ST) products. Yet, these products come in a variety of flavors with mint products being the most widely marketed. According to analyses conducted by Chen, Isabelle, Pickworth and Pankow (2010), ST products are utilizing “mint” and “wintergreen” at levels that are highly elevated compared with those found in candy and gum. They found that the average level of “mint” in the top five ST products was 50 percent higher than that of the top five brands of candy and levels of “wintergreen” were eight times higher (Chen et al., 2010). The role that mint flavoring plays in initiation and subsequent dependence has not been addressed. This study examines the choice of brand flavor in the course of ST product use, from initiation to regular use, in an intervention seeking population. Furthermore, we examined whether users of flavored ST products differ from nonflavored users on variables such as age of first use, age of regular use, amount of use, duration of use, level of dependence, and biomarkers of exposure.
Methods
Demographic, ST use history, and biomarker data were merged from five separate study populations of adult ST users. Only data collected during baseline from each study were used in the analysis. Studies 1–5 have been described elsewhere (Ebbert, Edmonds, Luo, Jensen, & Hatsukami, 2010; Hatsukami et al., 2007, 2008, 2004; Schiller, Luo, Anderson, Jensen, & Hatsukami, 2012). Briefly, Studies 1–4 recruited adult (18- to 70-year-old) ST users who were interested in reducing use but not quitting in the next 90 days; Study 1 only recruited those who were using Copenhagen or Kodiak Wintergreen brand ST products. Study 5 recruited adult (21- to 65-year-old) ST users, regardless of their desire to reduce or quit. The eligibility criteria for Studies 1 and 2 were: (a) use ST daily (≥6 dips/day) for the past 6 months, (b) apparent good health with no unstable medical condition, (c) good mental health, (d) no contraindications to nicotine replacement use, (e) not using other tobacco products, and (f) not pregnant or nursing. Studies 3 and 4 changed the required ST use to ≥2 tins/week and kept all other eligibility criteria. Study 5 required the use of at least one tin of moist snuff per week for a minimum of 1 year and additionally subjects could not be currently using any methods for quitting tobacco or cutting down on tobacco use. The sample sizes for the studies were: Study 1 (N = 66), Study 2 (N = 106), Study 3 (N = 102), Study 4 (N = 199), and Study 5 (N = 41).
All studies collected data from two baseline visits that were one week apart. (Subjects attended two baseline visits in order to determine reliability of our measures, to reduce subject burden of completing several questionnaires at each visit, and to collect subject diaries on ST use between visits). During each baseline visit, subjects provided a urine sample for cotinine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides (total NNAL, a biomarker for 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone or NNK, a potent lung carcinogen) analyses and completed a questionnaire regarding tobacco use history.
Statistical Methods
Flavors were placed into two categories: No Flavor (Classic, None, Straight) or Mint Flavor (Ice, Mint, Spearmint, Wintergreen). Subjects with missing data on current brand flavor (n = 12) were excluded. Additionally, those who reported ever using fruit-flavored ST products were excluded due to the small sample size (n = 34). Between-study heterogeneity was assessed by testing the differences in demographic and tobacco use variables across the five studies, using Chi-squared, Fisher’s exact and Kruskal–Wallis tests as appropriate. Significant differences between the five studies were found, most notably in age, ST use patterns, and biomarker levels.
The number and proportion of users of mint-flavored ST products (yes/no) were described over the course of use (first product, first regular product, first daily product, and current product). We compared the first ST product used (mint/no flavor) with the current product used to determine whether the first product influenced the current product choice. The proportions of subjects switching products were compared using a Z-test.
Demographic and tobacco use variables, including age at first dip and regular use, amount of use, duration of use, and measures of dependence (e.g., time to first dip, duration of use during the day, number of quit attempts) were summarized by flavor (mint, no flavor). Differences between flavored and nonflavored users, in biomarkers of exposure, were also determined. The urine biomarker outcomes of interest for this study were total NNAL (pmol/ml) and cotinine (ng/ml). Only cotinine levels were available for participants in Study 4; all biomarkers were available for the other studies. Only the first baseline biomarker value from each participant was analyzed unless it was missing, in which case the second baseline biomarker value was substituted if available. To ensure validity of the analyses, biomarkers were transformed using the natural logarithm to approximate normality and were summarized using geometric means. To compare each of these factors by flavor while adjusting for study differences, mixed-effects analysis of variance models were fit with flavor (yes, no) as a fixed effect and a random effect for individual study. Models additionally adjusting for years of regular use were considered.
