Abstract
This study was conducted to determine the presence of Acinetobacter and Rickettsia species DNA in lice and Melophagus ovinus (sheep ked) of animals from Oromia Regional State in Ethiopia. From September through November 2011, a total of 207 cattle, 85 sheep, 47 dogs and 16 cats were examined for ectoparasites. Results of morphological identification revealed several species of ectoparasites: Linognathus vituli (L. vituli), Bovicola bovis (B. bovis) and Solenopotes capillatus (S. capillatus) on cattle; B. ovis and Melophagus ovinus (M. ovinus) on sheep; and Heterodoxus spiniger (H. spiniger) on dogs. There was a significantly (p≤0.0001) higher prevalence of L. vituli observed in cattle than both S. capillatus and B. bovis. Molecular identification of lice using an 18S rRNA gene analysis confirms the identified lice species by morphological methods. We detected different Acinetobacter species among lice (11.1%) and keds (86.4%) including A. soli in L. vituli of cattle, A. lowffii in M. ovinus of sheep, A. pittii in H. spiniger of dogs, 1 new Acinetobacter spp. in M. ovinus and 2 new Acinetobacter spp. in H. spiniger of dogs using partial rpoB gene sequence analysis. There was a significantly higher prevalence of Acinetobacter spp. in keds than in lice (p≤0.00001). Higher percentage of Acinetobacter spp. DNA was detected in H. spiniger than in both B. ovis and L. vituli (p≤0.00001). Carbapenemase resistance encoding genes for blaOXA-23, blaOXA-24, blaOXA-58, blaNDM-1 and blaOXA-51 were not found in any lice and keds. These findings suggest that synanthropic animals and their ectoparasites might increase the risk of human exposure to zoonotic pathogens and could be a source for Acinetobacter spp. infections in humans. However, additional epidemiological data are required to determine whether ectoparasites of animals can act as environmental reservoirs and play a role in spreading these bacteria to both animal and human hosts.
Introduction
Lice and sheep keds (Melophagus ovinus) are two of the most common and economically important ectoparasites of domestic animals worldwide. They are responsible for a wide range of health problems in domestic animals [1]. In infested animals they cause losses in productivity; due to anemia, loss of wool or hair due to scratching, biting and rubbing, disruption in feeding, hide and skin damage, secondary skin infections and damage, reduced newborn birth weights, abortion in pregnant animals, and damage to fences, equipment and buildings due to excessive rubbing and scratching [2], [3].
Several species of lice have been found on Ethiopian cattle; one species of chewing lice, Bovicola (Damalinia) bovis, and three species of sucking lice, including Linognathus vituli (the long-nosed cattle louse), Solenopotes capillatus (the little blue cattle louse) and Haematopinus quadripertusus (the cattle tail louse) [4]. On sheep, the biting lice Bovicola (Damalinia) ovis; one species of sucking lice, Linognathus ovillus and one species of the fly, Melophagus ovinus, are common ectoparasites [5], [6]. On goats, one species of biting lice, Bovicola (Damalinia) caprae, and the sucking lice Linognathus stenopsis have been reported [6], [7]. On dogs, the biting lice Heterodoxus spiniger and Trichodectes canis as well as one species of sucking lice, Linognathus steosus, were reported. In contrast, there are no reports available on the lice from cats in Ethiopia [8].
All the previous reports on the lice and sheep keds of animals in Ethiopia resulted from studies of other ectoparasites, specifically ticks and mange mites [6], [7], [9], [10], [11]. The only lice-specific study in Ethiopia was carried out by Kumsa and Bekele (2008) on the lice of cattle in the Endegagn district. Earlier studies focus on the epidemiology, species composition, distribution and impact of ectoparasites on the skin and hides of food animals. Studies on the role of lice and keds of domestic animals as vectors of pathogens of veterinary and medical importance have not yet been conducted in Ethiopia.
Acinetobacter species are Gram-negative coccobacilli bacteria commonly found in water, soil, mud, living organisms, vegetables, as well as in the feces, urine and skin of humans and animals [12], [13], [14], [15]. Currently, the genus comprises 23 validly named species and 12 genomic species. Owing to difficulty of precise identification of all members to the species level with advancement and development of new techniques in molecular methods, the taxonomy of the genus Acinetobacter has been continually revised [16], [17], [18]. Recently, formerly Acinetobacter genomic sp. 3 was renamed as A. pittii, Acinetobacter genomic sp. 13TU as A. nosocomialis and Acinetobacter genomic sp. G13 as A.lwoffii [16].
In humans, members of the genus Acinetobacter have emerged as opportunistic pathogens, and are frequently implicated in various types of infections, especially in immunocompromised individuals and intensive health care units [14] throughout the world. In tropical countries, they are associated with severe community-acquired infections [13]. They have the capability to survive for prolonged periods under a wide range of environmental conditions [13]. A. baumannii is described as the most common species in this genus frequently associated with outbreaks and has been repeatedly reported to develop a high level of resistance against all available classes of antimicrobial drugs in many parts of the world [12]. A. pittii is reported as the second most commonly isolated Acinetobacter species after A. baumannii in human patients [16], [17]. More recently other less known species such as A. lwoffii and A. soli have been associated with serious infections and considered as emerging pathogens [14], [19], [20]. For instance A. lwoffii has been associated with acute gastroenteritis in USA [21], multidrug resistant A. lwoffii in southern Thailand [22], A. soli outbreak as a cause of infection in neonatal intensive health care unit in Korea [23] and nosocomial bloodstream infections due to A. pittii in United States were reported.
In animals infection due to Acinetobacter species is considered as an emerging problem due to escalating in the number of reports from many countries of the world. Nosocomial infection by A. baumannii in dogs and cats in intensive care unit in Switzerland [24], infection due to A. baumannii in pets and horses in Switzerland [25], multidrug resistant Acinetobacter spp. in veterinary clinics in Germany [12], detection of A. baumannii in samples from cattle and pigs slaughtered for human consumption in major Scottish abattoirs [26], carbapenemase producing Acinetobacter spp. in cattle from France [27] and OXA-23 producing Acinetobacter spp. from faeces of horses in Belgium [28] are some of the current reports that notify the growing importance of Acinetobacter spp. in veterinary medicine mirroring the situation happening in human medicine.
In arthropods Acinetobacter spp. have been detected in different species including tsetse flies, sand flies, mosquitoes, fleas and ticks in many countries of the world. Details on Acinetobacter spp. detected in arthropods, species of vectors and methods of detection is presented (Table 1). Also, recently several investigators detected A. baumannii in both body and head lice of humans from some countries [15], [29], [30], [31]. Despite the worldwide distribution and great economic significance of these ectoparasites, information is not available on the occurrence of Acinetobacter species in the lice and flies found on domestic animals. In addition, there is little known about Rickettsia species in the lice and flies of domestic animals in Ethiopia. Therefore, the current study investigated the presence of these bacteria in arthropods of domestic animals in six districts in Oromia Regional State, Ethiopia.
