Abstract
Introduction:
The prevalence of smoking among people with schizophrenia in the United States is about 3 times that of the general population. Novel approaches are needed to reduce rates of smoking-related morbidity and mortality among these smokers.
Methods:
This study used a within-subjects design to investigate the separate and combined effects of sensorimotor replacement for smoking (very low nicotine content [VLNC] cigarettes vs. no cigarettes) and transdermal nicotine replacement (42 mg nicotine [NIC] vs. placebo [PLA] patches) in smokers with schizophrenia (SS; n = 30) and control smokers without psychiatric illness (CS; n = 26). Each session contained a 5-hr controlled administration period in which participants underwent the following conditions, in counterbalanced order: VLNC + NIC, VLNC + PLA, no cigarettes + NIC, no cigarettes + PLA, usual-brand cigarettes + no patches. Next, participants completed measures of cigarette craving, nicotine withdrawal, smoking habit withdrawal, and cigarette subjective effects, followed by a 90-min period of ad libitum usual-brand smoking.
Results:
Smoking VLNC cigarettes during the controlled administration periods reduced cigarette craving, nicotine withdrawal symptoms, habit withdrawal symptoms, and usual-brand smoking in SS and CS relative to the no cigarette conditions. VLNC cigarettes were well accepted by both groups and did not affect psychiatric symptom levels in SS. Transdermal nicotine significantly reduced cigarette craving but did not affect usual-brand smoking.
Conclusions:
These findings suggest that reducing the nicotine content of cigarettes to nonaddictive levels may be a promising approach for reducing nicotine dependence among people with schizophrenia.
Introduction
The prevalence of smoking among people with schizophrenia in the United States is about 60%, which is 3 times that of the general population (de Leon & Diaz, 2005; McClave et al., 2010). Schizophrenia is associated with a 20% reduction in lifespan, due primarily to cardiovascular and other smoking-related illnesses (Brown, Kim, Mitchell, & Inskip, 2010; Hennekens, Hennekens, Hollar, & Casey, 2005; Parks, Svendsen, Singer, Foti, & Mauer, 2006; Saha, Chant, & McGrath, 2007). Although almost 40% of smokers with schizophrenia (SS) in the 2007 National Health Interview Survey reported having tried to quit smoking in the past year (McClave et al., 2010), SS have high relapse rates even when they have access to combined pharmacological and psychosocial smoking treatments (e.g., Evins et al., 2007; George et al., 2002). Thus, there is a need for innovative and effective approaches to improving smoking cessation rates among these smokers.
A recent human laboratory study found that acute use of 42 mg transdermal nicotine replacement reduced nicotine withdrawal symptoms but not usual-brand smoking in SS (Tidey, Rohsenow, Swift, Kaplan, & Adolfo, 2008), suggesting that complementary strategies may be needed to reduce smoking in this population. Very low nicotine content (VLNC) cigarettes (i.e., cigarettes with less than 0.2 mg Federal Trade Commission nicotine yield) provide sensorimotor replacement for usual-brand smoking by imitating the taste, aroma, and respiratory effects of normal nicotine content cigarettes. Among smokers without psychiatric illness, VLNC cigarettes consistently reduce cigarette craving, negative affect, and usual-brand smoking in laboratory and treatment studies (e.g., Buchhalter, Acosta, Evans, Breland, & Eissenberg, 2005; Butschky, Bailey, Henningfield, & Pickworth, 1995; Dallery, Houtsmuller, Pickworth, & Stitzer, 2003; Gross, Lee, & Stitzer, 1997; Johnson, Bickel, & Kirshenbaum, 2004; Pickworth, Fant, Nelson, Rohrer, & Henningfield, 1999; Rose, Behm, Westman, & Johnson, 2000; Rose, Behm, Westman, Bates, & Salley, 2003; Westman, Behm, & Rose, 1996). As sensorimotor stimuli that are repeatedly associated with nicotine delivery acquire, through classical conditioning processes, conditioned reinforcing effects that help to maintain smoking (Palmatier et al., 2007; Rose & Levin, 1991), protracted use of VLNC cigarettes in combination with nicotine replacement may improve smoking cessation rates by uncoupling the pharmacological effects of nicotine from these sensorimotor stimuli, thus facilitating the extinction of these conditioned reinforcing effects (e.g., Becker, Rose, & Albino, 2008; Donny & Jones, 2009). Two studies have compared the effects of VLNC versus high nicotine cigarettes on psychiatric symptoms and cognitive performance in SS (Smith, Infante, Ali, & Nigam, 2001; Smith, Singh, Infante, Khandat, & Kloos, 2002). However, neither study investigated the effects of VLNC cigarettes on cigarette craving, nicotine withdrawal, or smoking behavior. To our knowledge, the current study is unique in its focus on the effects of VLNC cigarettes on smoking measures in SS.
The current study investigated the separate and combined effects of acute nicotine replacement and sensorimotor smoking replacement, in the form of VLNC cigarettes, on cigarette craving, withdrawal symptoms, and usual-brand smoking in SS and control smokers (CS). We hypothesized that smoking VLNC cigarettes would reduce cigarette craving, nicotine withdrawal symptoms, smoking habit withdrawal symptoms, and usual-brand cigarette smoking in both SS and CS, and that nicotine replacement would enhance these effects.
