Abstract
Introduction:
Difficulty concentrating is a symptom of nicotine withdrawal that can contribute to relapse in individuals trying to quit smoking. The purpose of this study was to determine the effects of nicotine on executive and alerting attention in smokers and nonsmokers.
Methods:
Thirty daily smokers who were not tobacco deprived and 30 nonsmokers participated in the study. Participants received a single dose of intranasal nicotine (0, 0.5, or 1.5 mg) at each of 3 experimental sessions on separate days. Participants completed subjective ratings and 3 attention tasks before and after nicotine administration.
Results:
Nicotine had no effect on executive attention as assessed by a Rapid Serial Visual Presentation (RSVP) task or the Attention Network Test in smokers and nonsmokers. In contrast, nicotine enhanced alerting attention by decreasing errors on a Continuous Performance Test (CPT) in nonsmokers and improving the correct identification of target words on the RSVP task in smokers. Nonsmokers were more sensitive than smokers to the subjective, but not the cardiovascular, effects of nicotine.
Conclusions:
The acute administration of intranasal nicotine improved alerting attention in nonsmokers as measured by the CPT, and in smokers as measured by the RSVP. Understanding the elements of attention enhanced by nicotine might guide the development of novel medications for tobacco dependence.
Introduction
A major impediment to quitting smoking is the nicotine withdrawal syndrome, which includes difficulty concentrating (American Psychiatric Association, 2000). The reversal of abstinence-induced cognitive deficits by smoking or nicotine has been documented (Heishman, Taylor, & Henningfield, 1994; Sherwood, 1993). Additionally, nicotine enhances some elements of attention in nondeprived or minimally deprived smokers and nonsmokers (Heishman, Kleykamp, & Singleton, 2010).
Attention is a multidimensional process postulated to comprise three anatomically distinct networks that are involved in the functions of alerting, orienting, and executive attention (Fan et al., 2009; Posner & Rothbart, 2007). Numerous studies have shown that nicotine enhances the alerting and orienting networks, as measured by continuous performance tests (CPTs) and cued-detection tasks (Lawrence, Ross, & Stein, 2002; Myers, Taylor, Moolchan, & Heishman, 2008; Thiel & Fink, 2008).
In contrast, the effect of nicotine is less clear on executive attention, which involves detecting and resolving conflict (Fan et al., 2009). Nicotine gum had no effect on executive attention in nonsmokers (Kleykamp, Jennings, Blank, & Eissenberg, 2005), whereas AhnAllen, Nestor, Shenton, McCarley, and Niznikiewicz (2008) reported that transdermal nicotine improved executive attention in smokers.
To clarify the effect of nicotine on executive attention, we administered intranasal nicotine (0, 0.5, 1.5 mg) to smokers and nonsmokers and assessed measures of executive and alerting attention. We also assessed subjective and cardiovascular effects of nicotine. We hypothesized that nicotine would (a) dose-dependently enhance executive and alerting attention similarly in smokers and nonsmokers and (b) elicit greater subjective and cardiovascular effects in nonsmokers than smokers because of chronic nicotine tolerance in smokers.
Methods
Participants
Male and female cigarette smokers (n = 30) and nonsmokers (n = 30) were recruited from the Baltimore, Maryland area. Smokers averaged (mean ± SD) 21 ± 6 cigarettes/day, had smoked for 15.8 ± 10.2 years, were moderately nicotine dependent (Fagerström Test for Nicotine Dependence = 5.2 ± 1.6) and were 30.8 ± 9.2 years old. Nonsmokers reported smoking less than 10 cigarettes ever and were 29.8 ± 8.5 years old. Smokers and nonsmokers did not differ significantly in age, education, and estimated IQ. All participants provided written informed consent and were paid for their participation. The National Institute on Drug Abuse (NIDA) Institutional Review Board approved the study.