Table 1. Summary of Acinetobacter spp. detected in various species of arthropods from different countries of the world.
| Acinetobacter sp. | Arthropod sp. | Detection method | Country | Reference |
| A. radioresistans | Ctneocephalides felis | Culture and PCR | Australia | [53] |
| A. johnsonii | Ct. felis | Culture and PCR | Australia | [53] |
| A. junii | Ct. felis | Culture and PCR | Australia | [53] |
| A. lwoffii | Ixodes holocyclus | Culture and PCR | Australia | [53] |
| A. lwoffii | Boophilus microplus | Culture and PCR | Australia | [53] |
| A. johnsonii | Boophilus microplus | Culture and PCR | Australia | [53] |
| A. junii | Boophilus microplus | Culture and PCR | Australia | [53] |
| A. radioresistans | Boophilus microplus | Culture and PCR | Australia | [53] |
| A. baumannii | Lutzomyia longipalpis | Culture and PCR | Brazil | [39] |
| A. juni | Culex quinquefasciatus | PCR | India | [54] |
| A. calcoaceticus | Culex quinquefasciatus | PCR | India | [54] |
| A. calcoaceticus | Anopheles stephensis | PCR and biochemical | Iran | [55] |
| A. calcoaceticus | Anopheles maculipennis | PCR and biochemical | Iran | [55] |
| Acinetobacter sp. | Lutzomyia longipalpis | PCR | Brazil | [56] |
| Acinetobacter sp. | Bemisia tabaci | PCR | Iran | [44] |
| Acinetobacter sp. | Glossina palpalis palpalis | Culture and PCR | Cameroon | [57] |
| Acinetobacter sp. | Bactericera cockerelli | PCR | USA | [42] |
| A. genomosp. 3 | Aedes albopictus | PCR | Madagascar | [58] |
| A. genomosp. 13U | Aedes aegypti | PCR | Madagascar | [58] |
| A. baumannii | Glossina palpalis palpalis | Culture and PCR | Angola | [40] |
Materials and Methods
Study areas and animals
Lice and Melophagus ovinus were collected from indigenous cattle, sheep and dogs in six different districts in Oromia Regional State, Ethiopia: Asalla, Walmara, Shano, Ada'a, Bedele and Gachi. The districts are located in 5 zones in the central, southeast and southwestern parts of the country, with various climates and agroecology (Table 2). Ectoparasite collections were performed from September through November of 2011. A long rainy season from July to September, a short rainy season from March to May and a dry season from November to April prevail in all the study districts. In addition, October is a post rainy month with only few days of raining while November is a month during which the dry season commences hence is without raining or rarely for some days. The farming system in all the districts is characterized by a mixed crop-livestock production system. The livestock in the study areas are traditionally managed under extensive production systems [32].
Table 2. Description of study districts and number of study animals in Oromia Regional State.
| District | Zone | Km from Addis Ababa | Agroecology | Altitude in m.a.s.l | Coordinates | Annual rainfall in mm | Av. Annual Temp in °C | No. Animals examined |
| Asalla | Arsi | 175 southeast | Highland | 2500 | 7°56′58′.57′′N 39°8′23.42′′ E | 2000–4000 | 20–30 | Sheep = 39 |
| Shano | North Showa | 70 Northeast | Highland | 2861 | 9°20.00.00′′N 39°18′00.00′′E | 945.4 | 6.1–18.5 | Cattle = 38 Sheep = 19 |
| Walmara | West Showa | 45 west | Highland | 2500 | 9°7′50.64′′N 38°28′38.46′′E | 1060 | 4.6–23.3 | Cattle = 42 |
| Ada'a | East Showa | 47 east | Midland | 1911 | 8°44′37.69′′N 38°59′19.28′′E | 1911 | 13–26.5 | Cattle = 53 Dogs = 47 Cats = 16 |
| Bedele | Illubabora | 483 southwest | Midland | 1974 | 8°,27′1.76′′N 36°21′5.08′′E | 1400 | 12.5–27.5 | Cattle = 32 Sheep = 21 |
| Gachi | Illubabora | 460 southwest | Midland | 1751 | 9°14′2.57′′N 35° 4′48.46′′E | 1300 | 13–28 | Cattle = 42 Sheep = 6 |
m.a.s.l = meters above sea level; Av. = average; °C = degrees Celsius.
Collection and morphological identification of ectoparasites
Lice and flies were carefully removed manually, using forceps or by hand, to avoid any damage to the body and were then placed in vials containing 70% ethanol for subsequent identification. In the case of lice collected from dogs, the dog's body was brushed for ten minutes with a flea comb as previously described [8]. All lice and flies from the same animal were put in the same vial and transported to the Laboratory of the World Health Organization Collaborative Center for Rickettsial Diseases and Arthropod-borne Bacterial Diseases located in Marseille, France. Morphological identification of ectoparasites and molecular studies were performed from the end of 2011 through 2012. All of the ectoparasites were identified to the species level using a microscope and the morphological identification keys described [1], [3]. Photographs of the dorsal and ventral body parts of each ectoparasite were captured (Fig. 1), and the number and sex of each louse and fly was determined.
Figure 1. Photographs of morphologically identified 5 species of lice and Melophagaus ovinus collected from domestic animals.
A, Bovicola ovis of sheep; B, Bovicola bovis of cattle; C, Heterodoxus spiniger of dog; D, Linognathus vituli of cattle; E, Solenopotes capillatus of cattle; F, Melophagus ovinus (sheep ked) of sheep.
Molecular identification of ectoparasites
Prior to DNA extraction, each specimen was rinsed twice in sterile water for 15 minutes and then dried on sterile filter paper. Each specimen was longitudinally cut into two equal halves. Genomic DNA was extracted from each specimen using the QIAamp DNA tissue extraction kit (Qiagen, Hilden, Germany) as per the instructions of the manufacturer. DNA from each ectoparasite was eluted in 200 µl of TE buffer and stored at −20°C under sterile conditions to preclude any contamination until the sample was used for PCR. The second half of each louse and fly was kept at −80°C as a backup sample.
For molecular species identification, DNA samples of 3 to 8 randomly selected individual lice per species were subjected to standard PCR in an automated DNA thermal cycler to amplify a fragment of the 18S rRNA gene as described [33]. The PCR was carried out in a Peltier PTC-200 model thermal cycler (MJ Research Inc, Watertown, Mass.). The amplified products were detected by electrophoresis on 2% agarose gels in TBE 0.5× buffer, stained with ethidium bromide and visualized using ultraviolet (UV) transillumination. A DNA molecular weight marker (Boehringer-Mannheim VI, Germany) was used to estimate the size of the products. The positive controls consisted of one sample from Pediculus humanus capitis collected in Mali and one sample of P. humanus humanus collected in Algeria. These samples were included in the PCR assay, and sterile water was used as negative control.
The PCR products were cleaned of excess primers and nucleotides using a QIAquick Spin PCR Purification Kit (Qiagen) as per instructions of the manufacturer. Purified DNA was sequenced using the BigDye Terminator Cycle Sequencing Ready Reaction Kit (ABI PRISM, PE Applied Biosystems, Foster City, CA). All obtained sequences were assembled and edited using Chromas Pro1.34 (Technelyium Pty. Ltd., Tewantin). The sequences of the 18S rRNA genes were then subjected to BLAST analysis to determine similarities to those available in GenBank and to construct a phylogenetic tree using Mega 5 software (Molecular Evolution Genetic Analysis; The Biodesign Institute, Tempe, AZ).