Methods
Participants
Participants were required to either have a diagnosis of schizophrenia or schizoaffective disorder (SS) or no Axis I disorder (CS), based on the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1994), to be at least 18-years old, to have smoked 20–50 cigarettes/day for at least the past year, to have a score of at least 6 on the Fagerström Test for Nicotine Dependence (FTND), indicating a high level of dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), and to indicate an interest in quitting smoking someday. Exclusionary criteria included medical conditions contraindicating transdermal nicotine use, severe levels of disorientation or uncooperativeness, positive urine drug or pregnancy tests at baseline, or positive breath alcohol level at any session. Procedures were approved by the Institutional Review Board of Brown University. Thirty-seven SS and 38 CS enrolled, and 30 SS and 26 CS completed the study.
Design Overview
A within-subjects design was used to investigate the separate and combined effects of sensorimotor replacement for smoking (VLNC cigarettes vs. no cigarettes) and transdermal nicotine replacement (42 mg nicotine [NIC] vs. placebo [PLA] patches) in SS and CS. In Session 1, participants completed individual difference measures and practiced smoking through the topography measurement device. In Session 2, participants smoked their usual cigarettes ad libitum for 5 hr through the topography device so that the rate and timing of their smoking behavior could be determined. In Sessions 3–7, participants underwent the following conditions during 5-hr controlled administration periods, with order counterbalanced across participants: VLNC + NIC, VLNC + PLA, no cigarettes + NIC, no cigarettes + PLA, usual brand cigarettes + no patches. After the 5-hr controlled administration periods, participants completed the subjective measures described below, followed by a 90-min period of ad libitum usual-brand smoking. Participants were compensated $500 for completing the study.
Procedure
All sessions began at approximately 9 a.m. Upon arrival for each session, participants provided breath samples for the assessment of expired-air carbon monoxide (CO) levels (Smokerlyzer, Bedfont Scientific Ltd., Kent, U.K.). In Session 1, participants completed the individual difference measures described below and then were asked to smoke 1 cigarette of their usual brand using the smoking topography measurement device (laboratory-based Clinical Research Support System [CReSS], Borgwaldt KC, Richmond, VA) in order to habituate to smoking through the CReSS mouthpiece. The CReSS, which was calibrated before each session, provides the following smoking variables: puffs per cigarette, puff volume, puff duration, maximum flow (peak puff intensity), and inter-puff-interval. In Session 2, participants smoked their usual-brand cigarettes using the CReSS equipment for 5 hr so that characteristics of their typical smoking behavior could be measured.
In Sessions 3–7, participants underwent the following conditions during 5-hr controlled administration periods, with order counterbalanced across participants: VLNC + NIC, VLNC + PLA, no cigarettes + NIC, no cigarettes + PLA, usual brand cigarettes + no patches. PLA or NIC patches (GlaxoSmithKline, Parsippany, NJ) were applied to participants’ upper arms (one per arm, for a total of 0 or 42 mg NIC) under double-blind conditions. The VLNC cigarettes used in this study (Quest 3; Vector Tobacco, Timberlake, NC) contained less than 0.05 mg nicotine and 10 mg tar. Usual-brand cigarettes were provided by the experimenters. During the 5-hr controlled administration periods, all cigarettes were smoked through the CReSS, and participants were cued to smoke according to the rate and timing of their smoking during Session 2. Participants were also able to read magazines and watch videos and were provided with a light lunch. Participants were under continuous observation throughout the sessions.
After the 5-hr controlled administration periods, cigarette craving levels, nicotine withdrawal symptoms, smoking habit withdrawal symptoms, cigarette acceptability, and psychiatric symptoms (in SS) were assessed using the measures described below. Assessments of cognitive performance and cigarette demand were also administered but are not described here. Next, breath CO level was measured and participants were instructed that they could smoke as little or as much as they wanted of their usual-brand cigarettes through the CReSS for the next 90 min. At the end of the smoking period, breath CO level was measured and patches were removed.
Measures
Baseline Characteristics
Individual difference measures collected in Session 1 included demographic characteristics, smoking history, and the Contemplation Ladder (Biener & Abrams, 1991), a 10-point scale that measures motivation to quit smoking. In SS, current psychiatric symptom levels were measured using the Positive and Negative Syndrome Scale (Kay, Fiszbein, & Opler, 1987). All participants provided a saliva sample for cotinine analysis (Salimetrics LLC, State College, PA).
Sessions 3–7: Behavioral Measures
The primary measure of smoking during the 90-min ad libitum usual-brand smoking periods was CO boost (CO level at the end of the 90-min ad libitum period minus CO level before the 90-min ad libitum period) as this was the most sensitive measure of smoking behavior in a previous study (Tidey et al., 2008). The secondary measure of smoking behavior was total volume of puffs smoked during the 90-min ad libitum period as prior studies indicated the sensitivity of this measure to VLNC cigarettes and nicotine replacement in nonpsychiatric smokers (Rose et al., 2003; Strasser, Lerman, Sanborn, Pickworth, & Feldman, 2007).
