Abstract
Introduction:
Varenicline (Chantix®) is an efficacious first-line medication for smoking cessation. Studies suggest that one mechanism by which varenicline facilitates sustained smoking abstinence is by reducing the likelihood of relapse to smoking when a lapse, or slip, occurs during a quit attempt. The present study extends this line of research by conducting a prospective laboratory study to examine the relapse prevention effects of varenicline following a programmed lapse.
Methods:
Daily smokers (N = 47) completed a 5-week outpatient study in which they were randomized to receive varenicline or placebo. The first week was a medication induction period that was immediately followed by a 4-week quit attempt. A programmed lapse (2 cigarettes smoked in the laboratory) occurred on the second day of the quit attempt.
Results:
Participants receiving varenicline were slower to relapse and had greater total abstinence rates following lapse exposure. Participants in the varenicline group rated lapse cigarettes lower on measures of reward and intoxication and showed increased behavioral economic demand elasticity for cigarettes (reduced cigarette purchasing at higher prices) compared with those receiving placebo.
Conclusions:
These results demonstrate a relapse prevention effect of varenicline following smoking lapse exposure and suggest that an attenuation of reward from smoking and the blunting of subjective effects of smoking may underlie and/or contribute to this effect.
Introduction
Nearly half of all smokers in the United States (44.2%) report making a quit attempt annually (Centers for Disease Control and Prevention, 2006). Successful quit rates, unassisted by smoking cessation aids, are low and historically range from 4% to 7% (Cohen et al., 1989; Hughes, 2003), but these rates can be boosted by use of evidence-based interventions. Varenicline (Chantix®), a partial agonist of the ∂4ß2 nicotinic acetylcholine (nACh) receptor, is an effective first-line smoking cessation medication (Gonzales et al., 2006; Jorenby et al., 2006; Oncken et al., 2006). A recent literature review suggests that varenicline increases the chances of long-term smoking cessation between two and threefold compared with quit attempts in which no medication assistance is used (Cahill, Stead, & Lancaster, 2011).
While the efficacy of varenicline in improving smoking cessation outcomes has been demonstrated, research exploring specific effects of varenicline that contribute to its clinical benefit is ongoing. Varenicline is reported to reduce craving and withdrawal, improve mood and cognition, and minimize the rewarding and subjective effects of cigarettes after a period of abstinence (Brandon et al., 2011; Gonzales et al., 2006; Jorenby et al., 2006; Oncken et al., 2006; Patterson et al., 2009; Perkins, Mercincavage, Fonte, & Lerman, 2010; West, Baker, Cappelleri, & Bushmakin, 2008). Blocking or attenuating the rewarding and subjective effects of nicotine may be an important and unique aspect of the psychopharmacology of varenicline. Research suggests that the majority of smokers (95%) who take even a few puffs from a cigarette early in their quit attempt (i.e., experience a smoking lapse) soon relapse and may return to prequit levels of smoking (Brandon, Tiffany, Obremski, & Baker, 1990; Kenford et al., 1994). Shiffman, Ferguson and Gwaltney (2006) found that higher hedonic ratings associated with a smoking lapse (i.e., pleasantness of the cigarette, satisfying) were predictive of smoking relapse. This suggests that a medication-produced attenuation of the subjective reward associated with a lapse cigarette may protect smokers who lapse from progressing to full relapse during a quit attempt.
In placebo-controlled clinical trials conducted with varenicline, patient reports suggest that varenicline indeed reduced the subjective rewarding effects of lapse cigarettes (Gonzales et al., 2006; Jorenby et al., 2006; Oncken et al., 2006). However, the lapse cigarette ratings obtained in these studies were retrospective, which introduces the potential for recall bias, and were restricted to those who experienced a smoking lapse, which introduces the potential for sample bias. Thus, a prospective evaluation of varenicline on lapse cigarette effects is required to validate this finding.
A model of smoking cessation and relapse has been developed in which a brief period of objectively verified abstinence is followed by a programmed smoking lapse in a laboratory setting (Chornock, Stitzer, Gross, & Leischow, 1992; Juliano, Donny, Houtsmuller, & Stitzer, 2006). In this model, all participants experience the smoking lapse, and subjective ratings of the cigarette(s) can be immediately completed. Using this lapse model, Patterson et al. (2009) showed that varenicline, compared with placebo, improved mood and cognition, decreased the subjective rewarding effects of a smoking lapse, and increased latency to relapse in a subsequent 1-week quit attempt. However, this study used a within-subjects design and an order effect was observed such that varenicline’s relapse prevention effects were more robust in participants who received varenicline after exposure to placebo, suggesting that repeated exposure to the smoking cessation protocol impacted the study outcomes. In a second study, Perkins et al. (2010) found decreases in smoking reward during varenicline administration compared with placebo but found no difference in abstinence rates during a subsequent 1-week quit attempt. It is possible that the sensitivity to detect between group relapse rate differences may have been hindered by the short (1-week) postlapse assessment period.
The current study was conducted to extend research with varenicline using the experimental lapse model. A between-subjects study design was selected to avoid order effects, and the postlapse quit attempt was extended to 4 weeks. The aims of the study were to assess the effects of varenicline versus placebo on (a) latency to relapse following the experimental lapse smoking procedure, (b) abstinence rates during a 4-week quit attempt following the lapse exposure, and (c) subjective and rewarding effects of smoking.
Methods
Study Design
This was a placebo-controlled, double-blind, between-subjects outpatient study, in which participants were randomized to receive varenicline or matching placebo for 5 weeks. The first week was medication induction with ad libitum smoking of the participant’s own brand of cigarettes. Beginning on study Day 7, participants engaged in a 4-week quit attempt. An experimental smoking lapse exposure procedure occurred on study Day 8, following the initial 24 hr of the quit attempt. The experimental procedures were approved by the Johns Hopkins University School of Medicine IRB.
Participants
Participants were recruited through media advertisements. Study inclusion criteria were (a) age 18–75, (b) self-reported smoking of 10 or more cigarettes/day and submission of a urine specimen positive for the nicotine metabolite, cotinine (>200 ng/ml as determined by the DRI® cotinine assay for urine [Microgenics Corp., Fremont, CA]), (c) contemplating a quit attempt within the next 6 months, and (d) willing to complete a short-term smoking quit attempt as part of the study. Participants were excluded from the study if they: (a) were seeking immediate treatment to quit smoking, (b) currently met DSM criteria for depression, bipolar disorder, or schizophrenia, (c) had a history of attempted suicide or expressed any current suicidal ideation, (d) were pregnant, breastfeeding, or planning to become pregnant, or (e) had severe impairment of renal function (Glomerular Filtration Rate; GFR < 30 ml/min).