Molecular detection of Acinetobacter and Rickettsia species
All the specimens were individually tested for the presence of Acinetobacter species DNA targeting the rpoB gene [30] by real-time quantitative (q) PCR as per the instructions of the manufacturer (Applied Biosystems, Foster City, CA). In addition, the ectoparasite DNA was tested for spotted fever group Rickettsia with primers targeting the gltA gene specific for this group [34] and for typhus group Rickettsia with primers targeting the Rpr274P gene, which encodes a hypothetical protein [35]. Sterile water was used as negative control; A. baumannii, R. montanensis and R. typhi DNA were used as positive controls. The samples were considered positive when cycle thresholds (Ct) were <35.
Molecular detection of carbapenemase encoding genes
All the DNA of lice (n = 82) and flies (n = 19) positive for Acinetobacter species were tested for the presence of carbapenemase encoding genes by qPCR targeting blaOXA-23, blaOXA-24, blaOXA-58 and blaNDM-1 using primers, probes and all conditions as has been described before [15], [36]. In addition, a total of 32 lice and 10 flies with sufficient amount of DNA were evaluated for carbapenemase encoding genes by standard PCR targeting blaOXA-51 and blaOXA-23 with primers and all conditions as described before [15].
Molecular identification of Acinetobacter spp. by partial rpoB gene
A total of 32 lice and 10 keds DNA of sufficient amount and positive for Acinetobacter spp. by qPCR were further subjected to standard PCR targeting partial rpoB gene (zone 1) to identify Acinetobacter spp. using the primers and all conditions as described before [17]. Sterile water and A. genomosp DNA were used as negative and positive controls, respectively. Detection of amplified products, cleaning of excess primers and nucleotides from DNA, sequencing, assembling and edition of sequences, BLAST analysis and rpoB gene phylogenetic tree construction with Maximum likelihood statistics of Mega 5 were all performed using similar methods as described for 18S rRNA gene for lice above.
Ethical statement
Ethical approval for the collection of lice and flies from domestic animals was obtained from the animal research ethics board (Agreement # 14/160/550/2011) of the College of Veterinary Medicine and Agriculture of Addis Ababa University. All necessary oral permits were obtained for the described field studies, including permission of administration and agricultural office of each Ethiopian district and from each animal owner. Ectoparasite collections are not harmful and are not against the welfare of animals. No collection had been done from privately-owned, wildlife, national park or other protected areas and endangered or protected species.
Data analysis
Microsoft Excel was used for data management. Descriptive statistics such as percentages and means were employed to summarize the proportions of infestations with lice and keds. Statistical analysis was performed with EpiInfoTM7 and a P-value of <0.05 was considered significant.
Results
Morphological identification of ectoparasites
A total of 207 cattle, 85 sheep, 47 dogs and 16 cats in six districts were examined for the presence of lice and sheep keds (Table 2). The results of the morphological identification of the collected lice and flies from infested animals revealed a total of 408 Linognathus vituli, 4 Bovicola bovis and 3 Solenopotes capillatus from cattle; 22 Heterodoxus spiniger from dogs; 298 Bovicola ovis and 22 Melophagus ovinus from sheep (Fig. 1). Furthermore, the study showed that out of all of the examined cattle, 19.3% (40/207) were infested with L. vituli, 0.5% (1/207) with S. capillatus and 0.5% (1/207) with B. bovis. In cattle, there was a significantly higher prevalence of L. vituli than both S. capillatus and B. bovis (p≤0.0001). Of the total examined sheep, 48.2% (41/85) were infested with B. ovis and 21.05% (4/19) were positive for M. ovinus. In addition, 19.1% (9/47) of the examined dogs were infested with H. spiniger. Alternatively, lice were not detected on any of the cats studied.
Molecular identification of ectoparasites
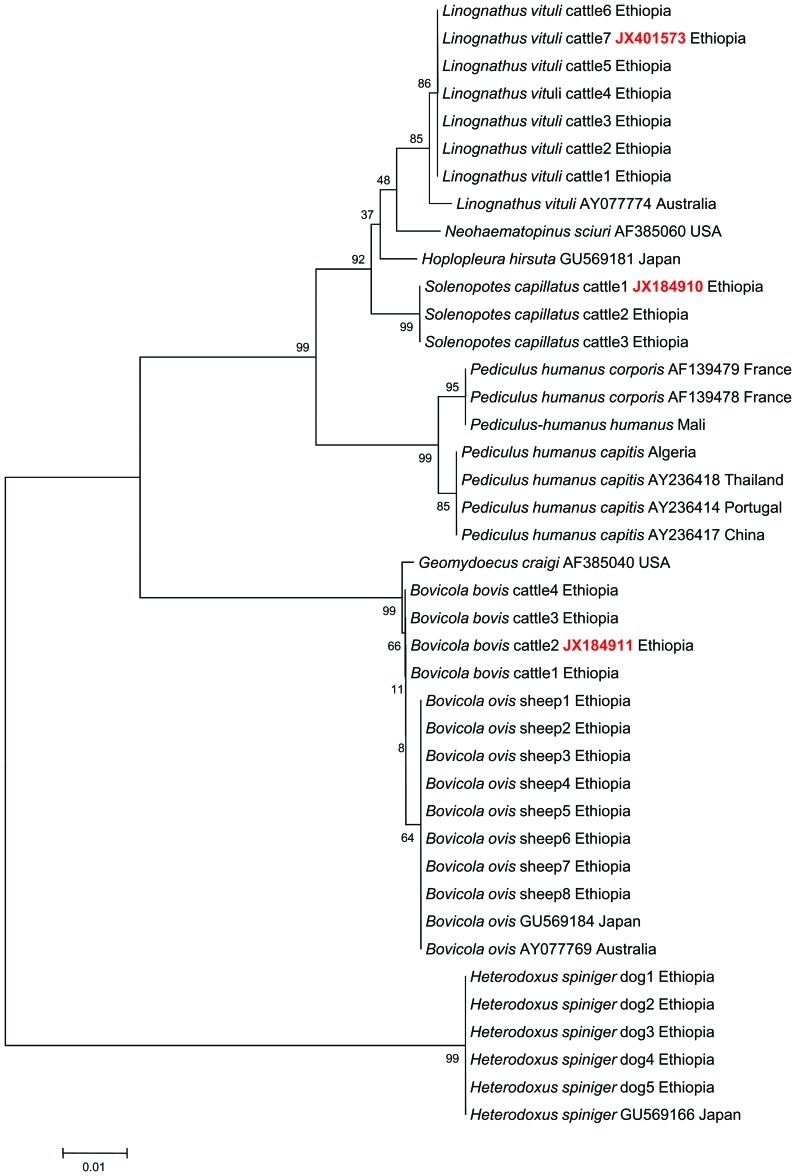
Molecular identification of the lice based on the 18S rRNA gene analysis was used to confirm the species of lice identified by morphological methods. A BLAST analysis of 18S rRNA gene sequences of lice from dogs morphologically identified as H. spiniger (n = 5) showed 100% (509/509) similarity to the GenBank reference of H. spiniger collected from Japan (GU569166) (Fig. 2). Likewise, a BLAST analysis of 18S rRNA gene sequences of lice that were collected from cattle and were morphologically identified as L. vituli (n = 7) showed 99.3% (553/557) similarity to Linognathus vituli collected in Australia (GenBank Access. No. AY077774). Four mutations were detected between our sequence and the reference (AY077774) at 283 bp (T-G), 327 bp (T-A), 352 bp (T-C), and 420 bp (T-C). This sequence was submitted to GenBank under accession number JX401573.