Procedures
Participants completed one adaptation and three experimental sessions. They were required to abstain from alcohol and other drugs (except caffeine, nicotine, and prescription drugs) 24 hr before each session. Smokers smoked ad libitum before sessions and smoked one preferred-brand cigarette 60 min before each session. During the adaptation session, participants practiced the attention tests and were familiarized with the subjective rating scales. At the end of the session, smokers were administered the highest nicotine dose (1.5 mg) to screen for adverse effects. Nonsmokers received 0.5 mg nicotine, and if tolerated, received 1.5 mg 30 min later. Five nonsmokers could not tolerate the nicotine doses and were discharged from the study.
Experimental sessions lasted 2 hr and were conducted at least 24 hr apart. At each session, a single dose of nicotine (0, 0.5, or 1.5 mg) was administered in randomized order. Participants performed a 30-min battery of attention, subjective, and cardiovascular measures before and after each dose. Nicotine was administered in the form of the marketed nasal spray Nicotrol® (Pfizer) and a placebo spray was formulated by the NIDA pharmacy, as described previously (Myers et al., 2008). The placebo solution was placed in empty Nicotrol bottles to allow double-blind administration.
Attention Measures
Executive attention was measured using a Rapid Serial Visual Presentation (RSVP) task and the Attention Network Test (ANT). The RSVP task can assess the attentional blink phenomenon in which two target words (T1 and T2) are presented with a varying number of intervening distracter words (lags). Participants are typically accurate at reporting T1; however when T2 follows T1 within 500 ms (early lags), identification of T2 is impaired, as if our attentional system “blinked” (Raymond, Shapiro, & Arnell, 1992). The RSVP task used in this study has been described (Heinz et al., 2007). Briefly, participants completed 48 trials, each of which contained 16 individually presented words (T1, T2, and 14 distracters). Target words were selected from a list of commonly used nouns whereas the 14 distracter words were less commonly used nouns. Across sessions, no word was presented more than once; all words were unrelated to smoking and emotionally neutral. The task lasted 10–15 min. Correct reporting of T1 measured alerting attention; identification of T2, especially at early T1–T2 lags, assessed executive attention. The ANT is a combination of a cued reaction time task and a flanker task (Fan, McCandliss, Sommer, Raz, & Posner, 2002). Participants completed 2 blocks of 96 trials. For each trial, the target was an arrow presented above or below a fixation cross on the monitor. Participants indicated whether the arrow was pointing left or right. The central arrow was flanked on either side by two arrows pointing in the same direction (congruent cue), the opposite direction (incongruent cue), or by straight lines. Subtraction of response times to congruent cues from incongruent cues yielded a measure of executive attention. Alerting attention was assessed by two warning conditions that cued the onset of the target arrow: fixation cross only (no cue) or an asterisk above and below the fixation cross (double cue). Subtraction of response time to double cue from no cue yielded a measure of alerting attention (Fan et al., 2002).
Alerting attention was also measured using a 6-min CPT (Myers et al., 2008). Participants viewed letters displayed on a monitor one at a time in rapid succession (100 ms presentation, 600 ms interstimulus interval) and pressed a button when they saw the letter “X.” There were 100 targets and 400 nontargets presented each session. To make the task more challenging, we used a degraded version in which 30% of the pixels of each letter were absent. Dependent variables were correct target responses, errors of commission, adjusted target responses (target responses minus errors of commission), mean response time, and response time variability.
Subjective and Cardiovascular Measures
The Positive and Negative Affect Schedule (PANAS) is a validated scale measuring positive and negative mood (Watson, Clark, & Tellegen, 1988). Individual items were averaged for a composite positive and negative mood score. PANAS items were measured 20-min postdose to evaluate participants’ mood near the end of each session. The following Visual Analog Scale (VAS) items were assessed: stimulated, jittery, dizzy, drug liking, drug strength, good drug effect, bad drug effect, and urge to smoke. Participants answered each item by placing a vertical mark along a 100-mm line anchored by not at all on the left and extremely on the right. VAS items were measured 5-min postdose to evaluate acute nicotine effects. Heart rate and blood pressure were measured 30 min before and 5 min after each nicotine dose.