Screening Assessment
Study volunteers were initially screened over the telephone for basic inclusion characteristics (age, smoking status, and desire for treatment) and those potentially eligible were invited to the laboratory for a screening assessment. During the laboratory assessment, participants completed locally developed medical and smoking history questionnaires, Beck Depression Inventory (BDI; Beck, Steer, & Brown, 1996), Symptoms Checklist-90 (SCL-90; Derogatis, Lipman, & Covi, 1973), and Fagerström Tobacco and Nicotine Dependence Questionnaire (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). The DSM Checklist (Hudziak et al., 1993) was used to assess current depression for participants who had scores on the BDI or Depression scale of the SCL-90 above normative values. Breath and urine specimens were collected to objectively assess markers of tobacco smoking behavior (breath carbon monoxide, CO; urinary cotinine, COT). A blood specimen was obtained to assess renal function.
One hundred and four daily smokers provided informed consent and met the study eligibility criteria. These participants were randomized to receive varenicline (N = 54) or placebo (N = 50). Among randomized participants, 37 dropped out of the study prior to completion (15 varenicline and 22 placebo). Among those 37 participants, 18 failed to return for study Days 7 or 8, which was the Lapse Exposure Session (7 varenicline and 11 placebo), and nine more failed to return for the first study visit of their quit attempt after lapse exposure (2 varenicline and 7 placebo). Ten additional participants dropped out through the remainder of the quit attempt (6 varenicline and 4 placebo). The larger number of early dropouts in participants receiving placebo (N = 18) versus varenicline (N = 9) suggests that medication side effects were not an issue but rather that some placebo participants may have been disappointed with their double-blind group assignment and/or found it more difficult to quit than those assigned to varenicline. A total of 67 participants completed all study procedures. Among study completers, 20 participants (10 varenicline and 10 placebo) were judged as not meeting criteria for overnight abstinence (self-reported smoking or CO > 6 ppm) prior to the lapse exposure session and were excluded from data analyses. The final study sample included 47 participants; 25 who received varenicline and 22 who received placebo.
Study Procedures
Medication
All participants received two pills (varenicline or matching placebo) daily with instructions to take one in the morning and one in the evening. Varenicline induction followed the clinically recommended dosing regimen of 0.5 mg once daily for 3 days (1 active dose and 1 placebo dose), 0.5 mg twice daily (1 mg/day) for 4 days, and 1.0 mg twice daily (2 mg/day) for the remainder of the study. Study medication and matched placebo encased in individual blister packs were provided by Pfizer, Inc. Pharmacy staff at the Behavioral Pharmacology Research Unit were responsible for medication distribution to nursing and research staff to ensure double-blind conditions.
Laboratory Visits
Participants completed 10 laboratory visits over a 5-week period.
Week 1 Prequit Visits: Visits on study Days 1 and 7 included brief smoking cessation counseling, study assessments, and assessments of smoking reward. A smoking cessation manual was used by trained staff to provide counseling for approximately 20 min at each prequit visit. The manual has been previously used in our laboratory studies (Juliano, Houtsmuller, & Stitzer, 2006) and includes modules on preparing to quit, actions to help initial quit success, expected craving/withdrawal effects, and cognitive and behavioral coping strategies. Breath and urine specimens were collected to assess CO and COT levels. Further, a battery of self-report assessments was administered that was repeated at each subsequent study visit. Assessments included the number of cigarettes smoked each day (based on a daily diary), Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986), the Positive and Negative Mood Assessment Scale (PANAS; Watson, Clark, & Tellegan, 1988), Schuh-Stitzer tobacco craving questionnaire (Schuh & Stitzer, 1995), a locally developed Medication Side Effects Questionnaire (all side effects listed in the package insert for Chantix rated on a four-point Likert scale), and Confidence to Quit Questionnaire (Juliano, Donny, et al., 2006).
Two behavioral measures were administered to assess smoking reward before (study Day 1) and after 1 week of medication exposure (study Day 7). The Cigarette Purchase Task (CPT; MacKillop et al., 2008) is based on a model of drug reward assessment that has been successfully used with several drugs including heroin (Jacobs & Bickel, 1999; Petry & Bickel, 1998), tobacco (Jacobs & Bickel, 1999; MacKillop et al., 2008; Madden & Kalman, 2010), and alcohol (MacKillop et al., 2008; Murphy & MacKillop, 2006; Murphy, MacKillop, Skidmore, & Pederson, 2009). Participants indicated how many cigarettes they would purchase at each of the following 18 prices: 0.01, 0.5, 0.1, 0.3, 0.5, 1, 2, 3, 4, 5, 6, 11, 35, 70, 140, 280, 560, and 1120 US $/cigarette. The simulated cigarette consumption data allow for analysis of behavioral economic demand curves. This method provides a framework for quantifying multiple dimensions of drug reinforcement (e.g., Bickel & Madden, 1999; Johnson & Bickel, 2006) and has been identified as an increasingly important framework for assessing drug abuse liability (Carter & Griffiths, 2009; Hursh, Galuska, Winger, & Woods, 2005). Specifically, demand functions were fit to these data (see Data Analysis section), resulting in two quantified parameters: demand intensity (Q0), which is the number of cigarettes purchased as price is close to zero (preferred level of consumption with no price constraint), and demand elasticity (α), which is price sensitivity (the extent to which increases in cigarette price result in decreases in cigarette purchases).
A Progressive Ratio Task (PRT) was also conducted on study Days 1 and 7. Progressive ratio procedures are standard drug reinforcement assessments, where research subjects must perform behavioral response requirements, on a schedule of increasing magnitude, in order to obtain a fixed dose of a test drug. Behavioral response requirements in this study were 100, 300, 600, 1,000, 1,500, 2,100, 2,800, 3,600, 4,500, 5,500, 6,600, and 7,800 computer mouse clicks, and participants immediately received one puff from a preferred-brand cigarette following the completion of each response requirement. Participants had 2 hr to complete as many responses as desired but could not leave the laboratory until the 2-hr time limit had expired. The primary task outcome is the breakpoint or highest response requirement completed in order to obtain a cigarette puff. Similar PRTs have been used as sensitive measures of cigarette reward with deprived and satiated smokers when puffs of a cigarette serve as the reinforcer (Donny, Houtsmuller & Stitzer, 2007; Rusted, Mackee, Williams, & Willner, 1998; Shahan, Bickel, Madden, & Badger, 1999; Willner, Hardman, & Eaton, 1995).