Figure 2. Phylogenetic tree based on 18S gene sequences of lice species collected from domestic animals.
Accession numbers in red color are 18S gene sequence of lice of animals from Ethiopia recently deposited in the GenBank. Minimum evolution method was used to build the phylogentic tree. Bootstrap values are indicated at the nodes.
Interestingly, a sequence analysis of the 18S rRNA gene of 3 lice from cattle morphologically identified as Solenopotes capillatus showed 92.2% (536/581) homology with the GenBank sequence of Neohaematopinus sciuri (AF423798) and 92% (520/565) homology with Hoplopleura hirsute collected in Japan from the cotton rat Sigmodon hispidus (GU569181).
A BLAST analysis of the 18S rRNA gene of 4 Bovicola bovis lice from cattle showed 99.8% (494/495) similarity to B. ovis (GenBank Access No. GU569184) and 99.4% (510/513) similarity to Geomydoecus craigi (GenBank Access No. AF385040) (Fig. 2). There are no 18S rRNA gene sequences available in GenBank for S. capillatus and Bovicola bovis. Thus, these two sequences were submitted to GenBank under accession numbers JX184910 and JX184911, respectively. An analysis of the 18S rRNA gene of Bovicola ovis (n = 8) from sheep revealed 100% (495/495) similarity with Bovicola ovis detected in Japan and Australia with the sequences GU569184 and AY077769, respectively.
An analysis of the 18S rRNA gene from Pediculus humanus humanus (n = 1) from Mali revealed 99.4% (496/499) similarity with Pediculus humanus corporis detected in France with Accessions Nos. AF139479 and AF139478, respectively. An analysis of the 18S rRNA gene from Pediculus humanus capitis (n = 1) from Algeria revealed 99.8% (518/520) similarity with the Pediculus humanus capitis detected in Thailand, Portugal and China with Accessions Nos. AY236418, AY236414 and AY236417, respectively.
A phylogenetic tree was constructed from the 18S rRNA gene sequences of lice collected in this study and the most similar sequences in the GenBank using the Neighbor-joining statistics of Mega 5. H. spiniger reference lice and our lice sample from dogs that belong to the family Boophidae in the suborder Amblycera formed one group on the phylogenetic tree (Fig. 2). Reference Linognathus vituli and our lice sample from cattle that belong to the family Linognathidae under the suborder Anoplura clustered together. The 3 individual S. capillatus lice from cattle, which shared the same family and suborder with L. vituli, clustered near the cattle louse. Both the control and the reference P. humanus capitis and P. humanus humanus isolated from human head and body lice, belonging to the family Pediculidae under the suborder Anoplura, formed a separate group on the phylogenetic tree that was close to L. vituli from cattle. The Bovicola ovis reference lice and our lice sample from sheep that belong to the family Trichodectidae in the suborder Ischnocera clustered in a separate group close to the four B. bovis cattle lice, which belong to the same genus, family and suborder (Fig. 2).
Detection of Acinetobacter and Rickettsia species in lice and M. ovinus
We detected Acinetobacter spp. in a total of 82 lice (11.1%) and 19 flies (86.4%) (Table 3). In our study, there was a significantly higher prevalence of Acinetobacter spp. in flies than in lice (82/735 vs. 19/22; p≤0.00001). The study showed that a higher percentage of Acinetobacter spp. DNA was detected in H. spiniger of dogs than in B. ovis and L. vituli (15/22 vs. 19/298; 47/408; p≤0.00001). The prevalence of Acinetobacter spp. in L. vituli collected from cattle was significantly higher than in B. ovis from sheep (47/408 vs 19/298; p = 0.02). Acinetobacter spp. was not detected in any of the 4 Bovicola bovis collected from cattle (Table 3).
Table 3. Percentage of Acinetobacter spp. detected by qPCR in lice and flies collected from domestic animals in six districts in Oromia.
| District (Number of positive samples for Acinetobacter spp./Number of tested samples) (%) | |||||||
| Lice spp. | Asalla (%) | Shano (%) | Walmara (%) | Ada'a (%) | Bedele (%) | Gachi (%) | Total (%) |
| B. ovis | 13/234(5.5) | 5/57(8.8) | - | - | 1/7(14.3) | - | 19/298(6.4) |
| L. vituli | - | 7/51(13.7) | 10/93(10.7) | 4/84(4.8) | 1/12(8.3) | 25/168(14.9) | 47/408(11.5) |
| S. capillatus | - | - | - | 1/3(33.3) | - | - | 1/3(33.3) |
| B. bovis | - | 0/4(0) | - | 0/0 (0) | - | - | 0/4(0) |
| H. spiniger | - | - | - | 15/22(68.2) | - | - | 15/22(68.2) |
| Total lice | 82/735(11.1) | ||||||
| Fly species | |||||||
| M. ovinus | - | 19/22(86.4) | - | - | - | - | 19/22(86.4) |
| Total flies | 19/22(86.4) | ||||||
Results of our study revealed that out of the total lice infested animals, 88.9% (8/9) of dogs were infested with H. spiniger, 45% (18/40) of cattle were infested with L. vituli and 34.4% (14/41) of sheep were infested with B. ovis and harbored at least 1 louse positive for Acinetobacter spp. (Table 4). Similarly, Acinetobacter spp. was detected at least in one M. ovinus in all the 4/4 (100%) infested sheep. Highest proportion of Acinetobacter spp. was observed in cattle infested with L. vituli in Walmara and Gachi districts whereas highest percentage of Acinetobacter spp. was noted in sheep infested with B. ovis in Shano and Asalla districts (Table 4).
Table 4. Proportion of infested animals positive for Acinetobacter spp. in lice and keds by qPCR in six districts in Oromia.
| District | No. animals positive for Acinetobacter spp./No. animals infested with lice or fly | |||||
| B. ovis (Sheep) | L. vituli (Cattle) | S. capillatus (Cattle) | B. ovis (Cattle) | H. spiniger (Dog) | M. vinus (Sheep) | |
| Asalla | 9/29 (31.0%) | - | - | - | - | - |
| Walmara | - | 6/9 (66.7%) | - | - | - | - |
| Shano | 4/10 (40%) | 2/9 (22.2%) | - | 0/1 | - | 4/4 (100%) |
| Ada'a | - | 3/9 (33.3%) | 1/1 (100%) | - | 8/9 (88.9%) | - |
| Bedele | 1/2(50%) | 1/4 (25%) | - | - | - | - |
| Gachi | - | 6/9 (66.7%) | - | - | - | - |
| Total | 14/41(34.4%) | 18/40 (45%) | 1/1 (100%) | 0/1 | 8/9 (88.9%) | 4/4 (100%) |
A molecular investigation of the 735 lice collected from domestic animals and 22 Melophagus ovinus collected from sheep using qPCR did not produced any positive results for either spotted fever or typhus group Rickettsia species.
