Abstract
Nearly all data regarding land-plant turnover across the Cretaceous/Paleogene boundary come from western North America, relatively close to the Chicxulub, Mexico impact site. Here, we present a palynological analysis of a section in Patagonia that shows a marked fall in diversity and abundance of nearly all plant groups across the K/Pg interval. Minimum diversity occurs during the earliest Danian, but only a few palynomorphs show true extinctions. The low extinction rate is similar to previous observations from New Zealand. The differing responses between the Southern and Northern hemispheres could be related to the attenuation of damage with increased distance from the impact site, to hemispheric differences in extinction severity, or to both effects. Legacy effects of the terminal Cretaceous event also provide a plausible, partial explanation for the fact that Paleocene and Eocene macrofloras from Patagonia are among the most diverse known globally. Also of great interest, earliest Danian assemblages are dominated by the gymnosperm palynomorphs Classopollis of the extinct Mesozoic conifer family Cheirolepidiaceae. The expansion of Classopollis after the boundary in Patagonia is another example of typically Mesozoic plant lineages surviving into the Cenozoic in southern Gondwanan areas, and this greatly supports previous hypotheses of high latitude southern regions as biodiversity refugia during the end-Cretaceous global crisis.
Introduction
The end-Cretaceous mass extinction, resulting from an asteroid impact in the Yucatán at 65.5 Ma, was the most recent great catastrophe for life on Earth [1]–[3]. Land plants may have been less affected than animals, but the only region with abundant, high-resolution stratigraphic data remains the Western Interior USA, relatively close to the Chicxulub crater [4]. There, both macrofloral and microfloral data record significant extinction and a recovery delayed for several million years [4]–[9]. In many sections, diverse latest Cretaceous floras are replaced by Paleocene floras of very low diversity. Many palynofloras from the basalmost Paleocene are dominated by a few species of ferns in a “fern spike” [5], usually interpreted as rapid colonization by pioneer species in a landscape denuded of seed plants.
In the Southern Hemisphere, many marine K/Pg-boundary sections have been recognized, and it is noteworthy that photosynthetic nannoplankton show significantly lower extinction rates and faster recovery in the southern than in the northern hemisphere [10]. To date, there are too few paleobotanical analyses to test whether this pattern is also true of terrestrial photosynthetic organisms, but the data that do exist are consistent with this idea. Sections from Seymour Island (Antarctica) and Australia show little change in the palynological record across the boundary, and no evidence of an abrupt event, although this could be due to relatively coarse sampling or the presence of hiatuses in these records [11], [12]. In contrast, studies from New Zealand, where the K/Pg interval is better defined, show a strong but brief disruption of the vegetation at the boundary, including an initial spike of fungal spores [13], a marked increase in fern spores, a reduction in the relative abundance of gymnosperms, and a temporary loss of angiosperm pollen [14]–[16]. The turnover patterns from New Zealand are completely different from North America, and more like the Southern nannoplankton record, in that no significant species extinctions occurred at, or near, the boundary, and the temporary loss of taxa was followed by an almost complete recovery of the pre-existing Cretaceous elements. In southern South America, early-middle Paleocene macrofloras (ca. 62 Ma) are much more diverse than North American counterparts, suggesting a relatively buffered extinction or faster recovery [17], but these floras date from ca. 4 Ma after the event, and neither macrofloras nor accurately dated palynofloras have previously been compared to latest Cretaceous assemblages in this region.
Here, we explicitly analyze plant turnover in southern South America through palynological examination of a single stratigraphic section in Patagonia from the K/Pg interval. We evaluate the magnitude of vegetation disturbance and examine the role of unexpected colonizer lineages. Although the K/Pg boundary layer itself is not preserved, the patterns nevertheless provide novel insights into geographic variation regarding the end-Cretaceous extinction and recovery.
Materials and Methods
Ethics statement
All necessary permits were obtained for the described field studies. Permits were issued by the Secretaría de Cultura de la provincia del Chubut, Argentina.
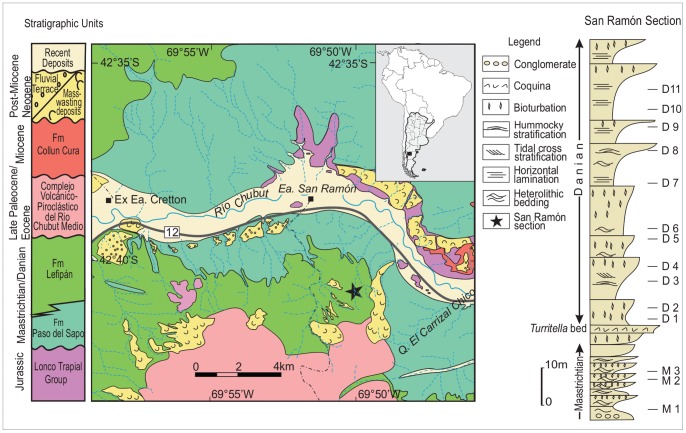
The stratigraphic package studied here is the San Ramón section of the Lefipán Formation, which crops out in northwestern Patagonia, Argentina (Figure 1; [18]). The section is up to 400 m thick, of which approximately 260 m are uppermost Cretaceous (Maastrichtian), and the remainder are lowermost Paleocene (Danian). The underlying unit is the Campanian-early Maastrichtian Paso del Sapo Formation, and the overlying unit is the late Paleocene Barda Colorada Ignimbrite. The Lefipán Formation consists of marine to marginal marine, fossiliferous sandstones and mudstones with some intercalated coquinas and conglomerates. Lithofacies analysis indicates the existence of coarse-grained, tide-dominated deltas with sediments input from braided rivers, formed at the western margin of a large marine embayment during the K/Pg boundary interval [18]. In the middle part of the sequence, a coquina bed rich in the gastropods Turritella and/or Pseudamaura marks the earliest Paleocene deposits in the area and represents a transgressive accumulation produced by wave-induced oscillatory flows, in a high-energy, shallow marine environment (Figure 1). The uppermost Maastrichtian is represented by tidal channel and tidal flat deposits with a low diversity Corbicula assemblage indicative of low salinity, high-stress environments unfavorable to most marine species that characterize the stable, normal marine, Maastrichtian infaunal guilds in most of the succession. During the early Danian coarsening-upward cycles reflect accumulation in tidal bars, mainly in a tide to wave-influenced distal delta-front environment with high sedimentation rates. These deposits bear molluscan faunal associations indicative of recurrent changes from normal marine to low-salinity marine environments with low-oxygen, fine-grained, soupy substrates [18].