Data Analysis
We assessed baseline performance on the participants’ first experimental session before drug was delivered. Because there was a significant group difference on CPT baseline performance (nonsmokers made more errors of commission than smokers, p < .05), we used this baseline session as a covariate on subsequent analyses of the CPT. There were two independent variables, group (smokers and nonsmokers) and nicotine dose (0, 0.5, 1.5 mg). The independent variables for the ANT, subjective, and cardiovascular data were group, dose, and trial (predrug vs. postdrug); the RSVP task had a fourth independent variable, T1–T2 lag positions. Because of missing data on some tasks, analyses were conducted using the Mixed Models procedure in SPSS, which allows for missing data without resorting to imputation. Analysis of RSVP data was conducted using GLM repeated measures in SPSS. Statistical tests were two tailed with alpha = .05. Post-hoc comparisons between means were conducted using the protected Fisher’s least significant difference test. This procedure was deliberately chosen to avoid the Type II error by minimizing alpha correction.
Results
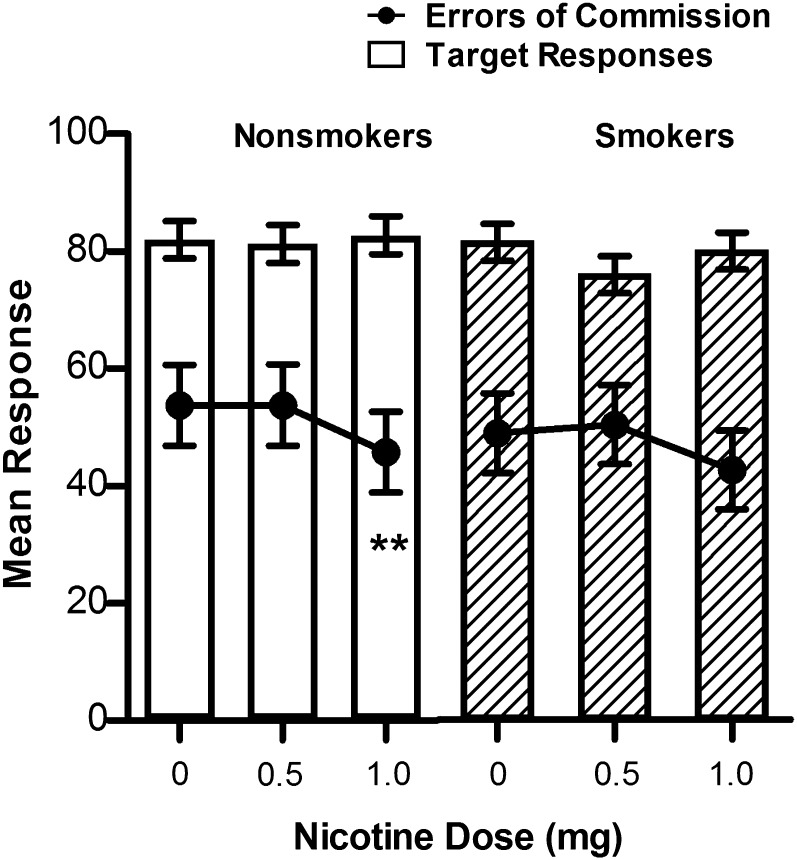
At baseline, nonsmokers made a higher percentage of errors of commission compared with smokers, t = 2.21, p < .05; 60.04 (5.22) versus 44.93 (4.44), respectively. Data shown are mean (SE). Nicotine improved performance on the CPT by reducing errors of commission, dose main effect F(2, 108.6) = 4.98, p < .01. Post-hoc tests showed that nonsmokers’ performance was significantly improved following the highest dose of nicotine compared with 0.5 mg, p < .05 and placebo, p < .05. Smokers showed a similar pattern but did not reach significance. Figure 1 shows that nicotine reduced errors of commission without concomitant reductions in target responses.