Molecular identification of Acinetobacter spp
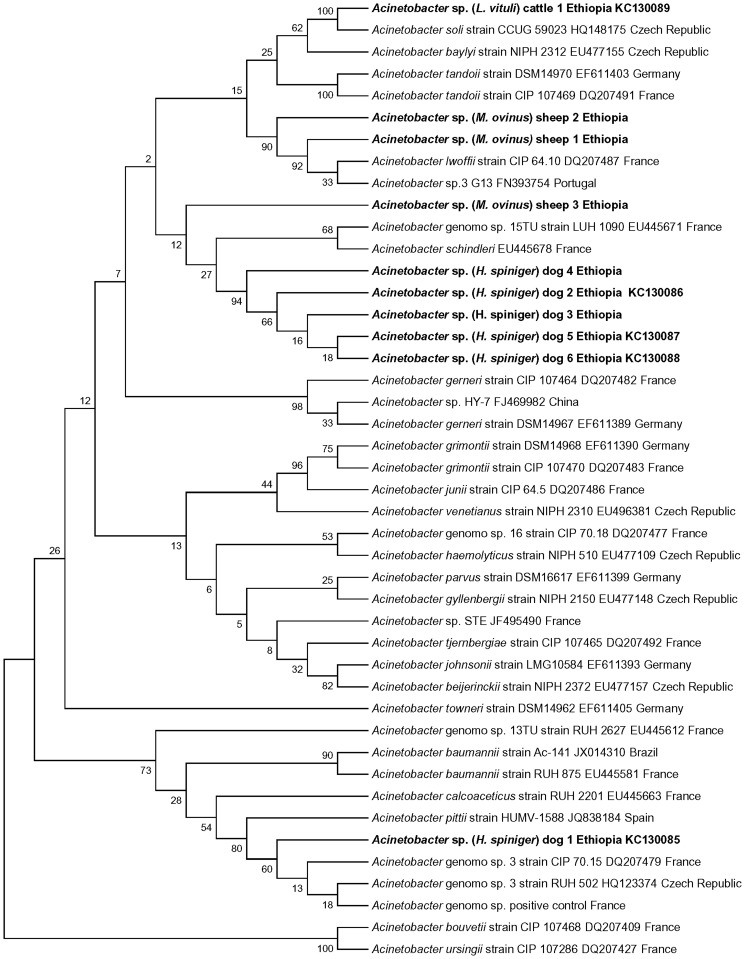
We succeeded in the amplification of 350 bp fragment of partial rpoB gene from a total of 10 samples including one from L. vituli of cattle, 3 from M. ovinus of sheep and 6 from H. spiniger of dogs (Table 5). BLAST analysis of partial rpoB gene sequence and rpoB phylogenetic tree showed the presence of A. soli in L. vituli of cattle, A. lwoffii in M. ovinus of sheep, 1 new Acinetobacter sp. in M. ovinus of sheep, A. pittii in H. spiniger of dogs and 2 new Acinetobacter sp. in H. spiniger of dogs (Table 5 and Fig. 3). Five of these 10 sequences were submitted to GenBank under accession numbers KC130085-89, respectively.
Table 5. Summary of BLAST analysis of partial rpoB gene sequences obtained from lice and keds of domestic animals in six districts in Oromia, Ethiopia.
| Lice/fly spp | Host spp | Length (bp) | Nearest match in GenBank | % similarity |
| H. spiniger | Dog | 383 | Acinetobacter genomosp. 3 (DQ207479) | 99.2% |
| H. spiniger | Dog | 351 | Acinetobacter sp.Hy-7 (FJ469982) | 90.8% |
| H. spiniger | Dog | 391 | Acinetobacter sp.Hy-7 (FJ469982) | 90.7% |
| H. spiniger | Dog | 310 | Acinetobacter sp. Hy-7 (FJ469982) | 86.5% |
| H. spiniger | Dog | 387 | Acinetobacter sp. Hy-7 (FJ469982) | 90.5% |
| H. spiniger | Dog | 388 | Acinetobacter sp. Hy-7 (FJ469982) | 90.5% |
| L. vituli | Cattle | 381 | Acinetobacter soli (HQ148175) | 98.9% |
| M. ovinus | Sheep | 365 | Acinetobacter sp.G13 (FN393754) | 98.9% |
| M. ovinus | Sheep | 293 | Acinetobacter sp.G13 (FN393754) | 94.39% |
| M. ovinus | Sheep | 328 | Acinetobacter sp-Hy-7 (FJ469982) | 90.2% |
| P. h. humanus (Control) | Man | 383 | Acinetobacter genomosp.3 (DQ207479) | 99.2% |
Figure 3. Phylogenetic tree based on partial rpoB gene sequences of Acinetobacter species.
Maximum Likelihood method was used to build the phylogentic tree. Bootstrap values are indicated at the nodes. Bold indicates the taxonomic position of Acinetobacter species identified in this study.
Detection of carbapenemase encoding genes in Acinetobacter species
A molecular investigation of 82 lice and 19 keds positive for Acinetobacter spp. by qPCR did not produced any positive results for blaOXA-23, blaOXA-24, blaOXA-58 and blaNDM-1 genes encoding for carbapenemase resistance. Likewise, investigation of 32 lice and 10 keds DNA by standard PCR never produced any positive results for both blaOXA-23 and blaOXA-51 genes encoding for carbapenemase resistance.
Discussion
In this study, we detected, for the first time, Acinetobacter spp. in the lice and flies of animals from Ethiopia. In addition, we obtained two new sequences of 18S rRNA genes from Bovicola bovis and Solenopotes capillatus collected from cattle. We believe that the results of this study are valid, as all of the negative control samples produced negative results and all the positive control samples tested positive for both Acinetobacter spp. and Rickettsia spp. detection in the lice and flies of these animals. These findings confirmed that our molecular study conditions precluded any accidental cross-contamination of samples during the study period. Similarly, in our 18S rRNA gene study of lice, we obtained sequences from both positive controls, Pediculus humanus capitis and Pediculus humanus humanus, confirming the appropriateness of our working conditions.
In the study, the species of lice morphologically identified as L. vituli, B. bovis and S. capillatus from cattle; H. spiniger from dogs; and B.ovis from sheep (Fig. 1) were confirmed molecularly using 18S rRNA gene sequences. The 18S rRNA gene has been used previously as an important tool to investigate human lice phylogeny [33], to study the evolution of sucking lice [37] and to study the phylogeny of lice [38]. The phylogenetic tree constructed from the 18S rRNA gene sequences of the collected lice and reference lice in the GenBank presented here supports the information from previous studies [37], [38].