Lapse Exposure Session: At the end of the study Day 7 session, participants were instructed to abstain from smoking overnight and return to the laboratory the following day. In order to facilitate compliance, half of the total compensation for participating in the Lapse Exposure Session ($30) was contingent on overnight abstinence (self-report verified by CO). Participants then completed a 7-hr period of supervised abstinence in the laboratory (9:00–16:00). At 16:00, following approximately 24 hr of abstinence (overnight included), participants smoked two cigarettes of their preferred brand spaced 45 min apart in an experimental simulation of lapse exposure. Following each lapse cigarette, participants completed a 19-item Cigarette Rating Questionnaire (based on Juliano, Donny, et al., 2006), in which they rated their experience of the lapse cigarette on a 100 point visual analog scale anchored on the left with not at all and on the right with extremely. The items to be rated fell into four categories: (a) rewarding and/or enjoyable effects of smoking (pleasant, tasted good, satisfying, relaxing, made me feel buzzed, liked effect, and stimulating), (b) physical sensations from smoking (enjoyable sensations in the throat and chest, enjoyable sensations on the lips and tongue, and smelled good), (c) removal of aversive stimuli (reduced craving, reduced withdrawal, and reduced irritability), and (d) unpleasant and/or punishing responses to smoking (harsh, strong, tasted different than usual brand, intensity, made me feel dizzy, and made me feel nauseous).
Quit Attempt: Following the lapse exposure visit on study Day 8, participants were instructed to resume their quit attempt and their smoking status was assessed during brief laboratory visits on study Days 10, 12, 14, 16, 21, 28, and 35. At each of these visits, participants provided breath and urine specimens and completed the self-reported smoking, withdrawal, craving, mood, and quit confidence questionnaires. Confirmation of self-reported abstinence was determined by evaluating quantitative urine COT results. Participants were judged abstinent if urine specimens were below 200 ng/ml or showed a 50% or greater reduction in COT from the prior specimen (first week of quit attempt only). Return to smoking was defined as any self-reported smoking or COT > 200 ng/ml. To promote engagement in the quit attempt, participants received bonus compensation on a decelerating schedule for remaining abstinent during the first week after the lapse exposure. Payment for meeting cotinine-based abstinence criteria was $24 on study Day 10, $18 on study Day 12, $12 on study Day 14, and $6 on study Day 16. No abstinence-based bonus compensation was provided on study Days 21, 28, or 35, and total study compensation was $371.
Medication Compliance
Urine specimens collected on study Days 7, 21, and 35 were tested for varenicline using liquid–liquid extraction by Alta Analytic Laboratories (El Dorado Hills, CA). Quantitative concentrations of varenicline (ng/ml) were returned; with the Lower Limit of Quantitation for the analyses established at 1.00 ng/ml.
Data Analysis
Time to first smoking occasion following the lapse exposure was analyzed using a Cox regression survival model and end of study group abstinence rates were compared using a Chi-squared test. Measures of abstinence (continuous days of abstinence, percentage of abstinent participants, number of negative COT samples, and hours of abstinence) were based on COT-validated self-report and were analyzed using Fisher’s exact test (only p values are reported). All variables with multiple time points were analyzed using repeated measures regressions with an autoregressive (a) covariance structure in SAS PROC Mixed. Pairwise comparisons between placebo and varenicline groups were conducted using planned comparison t tests at individual time points. A Cox regression survival model was used to assess the effects of possible moderators for abstinence duration following the lapse exposure (gender, race, education, FTND, CO and COT measures, cigarettes per day, and years of regular smoking).
For the CPT, three missing values were interpolated as the median of that participant’s data at the lower and higher surrounding prices. The median number of cigarettes purchased across participants in each group was determined for each of the two time points (study Days 1 and 7), resulting in four median datasets. For each median dataset, values less than1 and all but the first (lowest price) instance of 1 within a set were eliminated because in logarithmic coordinates zero is undefined and small median values of 0.5 and 1 provide little resolution. These four datasets were fit with nonlinear regression to the exponential equation by Hursh and Silberberg (2008): . The independent variable C represents cost (price per cigarette), the dependent variable Q represents consumption (cigarettes purchased at a particular price), and e is a constant known as Euler’s number. Scaling parameter k indicates the range of logQ and was set to 2 in this study because it was the lowest integer with an antilog (100) that covered the range of cigarettes purchased. Free parameters are Q0 (demand intensity) and α (demand elasticity). Statistical comparisons among the four median datasets (pre and postinduction for both groups) were performed with an extra sum-of-squares F test using GraphPad Prism® v. 5. As described elsewhere (Motulsky & Christopoulos, 2003), when comparing two datasets, this method calculated the error variance for data fit to the curve when: (a) assuming independent values for both free parameters in each of the two compared datasets and (b) assuming shared values across the two compared datasets for each of the two free parameters. Significant differences indicate that the curves as a whole significantly differed across the two compared datasets. To determine which parameters were responsible for any significant difference between curves, analogous procedures were used to determine if separate (as opposed to shared) values of a particular parameter were statistically justified across datasets while assuming individual fits for the other parameter across datasets.
Results
Participants
Demographic and smoking characteristics of the final study sample (N = 47) are shown in Table 1. Statistical comparisons revealed no differences on any characteristics between experimental groups.
Table 1.
Demographic and Smoking Characteristics
| Placebo (N = 22) | Varenicline (N = 25) | t/U-statistic | Significance (p) | |
| Gender, male (%) | 13 (59) | 10 (40) | a | .25 |
| Ethnicity, n (%) | ||||
| Black | 12 (55) | 17 (68) | ||
| Caucasian | 8 (36) | 8 (32) | ||
| Other | 2 (10) | a | .77 | |
| Education, n (%) | .98 | |||
| High school graduate or higher | 14 (64) | 16 (64) | −0.03 | |
| Age | 43 (10) | 45 (12) | −0.51 | .62 |
| Years smoked | 26 (11) | 25 (12) | 0.23 | .82 |
| Cigarettes per day | 18.4 (5) | 20.2 (6) | −1.0 | .30 |
| CO (ppm) | 15.5 (7), range: 5–35 | 14.8 (10), range: 4–46 | 219 | .33 |
| COT (ng/ml) | 1,930 (1,260), range: 466–5,220 | 1,912 (1,327), range: 404–6,756 | 261 | .77 |
| FTND | 5.5 (1.8) | 5.6 (1.6) | −0.11 | .91 |
| SCL-90 | 42 (10) | 43 (10) | −0.49 | .63 |
| BDI | 3.5 (3.5) | 2.7 (2.5) | 0.87 | .39 |
Note. Demographic and smoking characteristics of study completers (N = 47) at intake shown as percentages or group averages (SD). minimum and maximum range values are also shown for carbon monoxide (CO) and urinary cotinine (COT) following SD values. Fisher’s exact test was used for gender and ethnicity variables, while the Mann–Whitney Rank Sum Test was used for nonnormally distributed values found for CO and urinary COT. All other variables were analyzed with the use of t tests.