Analyses were carried out in SAS v. 9.2 (SAS Institute, Inc., Cary, NC) and all significance levels were set at 0.05.
Results
A total of 468 subjects had current brand-flavor information for this analysis. Table 1 shows the type of mint flavors used by the ST users during the progression from first product used to first daily product used to current product used. Approximately 60% used a mint-flavored product as their first product used or product that they first used regularly or daily. A similar percent reported current use of flavored products. The majority of the flavored-product users favored wintergreen flavors.
Table 1.
Flavors Across Smokeless Tobacco Use Spectrum (n = 468)
| First product | First regular product | First daily product | Current product | |||||
| N | % | N | % | N | % | N | % | |
| Any flavor | ||||||||
| No (none/classic/straight) | 195 | 42.21 | 167 | 35.91 | 174 | 37.42 | 193 | 41.24 |
| Yes (mint) | 267 | 57.79 | 298 | 64.09 | 291 | 62.58 | 275 | 58.76 |
| Missing | 6 | 3 | 3 | 0 | ||||
| Flavor type | ||||||||
| Ice | 1 | 0.22 | 1 | 0.22 | 0 | 0.00 | 1 | 0.21 |
| Mint | 55 | 11.90 | 66 | 14.19 | 58 | 12.47 | 46 | 9.83 |
| Spearmint | 9 | 1.95 | 6 | 1.29 | 4 | 0.86 | 1 | 0.21 |
| Wintergreen | 202 | 43.72 | 225 | 48.39 | 229 | 49.25 | 227 | 48.50 |
| Missing | 6 | 3 | 3 | 0 | ||||
| No flavor (none/classic/straight) | 195 | 42.21 | 167 | 35.91 | 174 | 37.42 | 193 | 41.24 |
Figure 1 shows first and current product use by flavor. Of the ST users who started using mint-flavored products, 64.4% reported current use of flavored products whereas 48.7% of those who started using nonflavored products continued to use nonflavored products. Of those who first used nonflavored products, 51.3% indicated switching to a flavored product as their current product. Of those subjects first using flavored products, 35.6% of ST users switched to a nonflavored product as their current product. Those ST users who started by using nonflavored products were more likely to switch to mint-flavored products compared with the other way around (p < .0001). ST users who started with a mint-flavored product were also more likely to currently use a mint-flavored product compared with those who continue with nonflavored products (p = .001).
Figure 1.
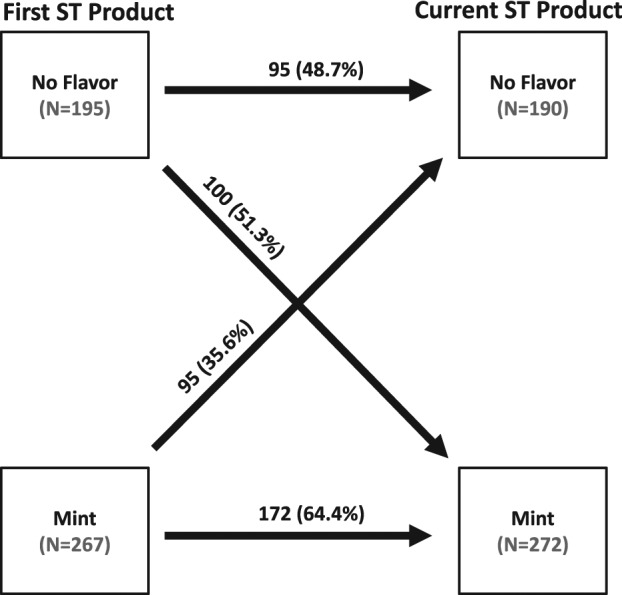
Comparison of first and current product use by flavor (n = 6 missing first smokeless tobacco product).