Sessions 3–7: Subjective Measures
Self-report measures of urge to smoke, nicotine withdrawal symptoms, smoking habit withdrawal symptoms and subjective effects of cigarettes were administered electronically. Urge to smoke was assessed using the Questionnaire on Smoking Urges-brief form (QSU-brief; Cox, Tiffany, & Christen, 2001) and the item “How much is your urge to smoke right now?”, which was rated on a 100 mm visual analogue scale with the anchors 0 = no urge at all and 100 = strongest urge you’ve ever had. Nicotine withdrawal symptoms were measured using the 8-item Minnesota Nicotine Withdrawal Scale (MNWS), with the insomnia item omitted as participants did not undergo overnight abstinence, and the craving item omitted to allow independent assessment of withdrawal versus craving (Hughes & Hatsukami, 1986, 1998). Each symptom was rated from 0 (not present) to 4 (severe), and a total symptom score was calculated by averaging scores of each item. Smoking habit withdrawal was measured using two items: “missed something to do with hands” and “missed having something in the mouth,” rated from 1 (not at all) to 7 (extremely; Rose, Behm, Westman, & Johnson, 2000). Subjective effects of cigarettes were measured using the Cigarette Effects Scale (CES; Rose et al., 2000), which consists of 11 items, rated from 0 (not at all) to 7 (extremely), forming the subscales Satisfaction, Psychological Reward, Nausea/Dizziness, Craving Relief, and Enjoyment of Airway Sensations. Finally, a trained rater assessed psychiatric symptoms in SS using the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962), an 18-item measure of positive and negative schizophrenia symptoms.
Data Analysis
Group comparisons on demographic and smoking history measures were conducted using independent-samples t tests for continuous variables and chi-square tests for categorical variables. CO boosts and total volume of puffs smoked during the 5-hr controlled administration periods were compared using 2 × 3 analyses of variance tests (ANOVAs) with the factors Diagnosis (SS, CS) and Cigarette Condition (VLNC + NIC, VLNC + PLA, usual brand). Comparisons of the effects of nicotine and sensorimotor replacement on QSU-brief, MNWS, Habit Withdrawal, and usual-brand smoking in SS and CS were conducted using mixed-factor 2 × 2 × 2 ANOVAs with the between-groups factor Diagnosis (SS, CS), and the within-subjects factors Nicotine Replacement (NIC, PLA) and Sensorimotor Replacement (VLNC cigarettes, No cigarettes). In addition, 2 × 2 ANOVAs were conducted to compare the NIC + VLNC and usual brand conditions in SS and CS. Comparisons of the subjective effects of VLNC, with and without nicotine replacement, and usual-brand cigarettes were conducted using 2 x 3 ANOVAs with the factors Diagnosis (SS, CS) and Cigarette Condition (VLNC + NIC, VLNC + PLA, usual brand). Effects of nicotine and sensorimotor replacement on BPRS scores in SS were analyzed using 2 × 2 ANOVAs, and t tests were conducted to compare BPRS scores from the VLNC + NIC and usual brand conditions. Analyses were conducted using PASW Statistics 17.0 for Windows (SPSS, Inc.). Differences were considered significant when p ≤ .05. Effect sizes (Cohen’s d) are also provided when p = .05–.10 (Cohen, 1988). Significant interactions were followed by simple effects tests. Due to technical errors or malfunctioning equipment, breath CO data were incomplete from two CS, subjective measures were incomplete from four SS and two CS, and puff volume data were incomplete from four SS and eight CS.
Results
Sample Characteristics
The groups did not differ significantly on any demographic or smoking history measure (Table 1). Overall, participants were 45.2 ± 9.5 (M ± SD) years old, 43% female, 70% White, 19% African American, and had completed 12.1 ± 2.0 years of education. At enrollment, participants smoked 25.1 ± 8.4 cigarettes/day, had been smoking daily for 28.2 ± 10.0 years, had FTND scores of 6.9 ± 1.7, indicating high levels of nicotine dependence, and had Contemplation Ladder scores of 4.9 ± 1.9, indicating that they were thinking about quitting smoking but did not have immediate plans to quit. SS were clinically stable with low to moderate psychiatric symptom levels, similar to those reported by other studies of smoking in SS (e.g., Fonder et al., 2005; George et al., 2000).
Table 1.
Baseline Characteristics of Study Participants
| SS (n = 30) | CS (n = 26) | |
| Age, M (SD) | 46.2 (8.0) | 44.5 (10.9) |
| Male (%) | 60 | 58 |
| Race (%) | ||
| White | 77 | 65 |
| African American | 13 | 23 |
| Hispanic ethnicity | 4 | 0 |
| Employed full- or part-time (%) | 7 | 23 |
| Years of education | 11.9 (2.2) | 12.2 (1.9) |
| Cigarettes per day | 25.9 (9.9) | 23.5 (6.5) |
| Nicotine dependence (FTND score) | 6.9 (1.5) | 6.7 (1.8) |
| Contemplation Ladder Score | 5.1 (2.0) | 4.7 (1.8) |
| Years of daily smoking | 27.4 (8.3) | 28.4 (11.9) |
| Salivary cotinine level (ng/ml) | 454 (267) | 434 (240) |
| Baseline CO level (ppm) | 30.6 (20.6) | 27.3 (15.2) |
| PANSS positive scale score | 11.6 (4.1) | |
| PANSS negative scale score | 14.6 (6.4) | |
| PANSS general scale score | 25.9 (5.9) | |
| PANSS total score | 52.1 (14.6) | |
| Antipsychotic drug class | 67% Atyp, 17% Typ, 10% both types |
Note. SS = smokers with schizophrenia; CS = control smokers; FTND = Fagerström Test for Nicotine Dependence; CO = expired-air carbon monoxide; PANSS = Positive and Negative Syndrome Scale; Atyp = atypical; Typ = typical.