Figure 1. Stratigraphic and geographic location with details of the San Ramón section of the Lefipán Formation (right).
Modified from Scasso et al. [16].
The identification of the K/Pg interval in the studied section (San Ramón) is based on three criteria. First, there is a major change in invertebrate faunal composition within a few meters of section wherein two successive, diagnostically Maastrichtian faunas are replaced by a succession of two Danian assemblages [18]–[20]. Second, latest Maastrichtian and earliest Danian dinoflagellate markers are present in the corresponding strata, including Cyclapophysis monmouthensis and Damassadinium californicum, respectively. Third, age-diagnostic continental palynomorphs are present in the corresponding beds, including the late Maastrichtian markers Grapnelispora evansii, Quadraplanus brossus, and Tubulifloridites lilliei, and the Danian marker Nothofagidites dorotensis. The boundary layer itself is apparently not preserved, but the biostratigraphic data indicate only a minimal temporal hiatus, and we consider the most likely bracket for the boundary interval to be approximately 4 m of strata within an 8.7 m thick, highly bioturbated, fine to medium-grained sandstone (Figure 1).
Fourteen outcrop samples were analyzed spanning 90 meters of the middle-upper Lefipán Formation. Samples of variable palynological content and preservation were obtained as close as 6.3 m to the suggested K/Pg boundary interval, on either side (Figure 1). Samples were prepared using standard palynological techniques, and most contained both marine (dinoflagellate cysts) and continental (spores, pollen grains, fungal remains, fresh water algae) palynomorphs. Pollen and spores were identified to species level whenever possible. A minimum of 300 continental specimens were counted in each sample, except in samples M1, M3 and D13, for a total of 5887 counted specimens. Slides are housed in the palynological collection of the Museo Paleontológico Egidio Feruglio, Trelew, Chubut, Argentina, under the prefix MPEF-Palin, numbers 100 to 113. Coordinates of the illustrated specimens refer to the England finder. Pollen terminology follows Punt et al. [21]. Groups of samples were determined by CONISS [22] stratigraphically constrained cluster analysis. The TGVIEW program was used for cluster analysis and plotting diagrams [22]. All other quantitative analyses were done using the R program, version 2.2.0 (http://www.r-project.org). A nonmetric multi-dimensional scaling analysis (NMDS) was used to ordinate the pollen and spore data. The data were arcsine square root transformed to improve normality [23], a dissimilarity matrix was computed using the Bray-Curtis distance metric [24], and R's “metaMDS” function performed the NMDS ordination. Diversity within a sample was estimated using the Shannon-Wiener Index and analytical rarefaction. Pielou's J metric was used to compare evenness among samples.
Results
One hundred and thirty spore and pollen species were recovered (Table S1), of which 40 were previously reported from the lower part of the Lefipán Formation [25], [26].
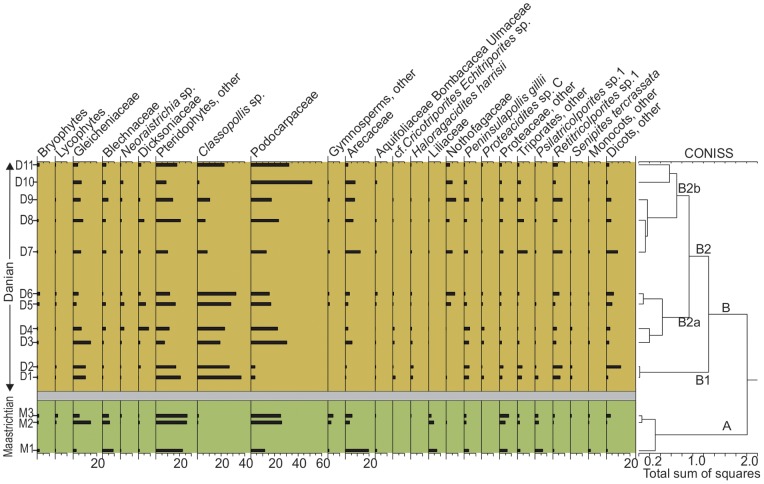
Cluster analysis (Q mode) revealed two stratigraphically well-ordered major groups of samples (Figure 2). Group A comprises the Maastrichtian samples (M1–M3) and Group B all early Danian samples, with two subgroups: B1 (samples D1 and D2) and B2 (samples D3–D11). Group A (Maastrichtian) is dominated by fern spores (mean 41%), followed by angiosperms (mean 34%) and gymnosperms (mean 24%; Table S2). Spores of bryophytes and lycophytes are uncommon (1.5%) but present in most Cretaceous samples (Table S1; Figure 2). Ferns are represented mainly by Gleicheniaceae, Cyathidites spp. (uncertain affinity), and Blechnaceae. There are also occurrences of aquatic ferns (Azollaceae). The most abundant angiosperm taxa are Arecaceae (including Nypa palms), Proteaceae, Liliaceae, and Psilatricolporites sp. (uncertain affinity). There are also low-frequency occurrences of Aquifoliaceae, Malvaceae (Bombacoideae), Chloranthaceae, and some monocots (Sparganiaceae). Gymnosperms are largely represented by Podocarpaceae (Podocarpus type), and other gymnosperms (Araucariaceae, extinct coniferophyte, and Cycadales–Bennettitales–Ginkgoales) occur at lower abundances.