Table 2 shows the results comparing the demographics, ST use history, and biomarkers of exposure between ST users using nonflavored versus mint-flavored products. ST users of flavored products were significantly younger in age (32.5 vs. 37.3 years; p < .0001), used fewer dips per day (8.8 vs. 9.9; p = .035) and had fewer years of regular use (13.0 vs. 17.8 years; p < .0001). As expected, age and years of regular use were highly correlated (Pearson correlation coefficient = .72, p < .0001). ST users of flavored products also had lower cotinine levels (p = .037), though this became nonsignificant once adjusted for dips per day (p = .745). A near significant difference was observed for total NNAL, with lower levels observed among those using the flavored product (p = .062), though this also became nonsignificant when adjusted for dips per day (p = .921). A higher proportion of ST users of nonflavored products endorsed a symptom associated with dependence (e.g., use of ST 30 min within awakening, 74.7% vs. 63.5%, respectively, p = .013, data not shown). Lower levels of dips per day as well as dependence may have been a function of the shorter duration of use. When duration of use was controlled, no significant difference by flavor was observed for dips per day (p = .666). After additionally adjusting for duration of use, use of ST 30 min within awakening was borderline significant, with those using nonflavored products showing more dependence (p = .073). Lastly, when Study 1 participants were excluded from all analyses because only Kodiak Wintergreen and Copenhagen users were recruited for this study, all comparisons remained the same except that current dips per day became less significant (p = .121, data not shown).
Table 2.
Comparison of Demographics and Smokeless Tobacco Use by Current Brand Flavor (n = 468)
| Variable | No flavor | Flavor | p Value | ||
| N | Mean (SD) | N | Mean (SD) | ||
| Total | 193 | 275 | |||
| Age | 193 | 37.3 (7.7) | 275 | 32.5 (7.8) | <.0001 |
| Age first dip | 191 | 16.8 (5.4) | 273 | 16.3 (5.5) | .358 |
| Age daily/regular use | 191 | 19.5 (5.9) | 273 | 19.6 (5.7) | .941 |
| Current tins/week | 193 | 4.0 (2.4) | 275 | 3.9 (2.0) | .606 |
| Current dips/day | 193 | 9.9 (5.7) | 275 | 8.8 (5.3) | .035 |
| Years of regular use | 191 | 17.8 (7.3) | 273 | 13.0 (6.6) | <.0001 |
| Duration use/day (hrs) | 176 | 12.2 (7.5) | 252 | 11.8 (7.2) | .610 |
| Median # quit attempts | 191 | 4.0 | 273 | 4.0 | .582a |
| N | Mean (95% CI) | N | Mean (95% CI) | p Value | |
| Cotinine (ng/ml) | 190 | 4,157 (3,510–4,922) | 273 | 3,838 (3,427–4,297) | .037 |
| Cotinine (ng/dip) | 190 | 475 (403–560) | 272 | 499 (447–557) | .745 |
| NNAL (pmol/ml) | 99 | 3.90 (3.36–4.52) | 147 | 3.20 (2.82–3.63) | .062 |
| NNAL (pmol/dip) | 99 | 0.42 (0.36–0.49) | 147 | 0.39 (0.34–0.45) | .921 |
Note. Mean and SD are presented for all demographic and smokeless tobacco use variables and geometric means and 95% CI are presented for all biomarkers. All p values are adjusted for differences across studies. NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
Kruskal–Wallis nonparametric test, not adjusted for study difference.
Discussion
In this intervention seeking population, a majority of subjects’ first and current choice of ST product was mint flavored. Furthermore, a significant number switched from nonflavored to a flavored ST product. These findings suggest support for the idea that flavored products may make tobacco more palatable, possibly contributing to the initiation and maintenance of ST use. On the other hand, the results showed that flavored products did not lead to greater dependence or more exposure to nicotine or to NNK. These results are consistent with what has been observed with menthol cigarettes (TPSAC, 2011), that is, there were insufficient data to support greater dependence or exposure on menthol cigarettes among established adult smokers.
The high rate of flavored ST product use and switching to flavored products is of concern given the most recent trends seen in the manufacturing of flavored products and trends in ST use in general. The use of flavors in ST products is not new. Mint, spearmint, and wintergreen have been available for many years with Skoal Cherry being the only fruit flavor offered in the past (World Health Organization, 2007). However, within the last decade, a number of new flavored products have emerged into the marketplace with Skoal now offering a selection of 10 varieties of flavoring (United States Tobacco Company, 2008). Advice to “newbies” on flavor selection even appears on websites’ blogs for the novice users (Dip-time: Brands of Smokeless Tobacco, 2011; Dip-time: New Dippers Page, 2011; Information About Chew and Different Brands, 2009).
If your [sic] going to try timberwolf I suggest you try Straight or if your [sic] just beginning try Peach or Apple.