SCL-90 = Symptoms Checklist-90; BDI = Beck Depression Inventory.
Fisher’s exact test.
Smoking Outcomes Following Lapse Exposure
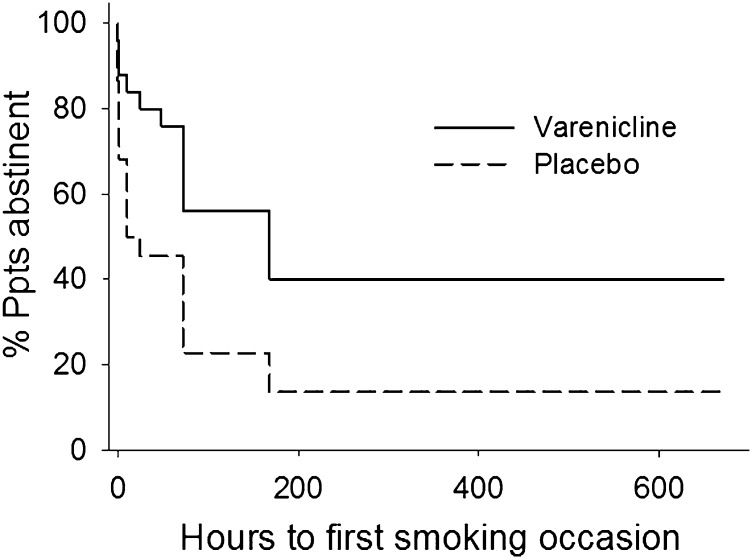
Abstinence survival analysis (Figure 1) indicated that time to first smoking occasion was delayed for those in the varenicline group compared with the placebo group (χ2 (1) = 6.3, p < .05). As shown in Table 2, mean latency to next smoking incident postlapse exposure was 14 days for varenicline versus 6.2 days for placebo (p = .02).
Figure 1.
Percentage of participants abstinent during the 4-week postlapse quit attempt based on self-reported hours to first smoking occasion and validated by urinary cotinine > 200 ng/ml.
Table 2.
Smoking Outcome Measures
| Abstinence measure | Placebo (N = 22) | Varenicline (N = 25) | t | Significance (p) |
| Days of continuous abstinence, M (SD) | 9.1 (9.7) | 16.4 (11.3) | −2.36 | .02 |
| Participants continuously abstinent, n (%) | 3 (13.6) | 10 (40.0) | a | .05 |
| Number of negative samples, M (SD) | 2.1 (2.5) | 4.1 (3.0) | −2.43 | .02 |
| Hours abstinent after lapse exposure, M (SD) | 148 (229) | 336 (290) | −2.44 | .02 |
Note. aFisher’s exact test
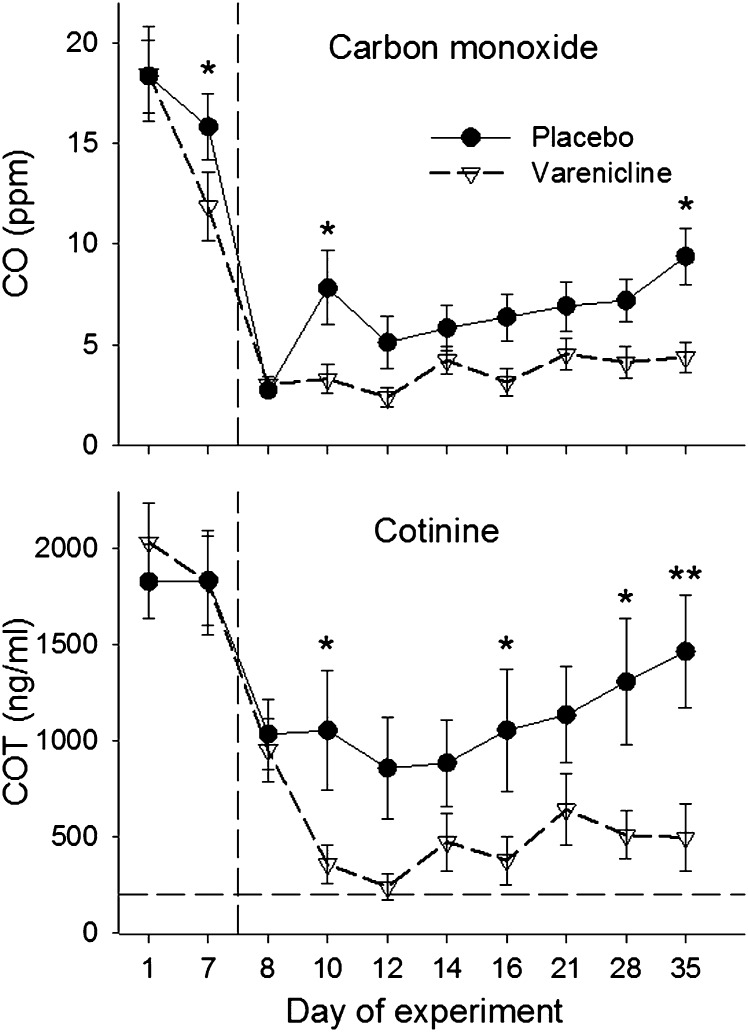
Main effects of Medication (F(9, 398) = 4.5, p < .05) and Time (F(9, 398) = 9.6, p < .0001) were observed for urinary COT, and a significant Medication × Time interaction was observed for breath CO (F(9, 397) = 2.6, p < .05). As shown in Figure 2, both groups showed large decreases in CO and COT from study Days 7–8, reflecting the initial 24-hr period of abstinence. However, biomarkers of smoking remained low for the varenicline group throughout the quit attempt whereas CO and COT steadily increased after study Day 12 in the placebo group. The time course of between group differences in CO and COT were further clarified by planned comparisons at each time point throughout the quit attempt.
Figure 2.
Carbon monoxide (CO; ppm) and urinary cotinine (COT; ng/ml) measures for each experimental session. Error bars represent SEM. The vertical dotted line indicates the start of the 4-week quit attempt. Incentive bonuses were given for abstinence during study Days 10-16 and removed for study Days 21-35. Significant group differences at each time point, determined by planned comparisons, are indicated by a single asterisk (p < .05) or double asterisks (p < .001).
Additional measures of self-reported and objectively verified abstinence obtained during the 4-week quit attempt are shown in Table 2. All variables favored the varenicline condition, including longer continuous abstinence rates, more participants continuously abstinent for all 4 weeks, twice as many COT negative urine specimens submitted, and more self-reported hours of abstinence following the programmed lapse.