Figure 2. Spore and pollen diagram shown as percent abundances per sample from the Lefipán Formation.
The CONISS cluster dendrogram at right determines distinct, stratigraphically ordered palynomorph groups. Note large Danian increase in Classopollis abundance.
Group B1 (basalmost Danian) is dominated by the gymnosperm Classopollis ( = Corollina) of the extinct conifer family Cheirolepidiaceae, which reaches up to 37% of the total pollen content, sharply up from less than 1% in latest Maastrichtian samples (Table S1, Figure 2). Other gymnosperms are scarce (3.5%) and exclusively represented by Podocarpaceae. Ferns (32%) decrease in both relative abundance and diversity: terrestrial Gleicheniaceae and Cyathidites spp. are the most abundant, but less so than during the Maastrichtian. Angiosperms (27%) are mainly represented by Penninsulapollis gilli (Proteaceae related to Beauprea), Retritricolporites sp. 1 (uncertain affinity), Haloragacidites harrisii (Casuarinaceae), and other triporate species (Cicotriporites sp., Echitriporites sp., Triatriopollenites spp.). Interestingly, some taxa well documented in Maastrichtian samples, particularly those of Liliaceae, Arecaceae, and some Proteaceae, suffered a general reduction in abundance in the basalmost Danian samples (Table S1).
Group B2 is divided into two subgroups B2a and B2b (Figure 2). These are both dominated by gymnosperms (mean 42.5%), including more than five species of Podocarpaceae and Classopollis, the latter showing a broad decline in abundance towards the levels containing subgroup B2 b (Table S1, S2; Figure 2). Angiosperms occupy the second rank in abundance (mean 30%), and prevalent elements include Nothofagaceae and Ericaceae. Some Arecaceae (including Nypa), several Proteaceae, Liliaceae, Aquifoliaceae, and Malvaceae (Bombacoideae) are recorded again, or have higher frequencies than before, in levels containing the B2 cluster. This trend is even more pronounced in subgroup B2b. There, ferns decrease to a mean value of 27% of the assemblages with an increased participation of ?Osmundaceae (Neoraistrichia sp.), and Dicksoniaceae (Trilites spp.), but the relative abundance of ferns remains lower than in group A; bryophytes are uncommon (1%) and lycophytes extremely scarce.
The NMDS analysis supports the clustering of samples defined above (Figure 3), again clearly separating the earliest Danian samples (D1–D2, Group B1) from those of the Maastrichtian (M1–M3, Group A). Samples D3 to D11 (Group B2) occupy an intermediate position between the Maastrichtian samples (M1–M3) and those of the earliest Danian (D1–D2).
Figure 3. Results of non-metric multidimensional scaling (NMDS) ordination of spore-pollen data from the Lefipán Formation.
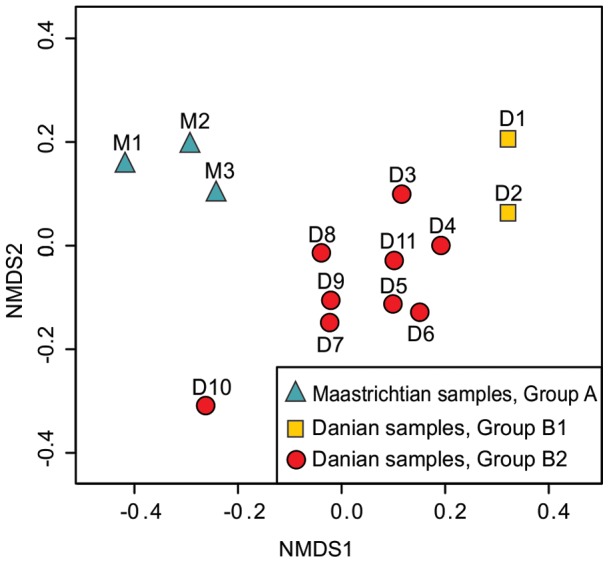
Sample numbers and cluster groups as in Figure 2. Stress = 9.99.