In this study, we sequenced the 18S rRNA genes of B. bovis and S. capillatus from cattle for the first time. B. bovis is the only biting lice species in cattle, and it has important morphological features such as a rounded head, reddish-brown color and dark transverse bands on the abdomen [1] (Fig. 1). S. capillatus from cattle are the smallest sucking lice, with morphological characteristics that include a hexagonal sterna plate on the thorax, a weak front pair of legs and prominent abdominal tubercles [3]. In Ethiopia, both species are typically reported with low prevalence [4]. The finding in this study that there was a significantly (p≤0.0001) higher prevalence of L. vituli than both S. capillatus and B. bovis in cattle is in agreement with previous work [4]. Our findings in sheep, dogs and cats of this study are also in line with previous reports [5], [6], [8].
The observation that 11.1% of the lice in the current study were found to contain Acinetobacter spp. is lower than the earlier report of 47% in human head lice and 71% human body lice from Ethiopia [31], 21% in human body lice [29] and 33% in human head lice from Paris [30]. Recently, a low prevalence (4%) of A. baumannii in head lice was reported from Senegal [15]. However, the overall percentage of 86.4% (19/22) of Acinetobacter spp. detected in M. ovinus of sheep in our study is greater than that found in the aforementioned reports. The variation among study districts in the percentage of Acinetobacter spp. in lice and keds in infested animals is most probably attributed to differences in agroecology, animal management and factors like age, sex, physiological status or presence of other concurrent diseases that may favor infection of lice or the animal by the bacteria. This observation coincides with the findings of differences in the prevalence of infection in lice of humans by A. baumannii among different study areas with different altitudes in south western Ethiopia [31].
In line with our findings, Acinetobacter spp. were detected in many species of arthropods in different parts of the world (Table 1). For instance, Acinetobacter spp. have been detected in 13% of Lutzomyia longipalpis (sand flies) in Brazil [39], 7.7% of Glossina palpalis palpalis (tsetse fly) in Angola [40], 8.7% in the gut of the Prionoplus reticularis larvae (wood feeding beetle) in New Zealand [41], up to 75% in Bacterria cockerelli (potato psyllid) in the USA [42], 18.9% in chewing lice of pocket gophers in the USA [43] and 1% in Bemisia tabaci (tobacco whitefly) in India [44]. Moreover, A. baumannii have been detected with a prevalence of 5.1% in the feces of domestic animals in Senegal [15].
Most earlier investigators suggested that it is still yet not exactly determined how both body and head human lice acquired A. baumannii infection [30], [31]. However, some authors argued undiagnosed transient A. baumannii bacteremia in infested patients as a source of infection for body lice but they stated it is not possible to rule out the possibility of acquiring A. baumannii infection in human body lice from external environmental contamination [29]. Other investigators pointed out that bacterial spp. such as Acinetobacter abundant in the environment reside in the gut of several species of arthropods as transient or natural flora [39] acquired commonly by vertical transmission [40] and also are maintained and spread by several mechanisms of horizontal transmission including mating, cofeeding, or contact with contaminated faeces [41], [42], [44]. Having all these facts in mind and due to the ubiquitous nature of Acinetobacter spp. in the environment including on the skin and in the faces of animals [15] lice and keds possibly had acquired Acinetobacter spp. infection from the skin, faces or transient Acinetobacter spp. bacteremia of their host animals. Moreover, it is not possible to rule out infection of lice and keds by vertical route and also probably animals may play a role as reservoirs for these bacteria.
We did not detect Rickettsia DNA in the lice and flies in our study. This finding contrasts with previous work that reported detecting R. helvetica in M. ovinus from sheep, in Linognathus stenopsis from goats, and Rickettsia spp. in Haematopinus eurysternus from cattle in Hungary [45], [46].
The absence of any positive results for blaOXA-23, blaOXA-24, blaOXA-58, blaNDM-1 and blaOXA-51 genes encoding for carbapenemase resistance by both qPCR and standard PCR in Acinetobacter spp. in the lice and keds of domestic animals in our study is in line with the previous finding of absence of resistance to several antimicrobials in A. soli in intensive health care units of neonates in Brazil [23], full susceptibility to several antibiotics of A. lwoffii from acute gastroenteritis in USA [21] and absence of blaOXA-like genes encoding for carbapenemase resistance in A. baumannii from faeces of domestic animals in Senegal [15]. On the other hand our findings contrast the previous multidrug resistance in A. baumannii reported from several countries of the world [12], [36], [47]. This finding also contradicts the observations of multidrug-resistant isolates of Acinetobacter spp. from a range of environmental sources in South Korea [20] and the presence of resistance to antibiotics in A. baumannii and other Acinetobacter spp. from blood cultures in Norway [48]. This variation is most probably attributed to differences in strains of the bacteria among various studies and other many factors contributing for emergence of resistance.
Acinetobacter spp. identification study from DNA of lice and keds of animals in Oromia uncovered the occurrence of 3 previously described species and 3 new Acinetobacter spp. (Table 5 and Fig. 3). All the 3 previously described species, we detected: A. soli from L. vituli of cattle, A. lowffii from keds of sheep and A. pittii from H. spiniger of dogs with high nucleotide sequence identities of 98–100% with their respective reference species in the GenBank (Table 5). This finding supports the criteria established for Acinetobacter spp. identification using partial rpoB gene sequence analysis [17], [18]. In line with our observation higher predominance of other Acinetoabacter spp. (24.8%) than A. baumannii (only 8.8%) from human blood culture isolates was recently reported from Norway [48]. Furthermore, these species are nowadays reported to cause various types of human infections worldwide [21], [23], [49], [50], [51] and are implicated as emerging Acinetobacter spp.
We also identified two new Acinetobacter species from H. spiniger of dogs and one from ked of sheep (Table 5 and Fig. 3). All these 3 new Acinetobacter spp. demonstrated low nucleotide homology with reference rpoB sequence in the GenBank and low bootstrap value in the rpoB phylogenetic tree. Results of our study suggest the presence of specific Acinetobacter species in lice and keds of domestic animals unlike A. baumannii in human lice. We believe that additional in depth epidemiological studies involving other species of lice and ectoparasites of different animal species from vast areas in the world are needed as has been done during the last decade for Bartonella species, ectoparasites and their animal hosts [52]. Further studies are also required to isolate and determine the human health significance of the new Acinetobacter spp. detected in the lice and keds from animals.
To our knowledge, this study is the first to report the presence of DNAs from different Acinetobacter spp. in various species of lice collected from domestic animals and in flies collected from sheep. Our study demonstrates that Acinetobacter spp. are not only common as hospital pathogens and in human lice but they can also be detected in the ectoparasites of animals. Our findings suggest that synanthropic animals and their ectoparasites might play a role to increase the risk of human exposure to zoonotic pathogens and could be a source for Acinetobacter spp. infections in humans. However, additional epidemiological data are required to justify the significance of this finding and to determine whether ectoparasites from animals can act as environmental reservoirs and play a role in spreading these bacteria to both animal and human hosts.