Figure 1.
Effect of nicotine on mean correct target responses and errors of commission on the 6-min Continuous Performance Test in nonsmokers and smokers. Each data point represents the mean (±SE) of the postdose trial (predose trial was a covariate). Significant dose effect post-hoc comparisons are as follows: **p < .05 different from 0 to 0.5 mg dose.
On the RSVP, there was a significant group × trial × dose interaction for T1 reporting, F(2, 112) = 4.53, p < .05. Smokers’ T1 identification improved following 0.5 mg nicotine as compared with the predrug condition, (p < .05). Identification of T2 was poorer at early lags than later lags, F(7, 392) = 119.5, p < .001, reflecting the attentional blink effect; however, there were no significant nicotine effects on T2 reporting (Table 1). There were no significant effects of nicotine on the ANT (Table 1).
Table 1.
Mean (SE) Performance on the Rapid Serial Visual Presentation task (RSVP) and the Attention Network Test (ANT)
| RSVP | Nonsmokers | Smokers | ||
| Predrug | Postdrug | Predrug | Postdrug | |
| %T1 report | ||||
| 0 mg | 86.7 (2.0) | 89.1 (1.8)* | 89.2 (1.8) | 88.0 (2.0) |
| 0.5 mg | 89.2 (1.6) | 89.2 (1.7) | 86.0 (2.3) | 88.6 (2.0)* |
| 1.5 mg | 88.3 (1.9) | 89.0 (1.6) | 88.0 (2.3) | 89.0 (2.0) |
| %T2 report | ||||
| 0 mg | 72.8 (3.1) | 72.7 (2.9) | 73.0 (3.6) | 70.6 (3.7) |
| 0.5 mg | 72.1 (3.2) | 72.8 (2.6) | 74.1 (3.6) | 75.3 (3.2) |
| 1.5 mg | 72.5 (3.1) | 73.5 (2.9) | 73.5 (3.1) | 74.4 (3.3) |
| ANTa | ||||
| Executive | ||||
| 0 mg | 95.2 (8.3) | 81.8 (8.5) | 96.6 (12.4) | 90.1 (8.0) |
| 0.5 mg | 94.3 (8.8) | 92.7 (7.4) | 97.4 (8.2) | 99.6 (8.9) |
| 1.5 mg | 86.6 (8.0) | 90.6 (7.7) | 103.4 (7.1) | 103.5 (8.0) |
| Alerting | ||||
| 0 mg | 48.3 (5.8) | 45.1 (6.4) | 30.9 (6.1) | 39.1 (7.2) |
| 0.5 mg | 43.1 (8.5) | 49.4 (7.6) | 41.7 (6.2) | 45.4 (7.9) |
| 1.5 mg | 43.3 (5.7) | 56.2 (6.5) | 29.8 (6.4) | 39.9 (6.5) |
| Orienting | ||||
| 0 mg | 37.6 (5.4) | 34.9 (5.6) | 35.9 (6.5) | 36.1 (5.1) |
| 0.5 mg | 33.1 (5.3) | 37.9 (7.1) | 33.9 (4.5) | 49.1 (5.5) |
| 1.5 mg | 39.2 (6.4) | 35.8 (6.0) | 46.2 (6.9) | 40.1 (4.8) |
Note. aData are subtractions of response times, as a measure of the efficiency of attention processing (Fan et al., 2009).
*p < .05. predrug versus postdrug.