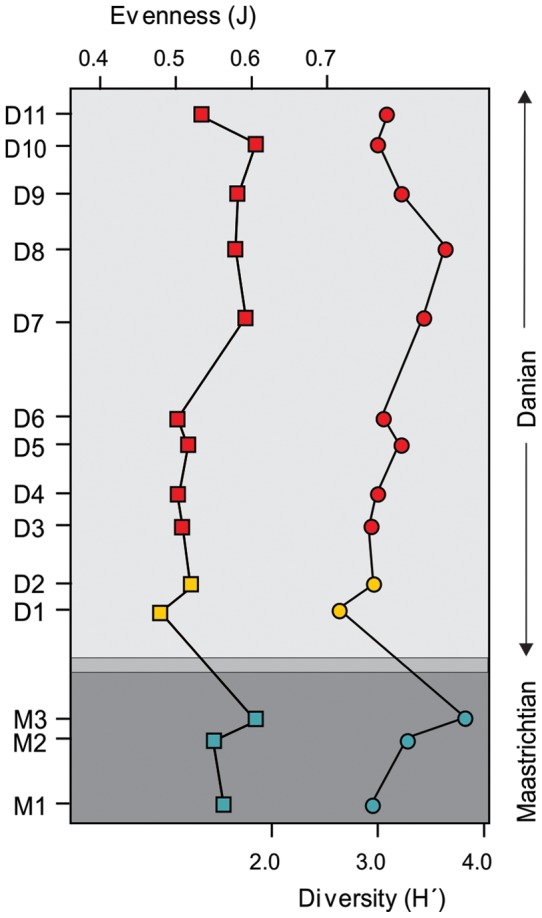
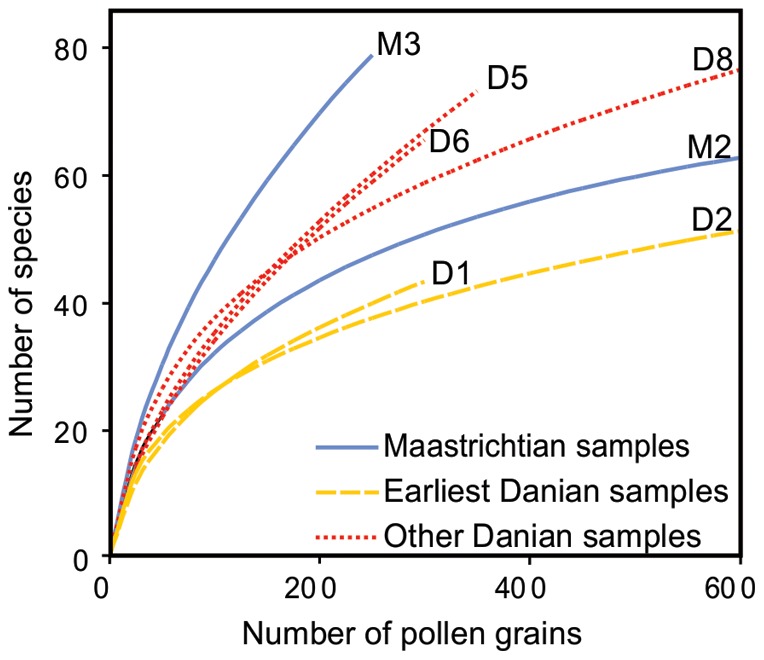
Measures of diversity (Shannon-Wiener index, rarefaction) and evenness (Pieleu's J) show a marked decrease from the latest Cretaceous to the Danian, and a minimum value in the lowest Danian sample (Figure 4, 5). The highest richness occurs in Sample M3, the uppermost Maastrichtian sample that yielded abundant palynomorphs, about 12.6 m below the Danian marker bed (Figure 4, 5). The rarefied richness at 250 specimens is 78 species in Sample M3 (Figure 5), whereas the lowest values of rarefied species richness occur in the first 14 m of the Danian (Samples D1 to D2) and range from 37 to 47 species. The rarefaction curves clearly indicate that a sustained recovery in diversity occurs at about 30 m above the boundary (sample D5) where several characteristic “Cretaceous species” re-appeared (ie. Echinosporis sp., Reticuloidosporites tenellis, Trisaccites microsaccatum, Araucariacites australis, Liliacidites variegatus, Nothofagidites saraensis) and other species are recorded for the first time (ie. Herkosporites elliottii, Trilites parvallatus, Nothofagidites fuegiensis, Propylipollis cf. tenuiexinus, Psilatricolporites salamanquensis, Striatricolporites gamerroi, Tricolpites sp. 1). Evenness sharply decreases across the K/Pg interval and increases again at Sample D7 (Figure 4).
Figure 4. Spore-pollen evenness (Pielou's J, left) and diversity (Shannon-Wiener Index, H', right), from the Lefipán Formation.
(Figure 1).
Figure 5. Rarefaction results from spore-pollen counts of selected samples from the Lefipán Formation ( Figure 1 ).
Discussion and Conclusions
Our results provide the first outline, on the basis of spores and pollen grains, of vegetational history across the K/Pg interval in central Patagonia. The analyses indicate a major floristic shift and decline in richness at the beginning of the Paleocene, but not a great extinction event.
As understood here, the presence of a fern-angiosperm dominated community during the Maastrichtian, with gymnosperms (podocarps) as common trees, diverse Proteaceae, aquatic ferns, and abundant palms suggests warm and humid adapted vegetation. Clearly, palms played a significant role in late Maastrichtian communities (Figure 6A–H, 7). The presence of Spinizonocolpites, related to the tropical mangrove palm Nypa, indicates specialized shore-line mangrove assemblages. Proteaceae related to Beauprea and Telopea, together with Aquifoliaceae, may have formed a lower stratum of forests or woodlands. The abundant monocots, primarily Liliaceae and some Sparganiaceae, Chloranthaceae and the diverse ferns may have grown in the understory, associated with ponds, small streams or rivers just landward of the shoreline.