As far as flavor selection goes try a flavor that you think sounds good. If you are confused I suggest trying a fruit flavor. SKOAL has a variety of fruit flavors including apple, peach, vanilla, berry blend, and cherry. Be sure to try more than one flavor if you decide you do not like the first flavor you try. SKOAL also offers wintergreen, mint, spearmint, classic (natural), and straight if fruit is not your thing.
Info On Rooster: Look for a new flavor coming out soon (already out in most markets) called “Icy Mint.” This flavor smells and tastes like candy, while still delivering a helluva buzz.
The ST industry has seen an “estimated growth rate of around 5 percent compared with the historic growth rate of around 2 percent in the first quarter of 2005” (Reid, 2005). During the same time period, some new flavors have appeared. According to Alpert, Koh, and Connolly (2008), there has been a drastic increase in the variety of sub-brands being offered from 2000 to 2006. For example, it was found that, “U.S. Smokeless Tobacco Company increased the number of sub-brands marketed by 140% from 20 in 2000 to 48 by 2006” (Alpert et al., 2008), with majority of the increase appearing to be driven mostly by increases in flavors. These new flavors are attractive not only to the new and experienced ST user but also potential new customer base—the smoker who may switch products or use ST in nonsmoking situations. Currently, the tobacco industry is seeing its biggest growth in moist snuff, up nearly double digits compared with 2009, and in pouches “which provide discreet usage for the consumer in the workplace or other banned smoking areas,” which now represent 10% of the market (Keller, 2010).
TobaccoRetailers.com stated, “Flavors continue to provide strong customer appeal and generate category growth…Some 27 percent [of retailers] believed it was the result of new customers or smokers switching to smokeless and 22 percent believed it was due to innovations (flavors/pouches) in the market.” (Reid, 2005).
How these flavored products actually impact the uptake of ST, both youth initiation and “switching” smokers remains to be seen. Despite the increase in many different flavored products, the majority of the intervention seeking ST users in our study used mint products over the other flavored products, potentially because the variety of flavored products were not in the market at the time they initiated ST use. Nonetheless, the presence of these flavored products, including the ones targeted to smokers, may in part account for the recent increases in use among young adults aged 18–25 (Centers for Disease Control and Prevention, 2010). According to testimony provided by Terry F. Pechacek, Ph.D., to the U.S. House of Representatives, which utilized data from the National Survey on Drug Use and Health, “From 2004 to 2007, the rates of ST use initiation increased significantly for males 12 to 17 and 18 to 25 years of age” (Pechacek, 2010) and coincidentally the increase in flavor varieties reported by Alpert et al. (2008) also occurred during that time.
The present study showed that flavored ST products did not result in greater nicotine dependence or toxicant exposure. In fact, when the analysis did not control for confounding variables, flavored ST brand users experienced less amount of use and exposure to nicotine and had a fewer number who were dependent. One possible explanation for the lack of significant differences between these two groups could be due to the fact that the sample included established ST users who were seeking an intervention. Similarly, in the studies on menthol cigarettes, established users were not found to be more dependent or have greater exposure to nicotine or toxicants. It was only in examining youth that greater dependence scores were observed (TPSAC, 2011). Future research should examine a population of users that have recently initiated use to determine if they tend to have greater exposure as a result of longer dip duration (due to the masking effect of flavored compared with nonflavored ST brands) and if they experience more rapid development of dependence or greater extent of dependence.
There are several limitations to this study, which include the use of convenience sample of nonrepresentative ST users (e.g., intervention seeking ST users meeting specific inclusion and exclusion criteria), thereby limiting the generalizability of the study results. Although the results of this study are not sufficient to provide support for the call of eliminating flavorants in ST products on the grounds that they are a more addictive or harmful product, the data suggest that flavoring, particularly mint, may make the product more appealing, thereby facilitating initiation and maintenance of use. Further research is clearly needed.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (5R01CA135884, R01CA141531, and P50CA13333) and the National Institute on Drug Abuse at the National Institutes of Health (5R01DA014404).
Declaration of Interests
DH was funded by Nabi Biopharmaceuticals and the National Institute on Drug Abuse to be a site for a nicotine immunotherapy trial. There are no other declarations.