On most subjective measures, the effect of nicotine differed between smokers and nonsmokers (Table 2). There were significant drug effects on all VAS items except urge to smoke. For example, following nicotine, participants felt more stimulated (trial × dose, F(2, 290) = 3.16, p < .05), more jittery (trial main effect, F(1, 290) = 10.87, p = .001) and dizzier (dose main effect, F(2, 290) = 7.6, p = .001). Post-hoc tests showed these effects were observed primarily in nonsmokers, although the highest dose also produced increased dizziness in smokers. Post-hoc tests showed that liking scores were significantly increased by nicotine in nonsmokers (p < .05) and nearly so in smokers (p = .051). Nicotine had no effect on the PANAS.
Table 2.
Mean (SE) Subjective Responses on the Visual Analog Scale (VAS) and Positive and Negative Affect Schedule
| VAS | Nonsmokers | Smokers | ||
| Predrug | Postdrug | Predrug | Postdrug | |
| Stimulated | ||||
| 0 mg | 43.7 (5.4) | 44.9 (5.4) | 36.9 (5.4) | 31.0 (5.4) |
| 0.5 mg | 39.9 (5.4) | 42.0 (5.4) | 35.9 (5.7) | 29.1 (5.4) |
| 1.5 mg | 35.1 (5.4) | 48.7 (5.4)* | 32.7 (5.4) | 36.5 (5.4) |
| Jittery | ||||
| 0 mg | 7.9 (3.8) | 10.5 (3.8) | 7.5 (3.8) | 11.5 (3.8) |
| 0.5 mg | 7.0 (3.8) | 11.9 (3.8) | 11.3 (3.8) | 12.8 (3.8) |
| 1.5 mg | 8.3 (3.8) | 22.2 (3.8)** | 11.0 (3.8) | 13.3 (3.8) |
| Dizzy | ||||
| 0 mg | 3.0 (3.1) | 6.5 (3.1) | 2.9 (3.6) | 5.5 (3.6) |
| 0.5 mg | 2.2 (3.1) | 10.9 (3.1)* | 7.3 (3.1) | 9.5 (3.1) |
| 1.5 mg | 2.7 (3.1) | 27.4 (3.1)** | 3.9 (3.1) | 11.1 (3.1)* |
| Drug liking | ||||
| 0 mg | 1.5 (3.2) | 9.8 (3.2)* | 10.1 (3.2) | 14.53 (3.2) |
| 0.5 mg | 1.5 (3.2) | 10.6 (3.2)* | 9.3 (3.2) | 12.3 (3.2) |
| 1.5 mg | 4.3 (3.2) | 12.3 (3.2)* | 7.3 (3.2) | 13.7 (3.2)+ |
| Drug strength | ||||
| 0 mg | 1.0 (4.5) | 27.5 (4.5)** | 8.8 (4.5) | 24.67 (4.5)** |
| 0.5 mg | 1.5 (4.5) | 27.0 (4.5)** | 8.13 (4.5) | 32.1 (4.5)** |
| 1.5 mg | 1.3 (4.5) | 37.13 (4.5)** | 9.8 (4.5) | 34.1 (4.5)** |
| Good drug effect | ||||
| 0 mg | 1.1 (3.8) | 16.0 (3.8)** | 7.7 (3.8) | 21.1 (3.8)** |
| 0.5 mg | 1.9 (3.8) | 21.8 (3.8)** | 9.7 (3.8) | 19.9 (3.8)* |
| 1.5 mg | 6.2 (3.8) | 21.1 (3.8)** | 7.7 (3.8) | 22.9 (3.8)** |
| Bad drug effect | ||||
| 0 mg | 0.9 (4.7) | 21.0 (4.7)** | 12.1 (4.7) | 24.3 (4.7)* |
| 0.5 mg | 1.9 (4.7) | 28.1 (4.7)** | 11.1 (4.7) | 32.2 (4.7)** |
| 1.5 mg | 2.7 (4.7) | 32.6 (4.7)** | 10.9 (4.7) | 23.7 (4.7)* |
| Urge to smoke | ||||
| 0 mg | 29.8 (3.8) | 29.0 (3.8) | ||
| 0.5 mg | 28.1 (3.8) | 18.9 (3.8) | ||
| 1.5 mg | 28.8 (3.8) | 23.9 (3.8) | ||
| PANAS | ||||
| Positive mood | ||||
| 0 mg | 30.7 (1.8) | 29.6 (1.8) | 28.2 (1.8) | 27.7 (1.8) |
| 0.5 mg | 30.2 (1.8) | 29.1 (1.8) | 27.7 (1.8) | 27.8 (1.8) |
| 1.5 mg | 30.6 (1.8) | 28.6 (1.8) | 29.4 (1.8) | 29.2 (1.8) |
| Negative mood | ||||
| 0 mg | 10.9 (.4) | 10.6 (.4) | 11.3 (.4) | 11.6 (.4) |
| 0.5 mg | 10.6 (.4) | 10.6 (.4) | 11.1 (.4) | 11.2 (.4) |
| 1.5 mg | 10.7 (.4) | 11.7 (.4) | 11.1 (.4) | 11.1 (.4) |
Note. + p = .051. *p < .05. **p < .001.