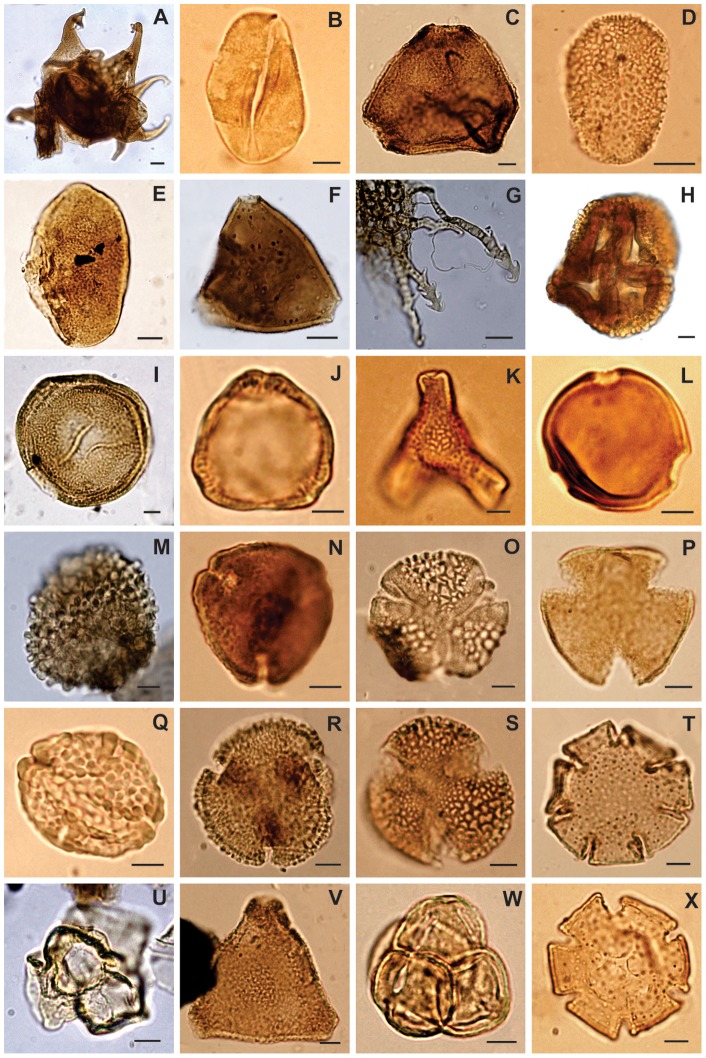
Figure 6. Selected characteristic species from the Maastrichtian (A–H) and Danian (I–X) samples.
Scale bar equals 5 µm, except in figures A, E, F and G where scale bar is equal 10 µm. Authorities are given in Table S1. A, Grapnelispora evansii (MPEF–Palin 102c: V32–4); B, Arecipites minutiscabratus (MPEF–Palin 101b: Q31–1); C, Lewalanipollis senectus (MPEF–Palin 101a: S47); D, Liliacidites regularis (MPEF–Palin 101b: N24–1); E, Longapertites aff. vaneendenburgi (MPEF–Palin 102a: P29); F, Propylipollis ambiguus (MPEF–Palin 102a: K33–1); G, Azollopsis tormentosa (MPEF–Palin 102a: Q33–4); H, Quadraplanus brossus (MPEF–Palin 102d: N37–1); I, Classopollis sp. (MPEF–Palin 103a: D43–4); J, Haloragacidites harrisii (MPEF–Palin 107b: F49); K, Proteacidites sp. C (MPEF–Palin 107b: P39–1); L, cf. Cicotriporites sp. (MPEF–Palin 103b: D33–1); M, Neoraistrichia sp. A (MPEF–Palin 103a: N39–3); N, Senipites tercrassata (MPEF–Palin 103b: M29); O, Retritricolporites sp. 1 (MPEF–Palin 108b: Y26–3); P, Peninsulapollis gillii (MPEF–Palin 109b: N28–4); Q, Ulmoideipites patagonicus (MPEF–Palin 108 b: M49–3); R, Bombacacidites cf. isoreticulatus (MPEF–Palin 108 b: L51–2); S, Rousea microreticulata (MPEF–Palin 104b: Z35/Z36); T, Nothofagidites dorotensis (MPEF–Palin 107a: V37); U, Rosannia manika (MPEF–Palin 106 b: W45); V, Propylipollis reticuloscabratus (MPEF–Palin 110d: M36–1); W, Ericipites microtectatum (MPEF–Palin 110d: F38–3); X, Nothofagidites fuegiensis (MPEF–Palin 109 b: F23–4).
Figure 7. Ancient landscapes across the Cretaceous/Paleogene time interval in central Patagonia.
Main floristic types from the: Maastrichtian (left): Ferns, palms, conifers; earliest Danian (middle): Cheirolepidiaceae, shrubs and other low–diversity flora; and Danian (right): Podocarps, Cheirolepidiaceae, palms.
Earliest Danian vegetation (Group B1) had low diversity (Figure 4, 5, 7) and was quite different in species composition and abundance from both the latest Maastrichtian (Group A) and the subsequent Danian (Group B2) assemblages (Figure 2, 3). The marked reduction in diversity affected all groups of plants, particularly ferns and monocots, most Proteaceae species except Beauprea-like form, and nearly all gymnosperms. The presence of marginal, shallow marine, somewhat stressed paleoenvironmental conditions on both sides of the K/Pg boundary indicates that changing depositional factors were unlikely to have had significant importance in driving the observed compositional changes in the palynological assemblages. Earliest Danian assemblages were characterized by the striking abundance of Classopollis, a pollen type linked to Casuarinaceae (Haloragacidites harrisii), the consistent presence of Beauprea (Peninsulapollis gillii), ferns of Gleicheniaceae (Figure 6I–P) and some taxa of unknown affinity (Retritricolporites sp. 1, Cicotriporites sp., Echitriporites sp., Triatriopollenites spp.). Moreover, the low values of evenness recorded in Sample D1 are consistent with a highly disturbed flora. Most early Danian taxa are not well understood in terms of their ecological requirements, but the common feature of many members of this group of plants is their notable adaptability to changing environments. The association of Classopollis with stressed (disturbed) environments is well known [27].