Smoking During the 5-hr Controlled Administration Periods
SS had higher smoke intake levels than CS during the 5-hr controlled administration periods, based on both CO boost (F(1, 54) = 4.00, p = .05) and total puff volume (F(1, 44) = 16.39, p < .01). Average CO boosts from the controlled administration periods were 15.7 ± 13.8 ppm in SS and 10.1 ± 11.5 ppm in CS; average total puff volumes from these periods were 7,145 ± 3,995 ml in SS and 3,574 ± 1,641 ml in CS. There was a significant main effect of Cigarette Condition on total puff volume from the controlled administration periods (F(2, 88) = 3.91, p < .05), with post-hoc comparisons indicating that average total puff volume from the VLNC + NIC condition (4,969 ± 3,594 ml) was significantly lower (p < .01) than that from the usual brand condition (5,841 ± 4,004 ml), whereas the total puff volume from the VLNC + PLA condition (5,268 ± 3,309 ml) was intermediate and did not significantly differ from either of those conditions. There was no significant effect of cigarette type on CO boost and no significant interaction between diagnosis and cigarette type on either measure.
Subjective Effects of Sensorimotor and Nicotine Replacement
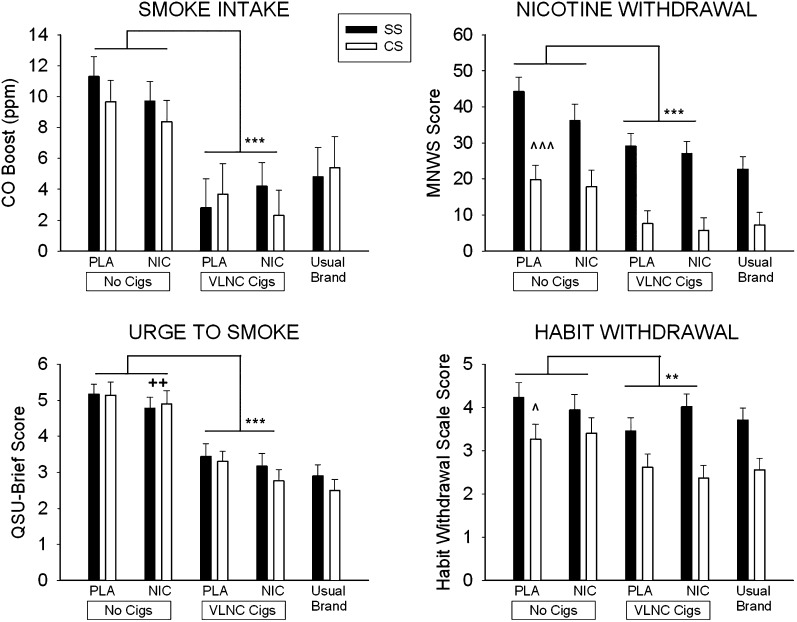
Figure 1 shows the effects of sensorimotor and nicotine replacement on QSU-brief, MNWS, and Habit Withdrawal scores in SS and CS. SS reported significantly higher MNWS and Habit Withdrawal scores than CS (F(1, 50) = 22.10, p < .001; F(1, 50) = 6.84, p < .05; respectively). Averaged across nicotine and sensorimotor replacement conditions, MNWS scores were 34.1 ± 23.1 in SS and 12.8 ± 12.7 in CS, and Habit Withdrawal scores were 3.9 ± 1.5 in SS and 2.9 ± 1.8 in CS. There was no effect of diagnosis on QSU-brief score.
Figure 1.
Effects of 5-hr exposure to 42 mg transdermal nicotine (NIC) or placebo patches (PLA) and very low nicotine content cigarettes (VLNC Cigs) or no cigarettes (No Cigs) on CO boost, urge to smoke, nicotine withdrawal symptoms and habit withdrawal symptoms in smokers with schizophrenia (SS; solid bars) and control smokers without psychiatric illness (CS; open bars). Bars represent M ± SEM. Significant main effects of sensorimotor replacement are indicated with asterisks (**p < .01; *** p < .001), significant main effects of nicotine replacement are indicated with plus signs (++ p < .01), and significant main effects of group are indicated with carets (^ p < .05; ^^^ p < .001).
There were significant main effects of sensorimotor replacement on QSU-brief, MNWS, and Habit Withdrawal scores (F(1, 50) = 84.75, p < .001; F(1, 50) = 38.77, p < .001; F(1, 50) = 13.36, p < .01; respectively), with lower scores from the VLNC cigarette conditions compared with the no cigarette conditions. Averaged across diagnostic groups and nicotine replacement conditions, QSU-brief scores were 5.0 ± 1.5 in the no cigarette and 3.2 ± 1.8 in the VLNC cigarette condition, MNWS scores were 29.5 ± 24.1 in the no cigarette and 17.3 ± 20.8 in the VLNC cigarette condition, and Habit Withdrawal scores were 3.7 ± 1.8 in the no cigarette and 3.1 ± 1.6 in the VLNC cigarette condition. There were no significant interactions between diagnosis and sensorimotor replacement on these measures.