Acknowledgments
The authors greatly acknowledge all animal owners in Oromia and Yonas Abiyi, Teferi Banti, Fikadu and Gurara Megerssa Veterinary professionals of Oromia Regional laboratories for their help during sample collection. Also Amina Boutellis and Seydina M. Diene PhD students at the Faculty of Medicine of Aix Marseille University, are gratefully acknowledged for their professional help during laboratory work of the study.
Funding Statement
The authors have no support or funding to report.
References
- 1.Wall R, Shearer D (1997) Veterinary Entomology; 1, editor. London: Chapman and Hall. 438 p.
- 2. Small RW (2005) A review of Melophagus ovinus (L.), the sheep ked. Vet Parasitol 130: 141–155. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MA, Coop RL, Wall R (2007) Veterinary Parasitology: Wiley-Blackwell. 847 p.
- 4. Kumsa B, Bekele S (2008) Lice Infestation On Cattle In Endegagn District, Southern Ethiopia: Species Composition, Prevalence And Seasonal Pattern. Bull Anim Hlth Prod Afr 56: 213–222. [Google Scholar]
- 5.Kumsa B, Beyecha K, Geloye M (2012) Ectoparasites of sheep in three agro-ecological zones in central Oromia, Ethiopia Onderstepoort J Vet Res 79(1): 7 pages. [DOI] [PubMed]
- 6. Sertse T, Wossene A (2007) A study on ectoparasites of sheep and goats in eastern part of Amhara region, Northeast Ethiopia. Small Rumin Res 69: 55–61. [Google Scholar]
- 7.Beyecha K, Beyene D, Kumsa B (2012) Ectoparasites of goats in three agroecologies in central Oromia, Ethiopia. Comp Clin Pathol DOI 10.1007/s00580-012-1563-x.
- 8.Kumsa B, Mekonnen S (2011) Ixodid ticks, fleas and lice infesting dogs and cats in Hawassa, southern Ethiopia. Onderstepoort J Vet Res 78: 4 pages. [DOI] [PubMed]
- 9. Yacob HT, ATAKLTY H, Kumsa B (2008) Major ectoparasites of cattle in and around Mekelle, northern Ethiopia. Entom Res 28: 26–30. [Google Scholar]
- 10. Chanie M, Negash T, Sirak A (2010) Ectoparasites are the major causes of various types of skin lesions in small ruminants in Ethiopia. Trop Anim Health Prod 42: 1103–1109. [DOI] [PubMed] [Google Scholar]
- 11. Abebayehu T, Kibrom M (2010) Study on ectoparasitic defects of processed skins at Sheba Tannery, Tigray, Northern Ethiopia. Trop Anim Health Prod 42: 1719–1722. [DOI] [PubMed] [Google Scholar]
- 12. Zordan S, Prenger-Berninghoff E, Weiss R, van der Reijden T, van den Broek P, et al. (2011) Multidrug-resistant Acinetobacter baumannii in veterinary clinics, Germany. Emerg Infect Dis 17: 1751–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21: 538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turton JF, Shah J, Ozongwu C, Pike R (2010) Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. J Clin Microbiol 48: 1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kempf M, Rolain JM, Diatta G, Azza S, Samb B, et al. (2012) Carbapenem Resistance and Acinetobacter baumannii in Senegal: The Paradigm of a Common Phenomenon in Natural Reservoirs. PLoS One 7: e39495. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Nemec A, Krizova L, Maixnerova M, van der Reijden TJ, Deschaght P, et al. (2011) Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Res Microbiol 162: 393–404. [DOI] [PubMed] [Google Scholar]
- 17. Gundi VA, Dijkshoorn L, Burignat S, Raoult D, La Scola B (2009) Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology 155: 2333–2341. [DOI] [PubMed] [Google Scholar]
- 18. La Scola B, Gundi VA, Khamis A, Raoult D (2006) Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol 44: 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narciso-da-Rocha C, Vaz-Moreira I, Svensson-Stadler L, Moore ER, Manaia CM (2012) Diversity and antibiotic resistance of Acinetobacter spp. in water from the source to the tap. Appl Microbiol Biotechnol. [DOI] [PubMed]
- 20. Choi JY, Kim Y, Ko EA, Park YK, Jheong WH, et al. (2012) Acinetobacter species isolates from a range of environments: species survey and observations of antimicrobial resistance. Diagn Microbiol Infect Dis 74: 177–180. [DOI] [PubMed] [Google Scholar]
- 21. Regalado NG, Martin G, Antony SJ (2009) Acinetobacter lwoffii: bacteremia associated with acute gastroenteritis. Travel Med Infect Dis 7: 316–317. [DOI] [PubMed] [Google Scholar]
- 22.Nakwan N, Wannaro J, Nakwan N (2011) Multidrug-resistant Acinetobacter lwoffii infection in neonatal intensive care units. Res and Reports in Neonatatology.
- 23. Pellegrino FL, Vieira VV, Baio PV, dos Santos RM, dos Santos AL, et al. (2011) Acinetobacter soli as a cause of bloodstream infection in a neonatal intensive care unit. J Clin Microbiol 49: 2283–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Francey T, Gaschen F, Nicolet J, Burnens AP (2000) The role of Acinetobacter baumannii as a nosocomial pathogen for dogs and cats in an intensive care unit. J Vet Intern Med 14: 177–183. [DOI] [PubMed] [Google Scholar]
- 25. Endimiani A, Hujer KM, Hujer AM, Bertschy I, Rossano A, et al. (2011) Acinetobacter baumannii isolates from pets and horses in Switzerland: molecular characterization and clinical data. J Antimicrob Chemother 66: 2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamouda A, Findlay J, Al Hassan L, Amyes SG (2011) Epidemiology of Acinetobacter baumannii of animal origin. Int J Antimicrob Agents 38: 314–318. [DOI] [PubMed] [Google Scholar]
- 27. Poirel L, Bercot B, Millemann Y, Bonnin RA, Pannaux G, et al. (2012) Carbapenemase-producing Acinetobacter spp. in Cattle, France. Emerg Infect Dis 18: 523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smet A, Boyen F, Pasmans F, Butaye P, Martens A, et al. (2012) OXA-23-producing Acinetobacter species from horses: a public health hazard? J Antimicrob Chemother. [DOI] [PubMed]
- 29. La Scola B, Raoult D (2004) Acinetobacter baumannii in human body louse. Emerg Infect Dis 10: 1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouvresse S, Socolovshi C, Berdjane Z, Durand R, Izri A, et al. (2011) No evidence of Bartonella quintana but detection of Acinetobacter baumannii in head lice from elementary schoolchildren in Paris. Comp Immunol Microbiol Infect Dis 34: 475–477. [DOI] [PubMed] [Google Scholar]
- 31.Kempf M, Abdissa A, Diatta G, Trape JF, Angelakis E, et al. (2012) Detection of Acinetobacter baumannii in human head and body lice from Ethiopia and identification of new genotypes. Int J Infect Dis. [DOI] [PubMed]
- 32.Central Statistics Agency (2008) Ethiopian agricultural sample survey report on livestock and livestock characteristics, Vol II, 2007/08, Addis Ababa, Ethiopia.