Later Danian vegetation (Group B2) was dominated by gymnosperms, including diverse Podocarpaceae related to Podocarpus, Microcachrys, Dacrydium, Lagarostrobos and Dacrycarpus. Classopollis remained abundant, but it shows a general reduction towards younger samples. Palms and tree ferns of Dicksoniaceae were also important components of the Danian vegetation. Other elements included Nothofagus, diverse eudicots of uncertain affinity, and new species of Proteaceae and Ericaceae, indicating that several typical components of extant austral forests were in place by the Danian (Figure 6Q–X, 7). Several Cretaceous taxa return again in this part of the sequence including Liliaceae and temperate to warmth-loving families: Aquifoliaceae, Malvaceae (Bombacoideae) and Arecaceae (Nypa type). The presence of Lactoridaceae, a monotypic family today restricted to subtropical forests of the Juan Fernández Archipelago, offshore from Chile in the South Pacific Ocean, is particularly striking. These assemblages are fairly diverse, although no Danian sample reaches the diversity recorded in the latest Maastrichtian (Figure 5, 6).
In broad terms, this analysis shows a reduction in diversity and relative abundance in almost all plant groups from the latest Maastrichtian to the Danian, although only a few true extinctions occurred. Many of the species that disappear at the boundary return higher in the sequence, indicating their survival in refugial areas. The overall extinction rate is difficult to estimate without more exhaustive studies, but it probably does not exceed 10% of the species. This pattern of recovery is comparable to that observed in New Zealand, where an abrupt disturbance of the vegetation across the K/Pg boundary occurred, but with a low overall extinction rate [14]–[16].
The different floral devastation patterns recorded in North America and the southern Hemisphere, now including Patagonia, may be related to the attenuation of damage with increased distance from the impact site, following the global pattern of decreasing sediment disturbance with increasing distance from Chicxulub [3]. This hypothesis is supported by paleobotanical data from the Paleocene of Colombia, which was near the impact site and appears to show a depressed ecosystem, and from France, which shows the opposite pattern [28], [29]. Alternatively, northern vs. southern hemispheric differences may have been the dominant effect, as shown in the global nannoplankton data [10]. Because Patagonia is very distant from Chicxulub and is in the Southern Hemisphere, our results are compatible with both hypotheses, alone or in combination. Our data also suggest that the elevated richness observed in Paleocene and Eocene macrofloras from Patagonia [17], [30] may, in part, be a legacy effect of a muted K/Pg extinction event.
The peak of Classopollis just above the K/Pg boundary is hypothetically a temporal correlate of the classic, short-lived fern spike seen elsewhere. However, there is not sufficient temporal resolution in the San Ramon section to determine whether a fern spike was absent versus non-sampled, and thus whether the Classopollis increase followed, or locally replaced, the widely distributed fern spike. Cheirolepidiaceae is a common component of Mesozoic floras, having last records in most regions in the Cenomanian and Turonian [27], [31], [32]. In southern South America, however, Classopollis showed a somewhat different behavior, reappearing in the record in the latest Maastrichtian and playing a significant role in Paleocene plant communities [33]–[36]. Even though it has not yet been found in situ in a Paleocene pollen cone, the Classopollis pollen type (Figure 6I) is so distinctive that it is very unlikely to belong to another group of plants besides Cheirolepidiaceae. Reworking has been sometimes invoked to explain comparable occurrences [31]. However, the Paleocene Classopollis are not corroded or otherwise degraded, showing no significant difference in color in comparison from the rest of the assemblages that would support reworking. Moreover these palynomorphs have been recorded in several Paleocene Argentinean basins occupying different paleoenvironments and at high relative abundances [33]–[36]. Thus, we find no evidence that Paleocene Classopollis was recycled from older strata, and instead we conclude that this genus persisted longer in southern South America than elsewhere, until its final Paleocene appearance. The terminal Cretaceous event caused significant changes in terrestrial ecosystems that appear to have favored the regeneration and spread of Classopollis in southernmost South America. Newly disturbed habitats with few competitors may have created suitable ecospaces for the re-establishment of this conifer, adapted to stressed environments [27].
It is also noteworthy that other dominant “Mesozoic” plant lineages considered to have disappeared globally at the end of the Cretaceous survived during the Paleogene in southern Gondwana. Particularly striking is the record of leaves resembling corystosperms in the Paleogene of Tasmania [37], along with the presence of extinct clades of Ginkgoales in Tasmania [38] and in Patagonia [39], [40]. Other records of this type include bennettitaleans from the Oligocene of Australia, following an extraordinarily long stratigraphic hiatus of more than 50 Ma [41]. Moreover, Araucaria is another interesting case; it shows a nearly worldwide distribution through the Mesozoic, but it disappeared from North America and Europe in the latest Cretaceous, continuing in the Southern Hemisphere only during the Cenozoic, where it has a relict distribution today [42], [43]. It is also remarkable that many groups of Cretaceous Patagonian vertebrates survived into the Paleogene. This is the case for several marsupial taxa that successfully crossed the K/Pg boundary in South America, while this group was severely reduced in North America [44]. The same is true for a dryolestoid mammal recorded in Patagonia in the Paleocene as a survivor of a Cretaceous South American radiation [45].