Subjective Effects of Lapse Exposure
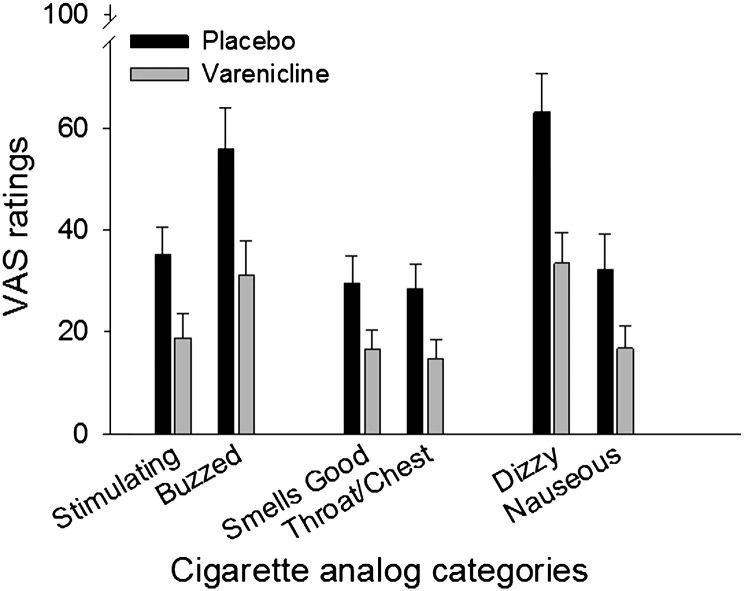
Varenicline attenuated several subjective effects of the lapse cigarettes. With both lapse cigarettes included, significant main effects of Medication were observed for participant ratings of “liked effect” (F(1, 45) = 4.4, p < .05), “stimulating” (F(1, 45) = 5.2, p < .05), “enjoyable sensations in the throat and chest” (F(1, 45) = 6.3, p < .05), “smelled good” (F(1, 45) = 4.8, p < .05), and “made me feel dizzy” (F(1, 45) = 5.2, p < .05), Since the magnitude of subjective effects was expected to decline from the first to the second lapse cigarette, planned comparisons were conducted to assess medication group differences on ratings of the first lapse cigarette only. Significant comparisons are shown in Figure 3. Participants receiving varenicline had significantly lower subjective ratings of stimulating (t(45) = 2.5, p < .05), made me feel buzzed (t(45) = 2.6, p < .05), smelled good (t(45) = 2.2, p < .05), enjoyable sensations in the throat and chest (t(45) = 2.5, p < .05), made me feel dizzy (t(45) = 3.2, p < .05), and made me feel nauseous (t(45) = 2.1, p < .05). These represent significant group differences for 2 out of 7 items relating to the rewarding/enjoyable effects of smoking, 2 out of 3 items relating to the physical sensations of smoking, 0 out of 3 items relating to reduced withdrawal and craving, and 2 out of 6 items relating to unpleasant/punishing effects of smoking.
Figure 3.
Mean subjective ratings on items in which significant group differences were observed following the first lapse cigarette exposure. Error bars represent SEM.
Behavioral Measures of Smoking Reward
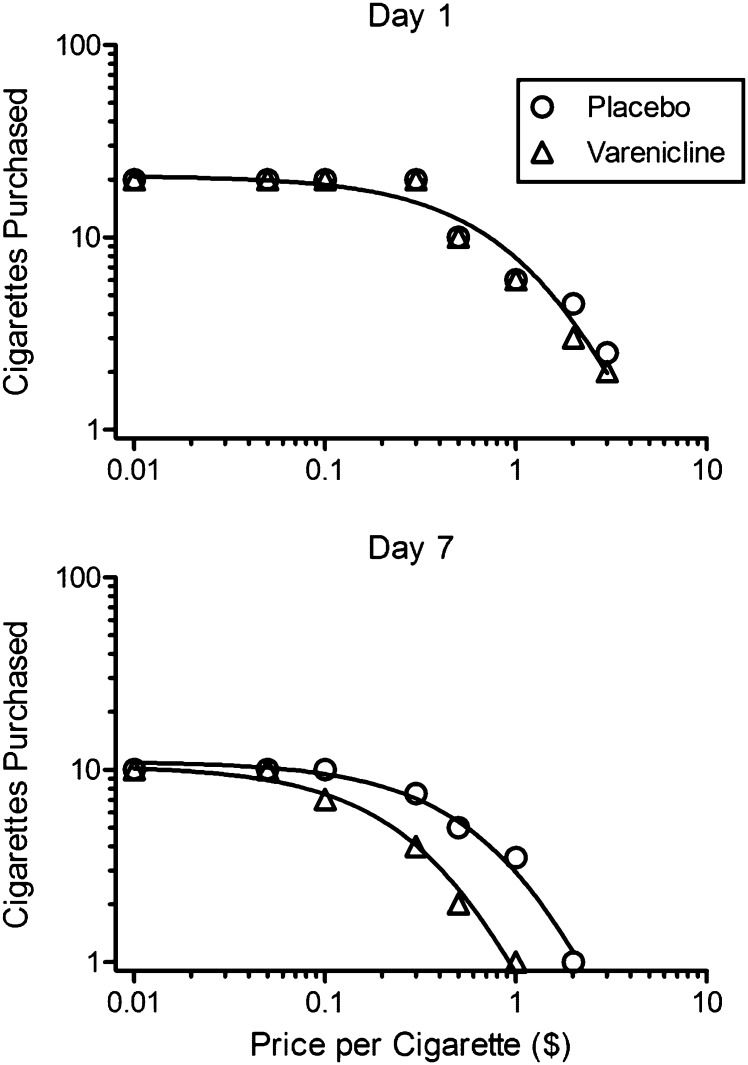
Behavioral economic demand curves derived from the CPT (median number of cigarettes purchased at each price) are shown in Figure 4. The exponential equation provided good fits to the data for the four median datasets (study Day 1 placebo: R 2 = .944; study Day 1 varenicline: R 2 = .969; study Day 7 placebo: R 2 = .984; study Day 7 varenicline: R 2 = .984) and for individual participants (median R 2 = .867 across all individual data for which model fit was possible). Both groups showed similar cigarette purchases on study Day 1 (premedication), with no significant difference between fitted demand curves (top panel; F(2, 12) = 1.06, p = .38; shared Q0 = 20.9, shared α = 0.0115). Both groups showed fewer purchases on study Day 7 compared with their own purchases on study Day 1 (placebo group: F(2, 11) = 49.36, p < .0001; varenicline group: F(2, 10) = 129.86, p < .0001). However, this decline was greater for the varenicline group than for the placebo group. This is shown by a significant varenicline versus placebo group difference on study Day 7 (F(2, 9) = 53.15, p < .0001; placebo Q0 = 11.1, varenicline Q0 = 10.5, placebo α = 0.0309, varenicline α = 0.0739). Analyses of individual purchase task parameters showed that neither Q0 (demand intensity) nor α (demand elasticity) parameters were significantly different between groups at baseline (Q0: F(1, 12) = .11, p = .75; α: F(1, 12) = 1.83, p = .20). However, on study Day 7, α (F(1, 9) = 105.47, p < .0001) but not Q0 (F(1, 9) = 0.25, p = .63) differed between groups indicating that varenicline decreased nicotine reward by increasing demand elasticity rater than decreasing demand intensity.