- 33. Yong Z, Fournier PE, Rydkina E, Raoult D (2003) The geographical segregation of human lice preceded that of Pediculus humanus capitis and Pediculus humanus humanus . C R Biol 326: 565–574. [DOI] [PubMed] [Google Scholar]
- 34. Socolovschi C, Barbarot S, Lefebvre M, Parola P, Raoult D (2010) Rickettsia sibirica mongolitimonae in traveler from Egypt. Emerg Infect Dis 16: 1495–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walter G, Botelho-Nevers E, Socolovschi C, Raoult D, Parola P (2012) Murine typhus in returned travelers: a report of thirty-two cases. Am J Trop Med Hyg 86: 1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kusradze I, Diene SM, Goderdzishvili M, Rolain JM (2011) Molecular detection of OXA carbapenemase genes in multidrug-resistant Acinetobacter baumannii isolates from Iraq and Georgia. Int J Antimicrob Agents 38: 164–168. [DOI] [PubMed] [Google Scholar]
- 37. Light JE, Smith VS, Allen JM, Durden LA, Reed DL (2010) Evolutionary history of mammalian sucking lice (Phthiraptera: Anoplura). BMC Evol Biol 10: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barker SC, Whiting M, Johnson KP, Murrell A (2003) Phylogeny of the lice (Insecta, Phthiraptera) inferred from small subunit rRNA. Zoologica Scripta 32: 407–414. [Google Scholar]
- 39. Gouveia C, Asensi MD, Zahner V, Rangel EF, Oliveira SM (2008) Study on the bacterial midgut microbiota associated to different Brazilian populations of Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae). Neotrop Entomol 37: 597–601. [DOI] [PubMed] [Google Scholar]
- 40. Geiger A, Fardeau ML, Grebaut P, Vatunga G, Josenando T, et al. (2009) First isolation of Enterobacter, Enterococcus, and Acinetobacter spp. as inhabitants of the tsetse fly (Glossina palpalis palpalis) midgut. Infect Genet Evol 9: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 41. Reid NM, Addison SL, Macdonald LJ, Lloyd-Jones G (2011) Biodiversity of active and inactive bacteria in the gut flora of wood-feeding huhu beetle larvae (Prionoplus reticularis). Appl Environ Microbiol 77: 7000–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nachappa P, Levy J, Pierson E, Tamborindeguy C (2011) Diversity of endosymbionts in the potato psyllid, Bactericera cockerelli (Triozidae), vector of zebra chip disease of potato. Curr Microbiol 62: 1510–1520. [DOI] [PubMed] [Google Scholar]
- 43. Reed DL, Hafner MS (2002) Phylogenetic analysis of bacterial communities associated with ectoparasitic chewing lice of pocket gophers: a culture-independent approach. Microb Ecol 44: 78–93. [DOI] [PubMed] [Google Scholar]
- 44. Singh ST, Priya NG, Kumar J, Rana VS, Ellango R, et al. (2012) Diversity and phylogenetic analysis of endosymbiotic bacteria from field caught Bemisia tabaci from different locations of North India based on 16S rDNA library screening. Infect Genet Evol 12: 411–419. [DOI] [PubMed] [Google Scholar]
- 45. Hornok S, de la Fuente J, Biro N, Fernandez de Mera IG, Meli ML, et al. (2011) First molecular evidence of Anaplasma ovis and Rickettsia spp. in keds (Diptera: Hippoboscidae) of sheep and wild ruminants. Vector Borne Zoonotic Dis 11: 1319–1321. [DOI] [PubMed] [Google Scholar]
- 46. Hornok S, Hofmann-Lehmann R, de Mera IG, Meli ML, Elek V, et al. (2010) Survey on blood-sucking lice (Phthiraptera: Anoplura) of ruminants and pigs with molecular detection of Anaplasma and Rickettsia spp. Vet Parasitol 174: 355–358. [DOI] [PubMed] [Google Scholar]
- 47. Higgins PG, Dammhayn C, Hackel M, Seifert H (2010) Global spread of carbapenem-resistant Acinetobacter baumannii . J Antimicrob Chemother 65: 233–238. [DOI] [PubMed] [Google Scholar]
- 48. Karah N, Haldorsen B, Hegstad K, Simonsen GS, Sundsfjord A, et al. (2011) Species identification and molecular characterization of Acinetobacter spp. blood culture isolates from Norway. J Antimicrob Chemother 66: 738–744. [DOI] [PubMed] [Google Scholar]
- 49. Wisplinghoff H, Paulus T, Lugenheim M, Stefanik D, Higgins PG, et al. (2012) Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J Infect 64: 282–290. [DOI] [PubMed] [Google Scholar]
- 50. Vaz-Moreira I, Novo A, Hantsis-Zacharov E, Lopes AR, Gomila M, et al. (2011) Acinetobacter rudis sp. nov., isolated from raw milk and raw wastewater. Int J Syst Evol Microbiol 61: 2837–2843. [DOI] [PubMed] [Google Scholar]
- 51. Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blumer N, et al. (2007) Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol 119: 1514–1521. [DOI] [PubMed] [Google Scholar]
- 52. Saisongkorh W, Rolain JM, Suputtamongkol Y, Raoult D (2009) Emerging Bartonella in humans and animals in Asia and Australia. J Med Assoc Thai 92: 707–731. [PubMed] [Google Scholar]
- 53. Murrell A, Dobson SJ, Yang X, Lacey E, Barker SC (2003) A survey of bacterial diversity in ticks, lice and fleas from Australia. Parasitol Res 89: 326–334. [DOI] [PubMed] [Google Scholar]
- 54. Pidiyar VJ, Jangid K, Patole MS, Shouche YS (2004) Studies on cultured and uncultured microbiota of wild culex quinquefasciatus mosquito midgut based on 16s ribosomal RNA gene analysis. Am J Trop Med Hyg 70: 597–603. [PubMed] [Google Scholar]
- 55. Dinparast Djadid N, Jazayeri H, Raz A, Favia G, Ricci I, et al. (2011) Identification of the midgut microbiota of An. stephensi and An. maculipennis for their application as a paratransgenic tool against malaria. PLoS One 6: e28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sant'Anna MR, Darby AC, Brazil RP, Montoya-Lerma J, Dillon VM, et al. (2012) Investigation of the bacterial communities associated with females of Lutzomyia sand fly species from South America. PLoS One 7: e42531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Geiger A, Fardeau ML, Njiokou F, Joseph M, Asonganyi T, et al. (2011) Bacterial diversity associated with populations of Glossina spp. from Cameroon and distribution within the Campo sleeping sickness focus. Microb Ecol 62: 632–643. [DOI] [PubMed] [Google Scholar]
- 58. Zouache K, Raharimalala FN, Raquin V, Tran-Van V, Raveloson LH, et al. (2011) Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiol Ecol 75: 377–389. [DOI] [PubMed] [Google Scholar]