References
- Alpert HR, Koh H, Connolly GN. Free nicotine content and strategic marketing of moist snuff tobacco products in the United States: 2000–2006. Tobacco Control. 2008;17:332–338. doi: 10.1136/tc.2008.025247. doi:10.1136/tc.2008.025247. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. State-specific prevalence of cigarette smoking and smokeless tobacco use among adults—United States, 2009. Morbidity & Mortality Weekly Report. 2010;59:1400–1406. doi: mm5943a2 [pii] [PubMed] [Google Scholar]
- Chen C, Isabelle LM, Pickworth WB, Pankow JF. Levels of mint and wintergreen flavorants: Smokeless tobacco products vs. confectionery products. Food and Chemical Toxicology. 2010;48:755–763. doi: 10.1016/j.fct.2009.12.015. doi:10.1016/j.fct.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Dip-time: Brands of Smokeless Tobacco. Retrieved from http://dip-time.tripod.com/smokeless/id1.html. [Google Scholar]
- Dip-time: New Dippers Page. Retrieved from http://dip-time.tripod.com/smokeless/id8.html. [Google Scholar]
- Ebbert JO, Edmonds A, Luo X, Jensen J, Hatsukami DK. Smokeless tobacco reduction with the nicotine lozenge and behavioral intervention. Nicotine & Tobacco Research. 2010;12:823–827. doi: 10.1093/ntr/ntq088. doi:10.1093/ntr/ntq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Ebbert JO, Anderson A, Lin H, Le C, Hecht SS. Smokeless tobacco brand switching: A means to reduce toxicant exposure? Drug and Alcohol Dependence. 2007;87:217–224. doi: 10.1016/j.drugalcdep.2006.08.021. doi:10.1016/j.drugalcdep.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Ebbert JO, Edmonds A, Li C, Lin H, Le C, et al. Smokeless tobacco reduction: Preliminary study of tobacco-free snuff versus no snuff. Nicotine & Tobacco Research. 2008;10:77–85. doi: 10.1080/14622200701704897. doi:10.1080/14622200701704897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Lemmonds CA, Zhang Y, Murphy SE, Le C, Carmella SG, et al. Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. Journal of the National Cancer Institute. 2004;96:844–852. doi: 10.1093/jnci/djh163. doi:10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Hatsukami DK, Zeller M, Peters E. Conference on abuse liability and appeal of tobacco products: Conclusions and recommendations. Drug and Alcohol Dependence. 2011;116:1–7. doi: 10.1016/j.drugalcdep.2010.12.009. doi:10.1016/j.drugalcdep.2010.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Information about chew and different brands. Retrieved from http://web.archive.org/web/20091022194547/ http://www.geocities.com/CollegePark/Library/9349/info.html. [Google Scholar]
- Keller M. Smokeless tobacco on the rise. 2010. Retrieved from http://www.specialty-retailing.com/ME2/Audiences/dirmod.asp?sid=C18F5EF3341948C3A17BD06E85D68045&nm=News+and+Features&type=MultiPublishing&mod=PublishingTitles&mid=8F3A7027421841978F18BE895F87F791&tier=4&id=4B93237E32BF45E794279DA8B1EC1F56&AudId=F67D06B89FCE4844AAF38E40C9F62E01. [Google Scholar]
- Pechacek TF. CDC Congressional Testimony. Smokeless tobacco: Impact on the health of our nation’s youth and use in major league baseball. 2010. United States House of Representatives Energy and Commerce Committee. Washington, DC: Centers for Disease Control and Prevention. [Google Scholar]
- Reid K. Less is more: New flavors, discounting and pouches continue to drive smokeless tobacco’s growth. 2005. Retrieved from http://web.archive.org/web/20070526224100/ http://www.tobaccoretailer.com/uploads/features/2005/0506_operations.asp. [Google Scholar]
- Schiller K, Luo X, Anderson A, Jensen J, Hatsukami D. Comparing an immediate cessation versus reduction approach to smokeless tobacco cessation. Nicotine & Tobacco Research. doi: 10.1093/ntr/ntr302. 2012. Advance online publication. doi:10.1093/ntr/ntr302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacco Products Scientific Advisory Committee. Menthol cigarettes and the public health: Review of the scientific evidence and recommendations. Washington, DC: U.S. Food and Drug Administration; 2011. [Google Scholar]
- U.S. Department of Health & Human Services. How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease. A report of the Surgeon General. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [PubMed] [Google Scholar]
- United States Tobacco Company. Skoal. 2008. Retrieved from http://web.archive.org/web/20080419071526/ http://www.ustinc.com/smokeless/skoal/ [Google Scholar]
- World Health Organization. The Scientific basis of tobacco product regulation: Report of a WHO study group. 2007. WHO Technical Report Series 945. Geneva, Switzerland: Author. [Google Scholar]