The expansion of Classopollis in Patagonia after the terminal Cretaceous event, together with a growing list of Mesozoic lineages persisting into the Cenozoic in southern Gondwanan areas, greatly supports the hypothesis of southern high-latitude regions as biodiversity refugia from the global environmental crisis at the end of the Cretaceous [37].
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
The helpful comments of two anonymous reviewers and the Editor (L.A. Newsom) are sincerely appreciated. We thank K. Johnson, A. Iglesias, L. Sarzetti, P. Puerta, W. Clyde, L. Canessa, and M. Caffa for field assistance, B. Nicoletti and C. San Martín for permission and hospitality during field work, A. González for drawing graphics, and F. Guillén for drawing landscape reconstructions.
Funding Statement
Support was provided through National Science Foundation grant DEB-0919071 and the Consejo Nacional de Investigaciones Científicas y Técnicas. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alvarez LW, Alvarez W, Asaro F, Michel HV (1980) Extraterrestrial cause for the Cretaceous–Tertiary extinction: experimental results and theoretical interpretation. Science 208: 1095–1108. [DOI] [PubMed] [Google Scholar]
- 2.Raup DM (1991) Extinction: Bad Genes or Bad Luck? New York, W.W. Norton. 210 p. [PubMed]
- 3. Schulte P, Alegret L, Arenillas I, Arz JA, Barton PJ, et al. (2010) The Chicxulub asteroid impact and mass extinction at the Cretaceous–Paleogene boundary. Science 327: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 4.Nichols DJ, Johnson KR (2008) Plants and the K–T Boundary. Cambridge University Press. 292 p.
- 5. Orth CJ, Gilmore JS, Knight JD, Pillmore CL, Tschudy RH, et al. (1981) An iridium anomaly at the palynological Cretaceous-Tertiary boundary in northern New Mexico. Science 214: 1341–1343. [DOI] [PubMed] [Google Scholar]
- 6. Tschudy RH, Pillmore CL, Orth CJ, Gilmore JS, Knight JD (1984) Disruption of the terrestrial plant ecosystem at the Cretaceous-Tertiary boundary, Western Interior: Science. 225: 1030–1032. [DOI] [PubMed] [Google Scholar]
- 7. Wolfe JA, Upchurch GR Jr (1986) Vegetation, climatic and floral changes at the Cretaceous–Tertiary boundary. Nature 324: 148–152. [Google Scholar]
- 8. Hotton CL (2002) Palynology of the Cretaceous-Tertiary boundary in central Montana: evidence for extraterrestrial impact as a cause of the terminal Cretaceous extinctions. Geol Soc Am Sp P 361: 473–501. [Google Scholar]
- 9. Wilf P, Johnson KR (2004) Land plant extinction at the end of the Cretaceous: a quantitative analysis of the North Dakota megafloral record. Paleobiology 30: 347–368. [Google Scholar]
- 10. Jiang S, Bralower TJ, Patzkowsky ME, Kump LR, Schueth JD (2010) Geographic controls on nannoplankton extinction across the Cretaceous/Palaeogene boundary. Nat Geosci 3: 280–285. [Google Scholar]
- 11. Askin RA (1988) The palynological record across the Cretaceous/Tertiary transition on Seymour Island, Antarctica. Geol Soc Am Mem 169: 155–162. [Google Scholar]
- 12. Macphail M (1994) Impact of the K/T event on the southeast Australian flora and vegetation: mass extinction, niche disruption or nil? Palaeoaustral 1: 9–13. [Google Scholar]
- 13. Vajda V, McLoughlin S (2004) Fungal proliferation at the Cretaceous-Tertiary boundary. Science 303: 1489. [DOI] [PubMed] [Google Scholar]
- 14. Vajda V, Raine JI, Hollis CJ (2001) Indication of global deforestation at the Cretaceous-Tertiary boundary by New Zealand fern spike. Science 294: 1700–1702. [DOI] [PubMed] [Google Scholar]
- 15. Vajda V, Raine JI (2003) Pollen and spores in marine Cretaceous/Tertiary boundary sediments at mid-Waipara River, North Canterbury, New Zealand. N Z J Geol Geophys 46: 255–273. [Google Scholar]
- 16. Vajda V, McLoughlin S (2007) Extinction and recovery patterns of the vegetation across the Cretaceous-Paleogene boundary– a tool for unravelling causes of the end-Permian mass-extinction. Rev Palaeobot Palynol 144: 99–112. [Google Scholar]
- 17. Iglesias A, Wilf P, Johnson K, Zamuner A, Cúneo R, et al. (2007) Paleocene lowland macroflora from Patagonia reveals significantly greater richness than North American analogs. Geology 35: 947–950. [Google Scholar]
- 18. Scasso RA, Aberhan M, Ruiz L, Weidemeyer S, Medina F, et al. (2012) Integrated bio- and lithofacies analysis of coarse-grained, tide-dominated deltaic marginal marine environments across the Cretaceous/Paleogene boundary in Patagonia, Argentina. Cretaceous Res 36: 37–57. [Google Scholar]
- 19. Medina FA, Camacho HH, Malagnino EC (1990) Bioestratigrafía del Cretácico superior – Paleoceno marino de la Formación Lefipán, Barranca de los Perros, Río Chubut, Chubut. 5° Cong Arg Paleontol Bioestrat 7: 137–142. [Google Scholar]
- 20. Medina FA, Olivero EB (1994) Paleontología de la Formación Lefipán (Cretácico-Terciario) en el valle medio del Río Chubut. RAGA 48: 100–106. [Google Scholar]
- 21. Punt W, Hoen PP, Blacmore S, Nilsson S, Le Thomas A (2007) Glossary of pollen and spore terminology. Rev Palaeobot Palynol 143: 1–81. [Google Scholar]
- 22.Grimm E (2004) Tilia software 2.0.2. Illinois State Museum Research and Collection Center Springfield.