Figure 4.
Behavioral economic demand curves derived from the CPT. Note the double logarithmic coordinates. Data points show the median number of cigarettes purchased for each group at each price prior to (study Day 1, upper panel) and after (study Day 7, lower panel) medication induction. Note that a single demand curve provided a good fit to both datasets on study Day 1 but significant group differences required separate curves for each group on study Day 7.
The PRT showed a significant main effect of Time for puffs earned (F(1, 44) = 25.2, p < .0001), reflecting a reduction in smoking self-administration in the laboratory from study Days 1–7, but no effect of Medication or Medication × Time interaction was found. Mean (SD) total responses (mouse clicks) on the task decreased from 2,380 (2,960) to 1,207 (3,633) for the placebo group and from 5,422 (8,101) to 3,416 (8,350) for the varenicline group on study Days 1 and 7, respectively. This corresponds to a mean decrease of 1.8 (2.0) puffs earned for the placebo group versus 2.0 (3.0) for the varenicline group following medication induction.
Subjective Effect Assessments
Subjective assessments of withdrawal, craving, mood, side effects, and quit confidence administered at all study visits were not sensitive to medication effects. These questionnaires consistently showed significant main effects of Time, with craving, withdrawal, and negative mood ratings decreasing over time in both groups. However, significant main effects of Medication were only observed for loss of balance on the Medication side effects questionnaire (F(1, 45) = 5.5, p < .05; varenicline > placebo), for “upset” (F(1, 45) = 9.7, p < .05), “ashamed” (F(1, 45) = 6.4, p < .05), and “jittery” (F(1, 45) = 10.0, p < .05) on the PANAS (varenicline < placebo), and for ratings of “how pleasant a cigarette would be right now” (F(1, 45) = 4.2, p < .05) on the Schuh–Stitzer questionnaire (varenicline < placebo).
Medication Compliance
Urine toxicology testing on study Days 7, 21, and 35 suggested a high rate of medication compliance for those randomized to receive varenicline. All samples tested had measurable amounts of varenicline but considerable variability within and across participants was noted. Mean (SD) concentrations of urinary varenicline were 371 ng/ml (263) on Day 7, 1,017 (1,052) on Day 21, and 570 (612) on Day 35.
Discussion
Relapse Prevention
The current study used a prospective between-subjects design to demonstrate the relapse prevention effects of varenicline following experimental exposure to a smoking lapse. Varenicline slowed rates of relapse (Figure 1), reduced objective biomarkers of smoking (Figure 2), and improved rates of abstinence at 4-weeks postlapse (Table 2) in this short-term model of smoking cessation, lapse, and relapse. The percentage of participants remaining continuously abstinent (COT verified) at the end of the 4-week quit attempt was 40% for the varenicline group versus 14% in the placebo group. These rates are remarkably similar to abstinence rates produced in much lengthier clinical trials of varenicline as a smoking cessation aid (Gonzales et al., 2006; Jorenby et al., 2006; Oncken et al., 2006). While the similarity in quit rates between the current study and previous reports may be simply coincidental, this observation lends credence to the validity of short-term models of cessation and relapse as tools to evaluate and predict clinical effects of smoking cessation medications.
Patterson et al. (2009) reported a similar relapse prevention effect of varenicline using an experimental model very similar to the one used here. In the present study, time to the first smoking episode following the experimental lapse exposure was about six days for placebo versus 14 days for varenicline (Table 2). These times are longer than those observed in the Patterson study, which were 2.6 versus 4.6 days for placebo and varenicline, respectively (among those receiving placebo first followed by varenicline). However, the magnitude of the varenicline effect on relapse delay is roughly comparable across the studies, specifically a doubling in the time to relapse. Longer latencies to relapse in the present study are likely due to differences in the amount of abstinence-contingent payments, as well as the decelerating schedule of payments, which promoted early abstinence during the quit attempt. Also, there were differences in the expected duration of the quit attempt (1 week vs. 4 weeks), which may have influenced relapse likelihood. In contrast, Perkins et al. (2010) found no differences in abstinence outcomes during a short-term 5-day quit attempt. Again, this may be due in part to differences in abstinence-contingent payment conditions (half the participants in Perkins et al. [2010] study did not receive abstinence-contingent payments and the remainder received $12 per day for meeting abstinence criteria) and/or the expected abstinence durations. These differences in magnitude and sensitivity suggest that the longer abstinence duration and/or the decelerating payment schedule used in the present study may be beneficial for magnifying varenicline’s effects on latency to relapse in short-term models of cessation.
Smoking Reward
The present study found that varenicline significantly reduced scores on subjective report items reflecting positive (liked, stimulated, and buzzed) and negative (dizzy and nauseous) effects of smoking during a period of abstinence and also seemed to attenuate ratings of some sensory aspects of smoking (smell, chest and throat sensations) that could operate as secondary or conditioned reinforcers associated with smoking. These findings replicate and extend previous reports of varenicline-induced reductions in subjective measures of smoking reward (e.g., cigarette liking and satisfaction) obtained both in clinical trials (Gonzales et al., 2006; Jorenby et al., 2006; Oncken et al., 2006) and laboratory studies (Patterson et al., 2009; Perkins et al., 2010).
An important advance in the understanding of varenicline’s effects on smoking reward was provided by the CPT, a task that models cigarette consumption at very low cigarette prices and at a range of higher prices. The data indicate that varenicline increased demand elasticity relative to placebo, producing a steeper decline in the number of cigarettes purchased at higher prices but not at lower prices. This pattern of results (increased elasticity but not intensity) on the CPT could suggest that varenicline’s effects on smoking reward become more apparent under conditions where there are higher costs associated with smoking. In the present study, for example, participants relinquished monetary incentives if they returned to smoking during the first week of the postlapse quit attempt. However, this study did not directly test that hypothesis, and future studies should explore the relationship between varenicline and relative costs associated with smoking versus abstinence. Another possible explanation for the CPT results is that the smoker becomes, generally, more sensitive to price for any commodity, which could also be tested by including other choice options besides cigarettes in future studies. Interestingly, a recent study showed that bupropion had no effect on demand elasticity or demand intensity for cigarettes (Madden & Kalman, 2010), which suggests that this effect may be unique to varenicline.