At 5-min postdose, nicotine increased blood pressure in smokers (p < .05) and nonsmokers (p < .05) and produced a dose-related increase in heart rate (p < .01). There were no differences between smokers and nonsmokers.
Discussion
The purpose of this study was to clarify the effect of nicotine in smokers and nonsmokers on executive attention, which involves detecting and resolving conflict among stimuli (Fan et al., 2009). We assessed executive attention in two tasks. In the RSVP task, attending to one target word interferes with the identification of a second word, and in the ANT, incongruent flanking arrows conflict with identifying the direction of the central arrow. Nicotine had no effect on the conflicting elements of either task in smokers and nonsmokers.
To our knowledge, this is the first study to investigate the effect of nicotine on the attentional blink phenomenon, a form of executive attention. Nicotine had no effect on T2 word identification at early T1–T2 lags when the competition for resources is greatest. We previously found impaired identification of T1 words in smokers after overnight tobacco deprivation when words were presented for 113 ms, but deprivation also had no effect on the attentional blink (Heinz et al., 2007). Consistent with our negative findings, Kleykamp et al. (2005) reported no effect of nicotine gum on ANT executive attention in nonsmokers. AhnAllen et al. (2008) reported improved performance in smokers following transdermal nicotine, but because subjects were tobacco deprived overnight, the improvement was likely due to withdrawal relief. Executive attention in the ANT also was not sensitive to the nicotinic antagonist, mecamylamine (Thienel et al., 2009).
In contrast to the lack of effect on executive attention, nicotine-enhanced alerting attention by decreasing errors of commission on the CPT in nonsmokers and improving the correct identification of T1 target words on the RSVP task in smokers. There was no nicotine effect on alerting as measured by the ANT, which might be a function of the tasks measuring alerting in different ways—that is, the ANT is a cued reaction time task. The differential responses to nicotine between smokers and nonsmokers on these tasks is not unusual in the literature (Heishman et al., 2010) and might reflect a combination of chronic nicotine exposure and preexisting trait differences (Pomerleau et al., 2009).
We hypothesized that nonsmokers would show greater subjective and cardiovascular effects of nicotine because of nicotine tolerance in smokers (Perkins et al., 2001). Nonsmokers were indeed more sensitive to negative effects such as jittery and dizzy; but they, as did smokers, rated all doses of nicotine as having a good drug effect. Both groups were similarly sensitive to the cardiovascular effects of nicotine.
In summary, the acute administration of intranasal nicotine improved alerting attention in nonsmokers as measured by the CPT, and in smokers as measured by the RSVP. Nicotine had no effect on executive attention as measured by the RSVP and the ANT. Empirical data on the elements of attention enhanced by nicotine might guide the development of novel medications for the treatment of tobacco dependence. Additionally, in a recent meta-analysis (Heishman et al., 2010), significant positive effect sizes of nicotine on cognitive performance were observed in both nonsmokers and smokers, with no difference between the two groups. The authors concluded that the results in nonsmokers is indirect evidence that cognitive enhancement might be one reason people decide to start smoking. However, whether or not the cognitive enhancing effects of nicotine reinforce cigarette smoking remains to be answered.