There was a significant main effect of nicotine replacement on QSU-brief score (F(1, 50) = 8.56, p < .01), and the effect of nicotine replacement on MNWS score approached significance (F(1, 50) = 3.21, p = .08; d = 0.16). Averaged across diagnostic groups and sensorimotor replacement conditions, QSU-brief scores were 3.9 ± 1.7 in the NIC condition and 4.3 ± 1.6 in the PLA condition, and MNWS scores were 21.7 ± 22.32 in the NIC condition and 25.2 ± 22.52 in the PLA condition. There were no significant Diagnosis × Nicotine Replacement or Sensorimotor Replacement × Nicotine Replacement interactions on these measures. However, there was a significant Diagnosis × Sensorimotor Replacement × Nicotine Replacement interaction effect on Habit Withdrawal score (F(1, 50) = 5.44, p < .05). Separate 2 × 2 ANOVAs conducted within each diagnostic group indicated that, in SS, there was a significant Nicotine Replacement × Sensorimotor Replacement interaction on Habit Withdrawal Scale score (F(1, 25) = 5.75, p < .05), and simple effects tests showed that in SS sensorimotor replacement reduced Habit Withdrawal Scale scores within PLA conditions (t (25) = 3.52, p < .01) but not NIC conditions (Figure 1). In CS, sensorimotor replacement reduced Habit Withdrawal Scale scores (F (1, 25) = 12.20, p < .005), with no main effect of Nicotine Replacement or interaction between these factors. There were no significant differences between the VLNC + NIC and usual-brand conditions on any of these measures.
Effects of Sensorimotor and Nicotine Replacement on Usual-Brand Smoking
Effects of sensorimotor and nicotine replacement on CO boost from the 90-min ad libitum usual-brand smoking periods are shown in Figure 1. There was no effect of diagnosis on CO boost from the ad libitum usual-brand smoking periods (SS: 6.98 ± 1.0 ppm; CS: 5.98 ± 1.1 ppm), however, there was a significant effect of diagnosis on total volume smoked during the ad libitum usual-brand smoking periods, with higher total puff volume in SS than CS (F(1, 44) = 8.81, p < .01; SS: 2,536 ± 1,656 ml; CS: 1,451 ± 835 ml; not shown). There were significant main effects of sensorimotor replacement on both CO boost and total volume smoked during the ad libitum usual-brand smoking periods (F(1, 54) = 47.05, p < .001; F(1, 44) = 12.93, p < .01, respectively). Means indicated that smoking VLNC cigarettes during the controlled administration periods reduced usual-brand smoking during the ad libitum periods, based on both CO boost (VLNC cigarette: 3.2 ± 0.2 ppm; No cigarette: 9.8 ± 7.1 ppm) and total puff volume (VLNC cigarette: 1,803 ± 1,420 ml; No cigarette: 2,184 ± 1,498 ml). There was no effect of nicotine replacement or significant interactions among factors on CO boost or total puff volume smoked during the ad libitum usual-brand periods.
VLNC Tolerability and Acceptability
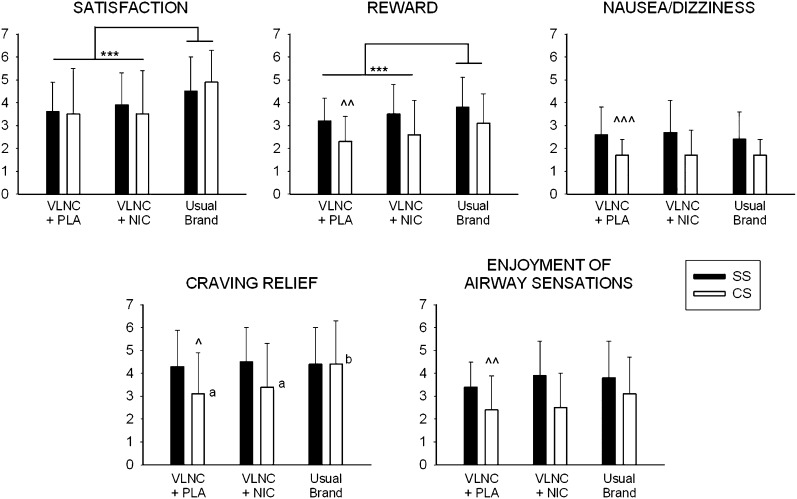
Comparisons between VLNC cigarettes with and without nicotine replacement and usual-brand cigarettes on CES scores are shown in Figure 2. There were significant main effects of Diagnosis on the Reward, Nausea/Dizziness, Craving Relief, and Enjoyment of Airway Sensations subscales of the CES, indicating that across cigarette types, SS provided higher ratings on these measures than CS (F(1, 50) = 7.94, p < .01, F(1, 50) = 16.04, p < .001; F(1, 50) = 4.14, p < .05; F(1, 50) = 10.77, p < .01; respectively). There were significant main effects of cigarette condition on the satisfaction and reward subscales of the CES (F(2, 100) = 12. 63, p < .001; F(2, 100) = 8.63, p < .001; respectively). Post-hoc tests indicated that, averaged across diagnostic groups, usual-brand cigarettes received higher satisfaction and reward ratings than VLNC + PLA or VLNC + NIC. There was a significant main effect of cigarette condition and a significant Diagnosis × Cigarette Condition interaction on craving relief (F(2, 100) = 3.34, p < .05; F(2, 100) = 3.33, p < .05; respectively). Separate ANOVAs and post-hoc tests conducted for each diagnostic group indicated that CS gave higher Craving Relief ratings to usual-brand cigarettes than either VLNC + PLA or VLNC + NIC (F(2, 50) = 6.74, p < .01), however, SS gave similar Craving Relief ratings under all conditions (Figure 2). BPRS scores in SS were not affected by nicotine or sensorimotor replacement and averaged 24.8 ± 6.2 across conditions (not shown).