In contrast to the CPT, differential medication effects were not observed for the PRT. This was surprising because PRT has been sensitive to reductions in smoking reward in our laboratory and others in prior studies (e.g., Donny et al., 2007). Significant reductions in responding from study Day 1 to study Day 7 were observed in both groups; therefore it is possible that participants were disinclined to smoke on study Day 7 because the quit attempt began immediately after that session. The motivation to abstain on that day may have minimized group differences in smoking reward that could have been obtained with the PRT on another study day. It is also possible that results reflect a greater overall sensitivity of the CPT (hypothetical cigarette consumption) versus the PRT (actual cigarette consumption and work requirement) in measuring smoking reward. Demand curves from the CPT also yield quantitative rather than dichotomous assessments from the PRT at each price, possibly related to increased sensitivity for the CPT. Additional research assessing the relative sensitivity of these and other measures of drug reward would be valuable.
Lapse and Relapse
Although there is a strong clinical connection between lapse and relapse in smoking cessation trials (see Brandon et al., 1990; Kenford et al., 1994; Shiffman et al., 2006), the mechanisms behind this association have remained obscure. The importance of reexposure to nicotine has been emphasized in nonhuman models of smoking relapse because, in these models, drug reexposure directly increases the probability of drug seeking and nicotine self-administration (Le et al., 2006; Martin-Garcia, Barbano, Galeote, & Maldonado, 2009; Shaham, Adamson, Grocki, & Corrigall, 1997; Shram, Funk, Li, & Le, 2008). The use of an experimentally programmed smoking lapse in the present human study is meant to simulate the nonhuman priming model. The fact that reexposure to smoking has been shown to facilitate or speed subsequent relapse (Juliano, Donny, et al., 2006) lends credence to the experimental lapse procedure as a model of the clinical association between lapse and relapse. Further, the association between blunted subjective effects of smoking and subsequent delay to relapse suggests that the former may be a mediating mechanism of the behavioral effect. Varenicline appears to reliably diminish the rewarding effects of cigarettes, which is consistent with its neurological actions when smoking occurs. Partial agonists of ∂4ß2 nACh receptors, such as varenicline, seem to exert antagonistic properties during smoking that serve to decrease the rewarding effects of smoking, making relapse far less likely (Rollema et al., 2007). This effect, along with the agonist properties that reduce craving and withdrawal during abstinence, is unique relative to other smoking cessation medications (Rollema et al., 2007) and is a likely mechanism by which varenicline can blunt the powerful chain between lapse exposure and relapse. Altering smoking reward has not been reliably demonstrated with other smoking cessation medications, such as bupropion (West et al., 2008), and in multiple studies using nicotine replacement therapy prior to a quit attempt (Lindson & Aveyard, 2011). Future research should continue to examine the lapse–relapse connection including its neuronal and subjective mediators and its modulation by medication.
Strengths and Limitations
The present study has many strengths including the prospective between-subjects design, random assignment, blind dosing, experimental manipulation of the smoking lapse, a 4-week postlapse quit attempt in which smoking status was determined, and use of a decelerating schedule of abstinence-contingent payments that may aptly model the declining motivation to remain abstinent during a prolonged quit attempt. Urine toxicology testing indicated excellent medication compliance by participants in this study. However, because the half-life of varenicline in urine is approximately 24 hr (Faessel et al., 2010), we were unable to differentiate diligent medication compliance throughout the study with more sporadic adherence. That said, participants were not told which specimens would be tested for varenicline, and there was no financial incentive placed on medication compliance that would have encouraged participants to misrepresent their medication use on these days.
Limitations of the study include testing medication effects on lapse exposure after a relatively short, 1-week induction period and a relatively short (24 hr) period of abstinence prior to the lapse exposure. As compared with 3 or 4-day prelapse abstinence periods used in previous studies (Juliano, Donny, et al., 2006; Patterson et al., 2009), the shorter 24-hr abstinence period was used in the current study in an attempt to reduce self-selection bias and increase generality of findings by maximizing the number of participants who could meet the initial abstinence criteria prior to the lapse exposure. We nonetheless experienced high dropout and abstinence failure rate, with 55% of those who were randomized to a medication condition failing to complete the study and/or be included in final data analysis due to failure to abstain for 24 hr. Thus, high rates of attrition were a limitation of this study. The fact that early dropout rate was higher in participants randomized to placebo versus varenicline suggests that medication side effects were not the reason for study dropout, but rather active medication was beneficial in retaining participants through the early stages of the study, when visits were longer and more effort was required. Since most participants were unreachable after missed visits, our ability to draw conclusions from high study attrition is limited. Future studies using this lapse model with nontreatment seeking smokers should consider additional refinements in monitoring or payment schedules to reduce dropout and increase compliance with initial abstinence requirements that occur prior to the lapse exposure.
A further limitation is that the use of abstinence-contingent payments during the first week of the postlapse quit attempt may have impacted relapse rates and patterns, producing relapse curves that may differ from those in clinical samples. In addition, the fact that varenicline did not reliably alter craving or withdrawal symptoms in this study is puzzling and dissimilar from findings in previous research (Brandon et al., 2011; Gonzales et al., 2006; Jorenby et al., 2006; Oncken et al., 2006; Patterson et al., 2009; Perkins et al., 2010; West et al., 2008). Finally, although the CPT provided orderly data and showed sensitivity to the experimental design, it is unknown whether the same results would be found with a CPT using a different set of prices.
Conclusions
Varenicline delayed relapse to smoking and increased rates of abstinence following exposure to a simulated smoking lapse delivered under controlled conditions. The study also showed that varenicline blunted the subjective effects of smoking (positive, negative and sensory) during the programmed smoking lapse, as well as increased sensitivity to price during a hypothetical cigarette purchase task. Overall, the findings support a relapse prevention effect for varenicline and suggest that varenicline is effective at least in part due to its ability to reduce the rewarding effects of smoking during postquit lapses.
Funding
This research was supported by an investigator-initiated grant from Pfizer, Inc. and T32-DA07209 from the National Institute on Drug Abuse.
Declaration of Interests
Funds for this study were provided by Pfizer, Inc.
Acknowledgments
The authors wish to thank Jessica Vanderhoff, Ashley Bathgate, and Erin Sullivan for execution of study procedures and data management. We also wish to thank the medical and pharmacy staff at the Behavioral Pharmacology Research Unit and Linda Felch for statistical consulting. Finally, we thank Krista Barbour, formerly of Pfizer, Inc.