Supplementary Material
Supplementary Table 1 can be found online at http://ntr.oxfordjournals.org/content/early/2012/05/09/ntr.nts108/suppl/DC1
Funding
This research was funded by the National Institutes of Health (NIH) Intramural Research Program , National Institute on Drug Abuse.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
We thank Drs. Jennifer Schroeder and David Epstein for statistical consultations and the National Institute on Drug Abuse clinical support staff for their assistance in conducting the study.
References
- AhnAllen CG, Nestor PG, Shenton ME, McCarley RW, Niznikiewicz MA. Early nicotine withdrawal and transdermal nicotine effects on neurocognitive performance in schizophrenia. Schizophrenia Research. 2008;100:261–269. doi: 10.1016/j.schres.2007.07.030. doi:10.1016/j.schres.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2000. doi:10.1176/appi.books.9780890423349. [Google Scholar]
- Fan J, Gu X, Guise KG, Liu X, Fossella J, Wang H, et al. Testing the behavioral interaction and integration of attentional networks. Brain and Cognition. 2009;70:209–220. doi: 10.1016/j.bandc.2009.02.002. doi:10.1016/j.bandc.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. doi:10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Heinz A, Waters AJ, Taylor RC, Myers CS, Moolchan ET, Heishman SJ. Effect of tobacco deprivation on the attentional blink in rapid serial visual, presentation. Human Psychopharmacology: Clinical and Experimental. 2007;22:89–96. doi: 10.1002/hup.826. doi:10.1002/hup.826. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berlin) 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. doi:10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: A review of effects on human performance. Experimental and Clinical Psychopharmacology. 1994;2:345–395. Retrieved from http://www.sciencedirect.com/science/journal/10641297. [Google Scholar]
- Kleykamp BA, Jennings JM, Blank MD, Eissenberg T. The effects of nicotine on attention and working memory in never-smokers. Psychology of Addictive Behaviors. 2005;19:433–438. doi: 10.1037/0893-164X.19.4.433. doi:10.1037/0893-164X.19.4.433. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. doi:10.1016/S0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. doi:10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Sanders M, Fonte C, et al. Dissociation of nicotine tolerance from tobacco dependence in humans. Journal of Pharmacology and Experimental Therapeutics. 2001;296:849–856. Retrieved from http://jpet.aspetjournals.org/content/296/3/849.short. [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Snedecor SM, Finkenauer R, Mehringer AM, Langenecker SA, et al. Substance use, trait measures, and subjective response to nicotine in never-smokers stratified on parental smoking history and sex. Nicotine & Tobacco Research. 2009;11:1055–1066. doi: 10.1093/ntr/ntp099. doi:10.1093/ntr/ntp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. doi:10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. doi:10.1037/0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Sherwood N. Effects of nicotine on human psychomotor performance. Human Psychopharmacology: Clinical & Experimental. 1993;8:155–184. doi:10.1002/hup.470080303. [Google Scholar]
- Thiel CM, Fink GR. Effects of the cholinergic agonist nicotine on reorienting of visual spatial attention and top-down attentional control. Neuroscience. 2008;152:381–390. doi: 10.1016/j.neuroscience.2007.10.061. doi:10.1016/j.neuroscience.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Thienel R, Voss B, Kellermann T, Reske M, Halfter S, Sheldrick AJ, et al. Nicotinic antagonist effects on functional attention networks. International Journal of Neuropsychopharmacology. 2009;12:1295–1305. doi: 10.1017/S1461145709990551. doi:10.1017/S1461145709990551. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. doi:10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.