Figure 2.
Comparisons of usual-brand cigarettes, very low nicotine content cigarettes plus 42 mg transdermal nicotine (VLNC + NIC) and very low nicotine content cigarettes plus placebo patches (VLNC + PLA) on subscales of the Cigarette Effects Scale in smokers with schizophrenia (SS, solid bars) and control smokers (CS, open bars). Bars represent M ± SEM. Significant main effects of cigarette type are indicated with asterisks (**p < .01; *** p < .001), significant main effects of group are indicated with carets (^ p < .05; ^^ p < .01; ^^^ p < .001), and different letters (a, b) represent a significant difference within the CS group only.
Discussion
The results of this study indicate that smoking VLNC cigarettes during the controlled administration periods reduced cigarette craving, nicotine withdrawal symptoms, smoking habit withdrawal symptoms, and usual-brand smoking in both SS and CS. Furthermore, VLNC cigarettes were well tolerated overall, and there was no indication that acute VLNC smoking affected psychiatric symptoms in SS. Transdermal nicotine substituted less effectively than sensorimotor replacement for usual-brand cigarettes under these study conditions, in that it reduced cigarette craving but to a lesser extent than did VLNC cigarettes, and had no effect on usual-brand smoking during the 90-min ad libitum smoking periods. These findings are consistent with previous laboratory reports in smokers without psychiatric illness (e.g., Johnson et al., 2004; Rose et al., 2003; Westman et al., 1996). Overall, the results of this study set the stage for longer-term studies of VLNC cigarettes in SS to determine whether reducing the nicotine yield in cigarettes to nonaddictive levels may be an effective strategy for improving smoking cessation outcomes in this population.
A likely explanation for the results of this study are that VLNC cigarettes provide sensorimotor effects that, after years of being paired with nicotine intake while smoking, have acquired conditioned reinforcing effects (Rose & Levin, 1991). Over longer periods of use, the conditioned reinforcing effects of VLNC cigarettes are expected to extinguish, leading to smoking reductions (Rose & Behm, 2004). The potential problem with this approach is that switching from normal nicotine content to VLNC cigarettes could increase carcinogen exposure if smokers compensate for the reduction in nicotine yield by smoking more intensely (reviewed in Scherer, 1999; Rose 2006). Results from the small number of studies that have examined this question indicate that total volume smoked of VLNC cigarettes increases relative to total volume smoked of higher-nicotine research cigarettes under acute smoking conditions (Strasser et al., 2007), but after 4–6 weeks of VLNC cigarette use, smoking rates, carcinogen biomarkers, and nicotine dependence severity decline, suggesting that compensatory smoking of VLNC cigarettes may not persist beyond a period of weeks or months in smokers without serious mental illness (Donny, Houtsmuller, & Stitzer, 2007; Donny & Jones, 2009; Hatsukami, Kotlyar, et al., 2010).
Although these results are promising, several differences between SS and CS suggest caution when generalizing the results of longer-term VLNC cigarette studies from CS to SS. First, despite reporting similar smoking rates and nicotine dependence levels at enrollment, SS in this study had higher levels of smoke intake from usual-brand and VLNC cigarettes than CS during the 5-hr controlled administration periods. This finding is consistent with previous reports from several laboratories that SS smoke more intensely than CS (Tidey, Rohsenow, Kaplan, & Swift, 2005; Tidey et al., 2008, Weinberger et al., 2007, Williams et al., 2005, 2011). Although the functional significance of their higher smoking intensity is unknown, it supports the hypotheses that the neuropathology of schizophrenia confers stronger reinforcing effects of nicotine in SS (Novak et al., 2010) and that beneficial cognitive effects of nicotine contribute to more intense tobacco use in SS (Martin & Freedman, 2007). Their more intense smoking of VLNC cigarettes in this study suggests that SS could be more prone to compensatory smoking than CS. Notably, we found that 42 mg transdermal nicotine reduced VLNC total puff volume in both SS and CS during the 5-hr controlled administration periods. This is consistent with findings from longer-term studies in nonpsychiatric smokers indicating that nicotine replacement reduces compensatory smoking (Becker et al., 2008; Donny & Jones, 2009).
Another difference between SS and CS that is relevant to the potential success of a treatment approach incorporating VLNC cigarettes is that SS were less sensitive to the effects of VLNC cigarettes on smoking habit withdrawal severity. This finding indicates that VLNC cigarettes substituted less effectively for normal-nicotine content cigarettes in SS compared with CS, and suggests that SS could be less compliant with longer-term VLNC cigarette use. On the other hand, the finding that SS found VLNC cigarettes to be as effective as their usual brand in relieving cigarette craving suggests that, if they are compliant with longer-term VLNC cigarette use, these cigarettes may be an effective method of reducing their craving for normal-nicotine content cigarettes.