- 23.Sokal RR, Rohlf FJ (1995) Biometry: Third Edition. W.H. Freeman and Co, New York. 887 p.
- 24. Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monog 27: 325–349. [Google Scholar]
- 25. Baldoni AM (1992) Palynology of the lower Lefipán Formation (Upper Cretaceous) of Barranca de Los Perros, Chubut Province, Argentina. Part I, cryptogam spores and gymnosperm pollen. Palynology 16: 117–136. [Google Scholar]
- 26. Baldoni AM, Askin RA (1993) Palynology of the Lower Lefipán Formation (Upper Cretaceous) of Barranca de los Perros, Chubut Province, Argentina. Part II. angiosperm pollen and discussion. Palynology 17: 241–264. [Google Scholar]
- 27. Alvin KL (1982) Cheirolepidiaceae: biology, structure and paleoecology. Rev Palaeobot Palynol 37: 71–98. [Google Scholar]
- 28. Wing SL, Herrera F, Jaramillo CA, Gómez NC, Wilf P, et al. (2009) Late Paleocene fossils from the Cerrejón Formation, Colombia, are the earliest record of Neotropical rainforest. Proc Natl Acad Sci U S A 106: 18627–18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wappler T, Currano ED, Wilf P, Rust J, Labandeira CC (2009) No post-Cretaceous ecosystem depression in European forests? Rich insect-feeding damage on diverse middle Palaeocene plants at Menat, France. Proc R Soc Biol Sci 276: 4271–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilf P, Johnson KR, Cúneo NR, Smith ME, Singer BS, et al. (2005) Eocene plant diversity at Laguna del Hunco and Río Pichileufú, Patagonia, Argentina. Am Nat 165: 634–650. [DOI] [PubMed] [Google Scholar]
- 31. Srivastava SK (1979) The fossil pollen genus Classopollis . Lethaia 9: 437–457. [Google Scholar]
- 32.Watson J (1988) The Cheirolepidiaceae. In: Beck CB, Origin and evolution of gymnosperms. Columbia Univ Press, New York, 382–447.
- 33. Archangelsky S (1973) Palinología del Paleoceno de Chubut. 1. Descripciones sistemáticas. Ameghiniana 10: 339–399. [Google Scholar]
- 34.Ruiz LC, Quattrocchio ME (1997) Estudio palinológico de la Formación Pedro Luro (?Maastrichtiano–Paleoceno), en la cuenca del Colorado, República Argentina. Parte 2: Turma Saccites, Plicates, Poroses e Insertae Sedis. Rev Esp Micropal 29:1 15–137.
- 35. Barreda V, Palamarczuk S, Chamberlain JA Jr (2004) Vegetational disruption at the Cretaceous/Paleogene boundary in Neuquén, Argentina: evidence from spores and pollen. 10° R Arg Sed 1: 185–186. [Google Scholar]
- 36. Volkheimer W, Scafati L, Melandi DL (2007) Palynology of a Danian warm climatic wetland in central northern Patagonia, Argentina. Rev Esp Micropal 39: 117–134. [Google Scholar]
- 37. McLoughlin S, Carpenter RJ, Jordan GJ, Hill RS (2008) Seed ferns survived the end-Cretaceous mass extinction in Tasmania. Am J Bot 95: 465–471. [DOI] [PubMed] [Google Scholar]
- 38. Hill RS, Carpenter RJ (1999) Ginkgo leaves from Paleogene sediments in Tasmania. Aust J Bot 47: 717–724. [Google Scholar]
- 39. Berry EW (1935) A Tertiary Ginkgo from Patagonia. Torreya 35: 11–13. [Google Scholar]
- 40.Cúneo NR, de Seoane LV, Gandolfo MA, Wilf P (2010) Last ginkgoalean record in South America. Bot Soc Am Ann Meet, Providence, Rhode Island, abstract: 507.
- 41. McLoughlin S, Carpenter RJ, Pott C (2011) Ptilophyllum muelleri (Ettingsh.) comb. nov. from the Oligocene of Australia: Last of the Bennettitales? Int J Plant Sci 172: 574–585. [Google Scholar]
- 42. Kunzmann L (2007) Araucariaceae (Pinopsida): Aspects in palaeobiogeography and palaeobiodiversity in the Mesozoic. Zool Anz 246: 257–277. [Google Scholar]
- 43. Panti C, Pujana RR, Zamaloa MC, Romero EJ (2012) Araucariaceae macrofossil record from South America and Antarctica. Alcheringa 36: 1–22. [Google Scholar]
- 44. Case JA, Woodburne MO (1986) South American marsupials: a successful crossing of the Cretaceous-Tertiary boundary. Palaios 1: 413–416. [Google Scholar]
- 45. Gelfo JN, Pascual R (2001) Peligrotherium tropicalis (Mammalia, Dryolestida) from the early Paleocene of Patagonia, a survival from a Mesozoic Gondwanan radiation. Geodiversitas 23: 369–379. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)