References
- Beck AT, Steer RA, Brown GK. Manual for beck depression inventory II (BDI-II) San Antonio, TX: Psychology Corporation; 1996. [Google Scholar]
- Bickel WK, Madden GJ. Similar consumption and responding across single and multiple sources of drug. Journal of the Experimental Analysis of Behavior. 1999;72:299–316. doi: 10.1901/jeab.1999.72-299. doi:10.1901/jeab.1999.72-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berlin) 2011;218:391–403. doi: 10.1007/s00213-011-2327-z. doi:10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. doi:10.1016/0306-4603(90)90013-N. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews (Online) 2011;(2) CD006103. doi:10.1002/14651858.CD006103.pub5. [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug and Alcohol Dependence. 2009;105(Suppl. 1):S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. doi:10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Tobacco use among adults-United States, 2005. Morbidity and Mortality Weekly Report. 2006;55:1145–1148. [PubMed] [Google Scholar]
- Chornock WM, Stitzer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: Effects on relapse. Psychopharmacology (Berlin) 1992;108:495–500. doi: 10.1007/BF02247427. [DOI] [PubMed] [Google Scholar]
- Cohen S, Lichtenstein E, Prochaska JO, Rossi JS, Gritz ER, Carr CR, et al. Debunking myths about self-quitting. Evidence from 10 prospective studies of persons who attempt to quit smoking by themselves. American Psychologist. 1989;44:1355–1365. doi: 10.1037//0003-066x.44.11.1355. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Covi L. SCL-90: An outpatient psychiatric rating scale–preliminary report. Psychopharmacology Bulletin. 1973;9:13–28. [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: Behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. doi:10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clinical Pharmacokinetics. 2010;49:799–816. doi: 10.2165/11537850-000000000-00000. doi:10.2165/11537850. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. doi:10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K.-O. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Helzer JE, Wetzel MW, Kessel KB, McGee B, Janca A, et al. The use of the DSM-III-R checklist for initial diagnostic assessments. Comprehensive Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Motivating and helping smokers to stop smoking. Journal of General Internal Medicine. 2003;18:1053–1057. doi: 10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods JH. The economics of drug abuse: A quantitative assessment of drug demand. Molecular Interventions. 2005;5:20–28. doi: 10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. doi:10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Jacobs E, Bickel W. Modeling drug consumption in the clinic using simulation procedures: Demand for heroin and cigarettes in opioid-dependent outpatients RID D-8898-2011. Experimental and Clinical Psychopharmacology. 1999;7:412–426. doi: 10.1037//1064-1297.7.4.412. doi:10.1037//1064-1297.7.4.412. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Replacing relative reinforcing efficacy with behavioral economic demand curves. Journal of the Experimental Analysis of Behavior. 2006;85:73–93. doi: 10.1901/jeab.2006.102-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:56–63. doi: 10.1001/jama.296.1.56. doi:10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. Journal of Abnormal Psychology. 2006;115:166–173. doi: 10.1037/0021-843X.115.1.166. doi:10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Houtsmuller EJ, Stitzer ML. A preliminary investigation of rapid smoking as a lapse-responsive treatment for tobacco dependence. Experimental and Clinical Psychopharmacology. 2006;14:429–438. doi: 10.1037/1064-1297.14.4.429. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. who will quit with and without the nicotine patch. Journal of the American Medical Association. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. doi:10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindson N, Aveyard P. An updated meta-analysis of nicotine preloading for smoking cessation: Investigating mediators of the effects. Psychopharmacology (Berlin) 2011;214:579–592. doi: 10.1007/s00213-010-2069-3. doi: 10.1007/s00213-010-2069-3. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG, Ray LA, Eisenberg DT, Lisman SA, Lum JK, et al. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Experimental and Clinical Psychopharmacology. 2008;16:57–65. doi: 10.1037/1064-1297.16.1.57. doi:10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Kalman D. Effects of bupropion on simulated demand for cigarettes and the subjective effects of smoking. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2010;12:416–422. doi: 10.1093/ntr/ntq018. doi:10.1093/ntr/ntq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Garcia E, Barbano MF, Galeote L, Maldonado R. New operant model of nicotine-seeking behaviour in mice. International Journal of Neuropsychopharmacology/Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2009;12:343–356. doi: 10.1017/S1461145708009279. doi:10.1017/S1461145708009279. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: A practical guide to curve fitting. San Diego, CA: GraphPad Software, Inc; 2003. [Google Scholar]
- Murphy JG, MacKillop J. Relative reinforcing efficacy of alcohol among college student drinkers. Experimental and Clinical Psychopharmacology. 2006;14:219–227. doi: 10.1037/1064-1297.14.2.219. doi:10.1037/1064-1297.14.2.219. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Skidmore JR, Pederson AA. Reliability and validity of a demand curve measure of alcohol reinforcement. Experimental and Clinical Psychopharmacology. 2009;17:396–404. doi: 10.1037/a0017684. doi:10.1037/a0017684. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. doi:10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. doi:10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Mercincavage M, Fonte CA, Lerman C. Varenicline's effects on acute smoking behavior and reward and their association with subsequent abstinence. Psychopharmacology (Berlin) 2010;210:45–51. doi: 10.1007/s00213-010-1816-9. doi:10.1007/s00213-010-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Bickel WK. Polydrug abuse in heroin addicts: A behavioral economic analysis. Addiction. 1998;93:321–325. doi: 10.1046/j.1360-0443.1998.9333212.x. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends in Pharmacological Sciences. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. doi:10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Mackee A, Williams R, Willner P. Deprivation state but not nicotine content of the cigarette affects responding by smokers on a progressive ratio task. Psychopharmacology (Berlin) 1998;140:411–417. doi: 10.1007/s002130050783. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology (Berlin) 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Adamson LK, Grocki S, Corrigall WA. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berlin) 1997;130:396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: A behavioral economic analysis. Psychopharmacology (Berlin) 1999;147:210–216. doi: 10.1007/s002130051162. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: Relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology (Berlin) 2006;184:608–618. doi: 10.1007/s00213-005-0175-4. doi:10.1007/s00213-005-0175-4. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: Evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33:739–748. doi: 10.1038/sj.npp.1301454. doi:10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berlin) 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. doi:10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- Willner P, Hardman S, Eaton G. Subjective and behavioural evaluation of cigarette cravings. Psychopharmacology (Berlin) 1995;118:171–177. doi: 10.1007/BF02245836. [DOI] [PubMed] [Google Scholar]