Smith and colleagues have conducted two previous studies of acute VLNC cigarette effects in SS (Smith et al., 2001, 2002). The initial study compared the effects of VLNC and high-nicotine content cigarettes on schizophrenia symptoms in 15 SS who underwent 6–12 hr smoking abstinence. Pre- versus postsmoking assessments indicated that smoking either VLNC or high-nicotine content cigarettes decreased negative symptoms to a similar extent; positive symptoms were unchanged (Smith et al., 2001). A subsequent study by this group examined the separate and combined acute effects of sensorimotor (VLNC or high-nicotine cigarettes) and nicotine replacement (nicotine or placebo nasal spray) on schizophrenia symptoms and cognitive performance in 31 SS who underwent 10–12 hr smoking abstinence (Smith et al., 2002). Consistent with their previous report, both VLNC and high-nicotine cigarettes improved negative symptoms, although the high-nicotine cigarette decreased negative symptoms to a greater extent, and positive symptoms were not affected. Cigarette type did not differentially affect cognitive task performance. In contrast, nicotine nasal spray improved cognitive performance to a greater extent than did placebo spray, but spray type did not differentially affect negative symptom levels. The current study replicates the findings of Smith et al. (2001, 2002) that acute use of VLNC cigarettes without concurrent nicotine replacement does not appear to adversely impact psychiatric symptoms.
Taking the above considerations into account, it is clear that the next step of this line of research should be to examine a longer period of VLNC cigarette use on measures of compliance, nicotine intake, carcinogen exposure, cognitive functioning, and psychiatric symptoms in SS. Given that cigarette craving levels and number of cigarettes smoked per day were reduced by approximately one-third in nonpsychiatric smokers after 6 weeks of VLNC smoking (Hatsukami, Kotlyar, et al., 2010), a 6-week period of VLNC cigarette use could provide an indication of whether this strategy may be effective in SS. Reducing the nicotine content of cigarettes to nonaddictive levels has been proposed as a public health approach for significantly reducing smoking-related morbidity and mortality in the United States (Benowitz & Henningfield, 1994; Hatsukami, Perkins, et al., 2010). The adoption of this strategy is now possible due to the passage of the 2009 Family Smoking Prevention and Tobacco Control Act, which gave the Food and Drug Administration the authority to reduce the level of nicotine in cigarettes. The findings of the current study suggest that this strategy may have potential for reducing smoking-related morbidity and mortality among SS. However, it is essential to examine the effects of VLNC cigarettes in SS over a longer period of time before this strategy can be fully considered.
Funding
This work was supported by National Institutes of Health grants R01DA14002 and P50DA031659 (JWT) and a Senior Research Career Scientist Award from the Department of Veterans Affairs (DJR).
Declaration of Interests
The authors have no conflicts of interest to report.
Acknowledgments
We thank Laura Dionne and Jennifer Schmidlin for their assistance with data management; Amy Adolfo Signore, Vera Mayercik, Netesha Reid, and Emily Xavier for their assistance with data collection; and the individuals who participated in this study for their contributions. This work was conducted at the Brown University Center for Alcohol and Addiction Studies, Providence, RI. Portions of these data were presented at annual meetings of the College on Problems of Drug Dependence and the Society for Research on Nicotine and Tobacco.
References
- Becker KM, Rose JE, Albino AP. A randomized trial with nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine & Tobacco Research. 2008;10:1139–1148. doi: 10.1080/14622200802123294. doi:10.1080/14622200802123294. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. New England Journal of Medicine. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. doi:10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. doi:10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. British Journal of Psychiatry. 2010;196:116–121. doi: 10.1192/bjp.bp.109.067512. doi:10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: The role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. doi:10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Butschky MF, Bailey D, Henningfield JE, Pickworth WB. Smoking without nicotine delivery decreases withdrawal in 12-hour abstinent smokers. Pharmacology, Biochemistry, and Behavior. 1995;50:91–96. doi: 10.1016/0091-3057(94)00269-o. doi:10.1016/0091-3057(94)00269-O. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU-Brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. doi:10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dallery J, Houtsmuller EJ, Pickworth WB, Stitzer ML. Effects of cigarette nicotine content and smoking pace on subsequent craving and smoking. Psychopharmacology (Berl) 2003;165:172–180. doi: 10.1007/s00213-002-1242-8. doi:10.1007/s00213-002-1242-8. [DOI] [PubMed] [Google Scholar]
- De Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. doi:10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: Behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. doi:10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug and Alcohol Dependence. 2009;104:23–33. doi: 10.1016/j.drugalcdep.2009.01.021. doi:10.1016/j.drugalcdep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12-week double-blind, placebo-controlled study of bupropion SR added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. Journal of Clinical Psychopharmacology. 2007;27:380–386. doi: 10.1097/01.jcp.0b013e3180ca86fa. doi:10.1097/01.jcp.0b013e3180ca86fa. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis-I disorders—Patient edition. (SCID -I/P, Version 2.0) New York: Biometric Research Department; 1994. [Google Scholar]
- Fonder MA, Sacco KA, Termine A, Boland BS, Seyal AA, Dudas MM, et al. Smoking cue reactivity in schizophrenia: Effects of a nicotinic receptor antagonist. Biological Psychiatry. 2005;57:802–808. doi: 10.1016/j.biopsych.2004.12.027. doi:10.1016/j.biopsych.2004.12.027. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biological Psychiatry. 2002;52:53–61. doi: 10.1016/s0006-3223(02)01339-2. doi:10.1016/S0006-3223(02)01339-2. [DOI] [PubMed] [Google Scholar]
- George TP, Ziedonis DM, Feingold A, Pepper WT, Satterburg CA, Winkel J, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. American Journal of Psychiatry. 2000;157:1835–1842. doi: 10.1176/appi.ajp.157.11.1835. doi:10.1176/appi.ajp.157.11.1835. [DOI] [PubMed] [Google Scholar]
- Gross J, Lee J, Stitzer ML. Nicotine-containing versus de-nicotinized cigarettes: Effects on craving and withdrawal. Pharmacology, Biochemistry, and Behavior. 1997;57:159–165. doi: 10.1016/s0091-3057(96)00309-7. doi:10.1016/S0091-3057(96)00309-7. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, et al. Reduced nicotine content cigarettes: Effects on toxicant exposure, dependence and cessation. Addiction. 2010a;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. doi:10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Perkins KA, LeSage MG, Ashley DL, Henningfield JE, Benowitz NL, et al. Nicotine reduction revisited: Science and future directions. Tobacco Control. 2010b;19:e1–e10. doi: 10.1136/tc.2009.035584. doi:10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. American Heart Journal. 2005;150:1115–1121. doi: 10.1016/j.ahj.2005.02.007. doi:10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. doi:10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. doi:10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Kirshenbaum AP. Substitutes for tobacco smoking: A behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes. Drug and Alcohol Dependence. 2004;74:253–264. doi: 10.1016/j.drugalcdep.2003.12.012. doi:10.1016/j.drugalcdep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. doi:10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Martin LF, Freedman R. Schizophrenia and the alpha-7 nicotinic acetylcholine receptor. International Review of Neurobiology. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. doi:10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- McClave AK, Whitney N, Thorne SL, Mariolis P, Dube SR, Engstrom M Centers for Disease Control and Prevention (CDC) Adult tobacco survey—19 states, 2003–2007. MMWR. Surveillance Summaries: Morbidity and Mortality Weekly Report. 2010;59:1–75. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5903a1.htm. [PubMed] [Google Scholar]
- Novak G, LeBlanc M, Zai C, Shaikh S, Renou J, DeLuca V, et al. Association of polymorphisms in the BDNF, DRD1 and DRD3 genes with tobacco smoking in schizophrenia. Annals of Human Genetics. 2010;74:291–298. doi: 10.1111/j.1469-1809.2010.00578.x. doi:10.1111/j.1469-1809.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. doi:10.2466/pr0.1962.10.3.799. [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology. 2007;195:235–243. doi: 10.1007/s00213-007-0897-6. doi:10.1007/s00213-007-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks J, Svendsen D, Singer P, Foti M, Mauer B. Morbidity and mortality in people with serious mental illness. National Association of State Mental Health Program Directors; 2006. Retrieved from http://www.nasmhpd.org/general_files/publications/med_directors_pubs/Technical%20Report%20on%20Morbidity%20and%20Mortaility%20-%20Final%2011-06.pdf. [Google Scholar]
- Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine & Tobacco Research. 1999;1:357–364. doi: 10.1080/14622299050011491. doi:10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. doi:10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: Pharmacological and behavioral treatments. Nicotine & Tobacco Research. 2004;6:523–532. doi: 10.1080/14622200410001696501. doi:10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED. Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. British Journal of Addiction. 1991;86:605–609. doi: 10.1111/j.1360-0443.1991.tb01816.x. doi:10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacology, Biochemistry, and Behavior. 2003;76:243–250. doi: 10.1016/j.pbb.2003.07.002. doi:10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacology, Biochemistry, and Behavior. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. doi:10.1016/S0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Archives of General Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. doi:10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Scherer G. Nicotine and compensation: A review of the literature. Psychopharmacology. 1999;145:1–20. doi: 10.1007/s002130051027. doi:10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- Smith RC, Infante M, Ali A, Nigam S. Effects of cigarette smoking on psychopathology scores in schizophrenic patients. An experimental study. Substance Abuse. 2001;22:175–185. doi: 10.1080/08897070109511457. doi:10.1080/08897070109511457. [DOI] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. doi:10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug and Alcohol Dependence. 2007;86:294–300. doi: 10.1016/j.drugalcdep.2006.06.017. doi:10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Swift RM, Kaplan GB, Adolfo AB. Effects of smoking abstinence, smoking cues and nicotine replacement in smokers with schizophrenia and controls. Nicotine & Tobacco Research. 2008;10:1047–1056. doi: 10.1080/14622200802097373. doi:10.1080/14622200802097373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched nonpsychiatric controls. Drug and Alcohol Dependence. 2005;80:259–265. doi: 10.1016/j.drugalcdep.2005.04.002. doi:10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Sacco KA, Creeden CL, Vessicchio JC, Jatlow PI, George TP. Effects of acute abstinence, reinstatement, and mecamylamine on biochemical and behavioral measures of cigarette smoking in schizophrenia. Schizophrenia Research. 2007;91:217–225. doi: 10.1016/j.schres.2006.12.007. doi:10.1016/j.schres.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacology, Biochemistry, and Behavior. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. doi:10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Lu S.-E., Kumar S, Steinberg ML, Cottler B, et al. Shorter interpuff interval is associated with higher nicotine intake in smokers with schizophrenia. Drug and Alcohol Dependence. 2011;118:313–319. doi: 10.1016/j.drugalcdep.2011.04.009. doi:10.1016/j.drugalcdep.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophrenia Research. 2005;79:323–335. doi: 10.1016/j.schres.2005.04.016. doi:10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]