Abstract
Bile acids (BAs) are important signaling molecules that are involved in the regulation of their own metabolism, lipid metabolism, energy expenditure and glucose homeostasis. The nuclear farnesoid X receptor (FXR) and the G-protein-coupled TGR-5 are the most prominent BA receptors. FXR is highly expressed in liver and activation of liver FXR profoundly affects glucose homeostasis. Strikingly, the effect of FXR activation on glucose metabolism seems to depend on the nutritional status of the organism, i.e., slimness or obesity. Recently, it became evident that FXR is present in pancreatic β cells and that activation of β cell FXR contributes to the regulation of glucose homeostasis. Interestingly, FXR activation increases glucose-induced insulin secretion by non-genomic effects on stimulus-secretion coupling. The first chapter of this review shortly introduces the role of liver FXR in glucose metabolism, the second part focuses on the impact of FXR in lean and obese animals, and the third chapter highlights the significance of FXR in β cells.
Keywords: FXR, KATP channel, insulin secretion, nuclear receptor, stimulus-secretion coupling, β cell
1. The FXR as a Key Regulator of Glucose Metabolism
BAs act as signaling molecules with systemic endocrine functions. They influence lipid metabolism, energy balance and glucose homeostasis of the body.1 The BA plasma concentration increases quickly after food intake and can be found in concentrations up to 15 µM.2 As BA receptors are predominantly expressed in liver, gallbladder and intestine as well as in brain and pancreas,3-5 it seems likely that BAs are coordinators of postprandial responses in the body.
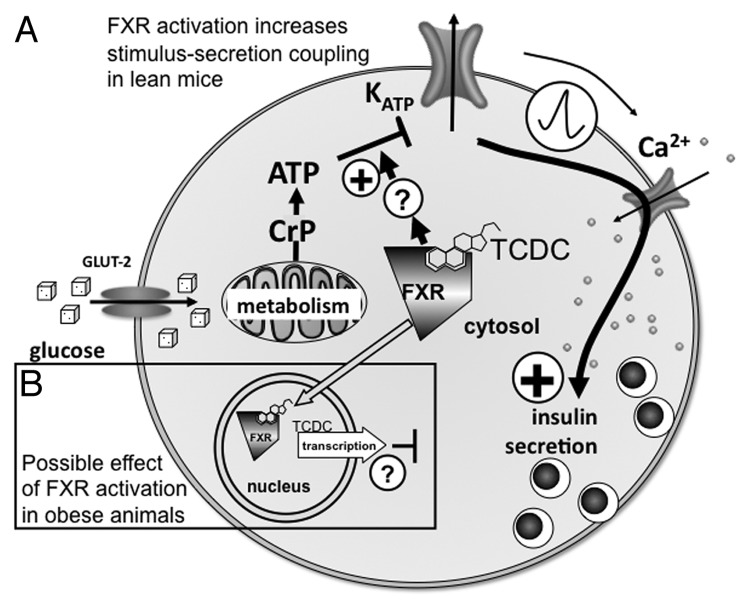
One of the most important BA receptors for glucose homeostasis is the nuclear farnesoid X receptor (FXR). This receptor is a ligand-modulated cytosolic transcription factor which is usually translocated to the nucleus after activation.6 However, in pancreatic β cells, FXR acts as a cytosolic and non-genomic effector of KATP currents (see Fig. 1A and ref. 5). The cellular location of FXR in β cells seems to be dependent on the body mass and thus may determine the effect of BAs on stimulus-secretion coupling. In lean mice FXR localization is predominant in the cytosol and in obese animals in the nuclei (Fig. 1 and ref. 7) where FXR may exert function(s) different to the cytosolic form. In β cells of lean mice (Fig. 1A), a TCDC-induced FXR activation augments the effect of glucose metabolism on the KATP-current, thus increasing electrical activity, Ca2+-influx and eventually insulin secretion.5 Section 3 of this review focuses on this topic. Figure 1B (black-rimmed box) shows in addition that the situation may be completely different in obese animals, i.e., that in this case genomic effects of FXR activation may inhibit insulin secretion.
Figure 1. Effects of FXR activation in β cells: dependence on intracellular receptor localization.
Most studies about the relation between FXR and glucose homeostasis have been performed on FXR in the liver and other insulin-sensitive peripheral organs. BAs seem to modulate the expression of genes for gluconeogenesis in the liver via a complex network.1 However, it is at present unclear whether FXR activation increases or decreases gluconeogenesis, because the nutritional status of the organism and the duration of the treatment with FXR agonists may be decisive for the observed effects.8 It has been reported that in murine hepatocytes activation of small heterodimer partner (SHP) by FXR decreases the expression of the phosphoenolpyruvate carboxy kinase (PEPCK) and other rate-limiting enzymes thus leading to decreased gluconeogenesis.9,10 By contrast, Stayrook, et al.11 observed an FXR-dependent upregulation of PEPCK expression in primary rat and human hepatocytes. Thus, the discrepancies in the observed effects might be owing to different study models (mice, rats, humans, cell lines), too.8,12 Despite the controversy of the available data, BAs clearly impact regulation of hepatic glucose metabolism.13 Interestingly, the second highest expression of FXR is found in the kidney;3 however, it is unclear whether FXR controls gluconeogenesis in this organ. FXR activation by the synthetic agonist GW4064 also enhances insulin sensitivity in adipocytes leading to increased uptake of glucose into adipose tissue.14
FXR concentration in the liver is decreased in diabetic animal models.15 FXR activation by GW4064 promotes insulin sensitivity in liver and skeletal muscle in genetically induced obesity in mice.10,14 This finding apparently contrasts with the observation that glucose tolerance is markedly improved by genetic ablation of FXR (FXR-KO) in obese animals,16 while FXR-KO decreased glucose tolerance and insulin sensitivity in lean mice(see Section 2).10,12,14
In humans, the plasma concentration of BAs not only increases after a meal, but also after an oral glucose tolerance test,17,18 reemphasizing the role of BAs in glucose homeostasis. This is underlined by the findings that BA metabolism is altered in patients with type-2 diabetes mellitus (T2DM)19 and, conversely, that a change in the BA pool using BA sequestrants improves the glycemic control of these patients.13 Evidently, changes in the composition of the BA pool during the development of T2DM and corrections of the pool composition by sequestrants are much more important than alterations in the bile fluid volume.13 The observation that FXR activation by BAs directly enhances glucose-induced insulin secretion of pancreatic islets shows that the FXR-dependent control of glucose homeostasis is of great importance.5 Noteworthy, one effect of BAs on blood glucose control that occurs independently of FXR is the release of GLP-1 by the L-cells of the gut, which is brought about by activation of the G-protein-coupled receptor TGR-5.20-22
2. Significance of FXR in the Maintenance of Glucose Tolerance in Lean and Obese Animals
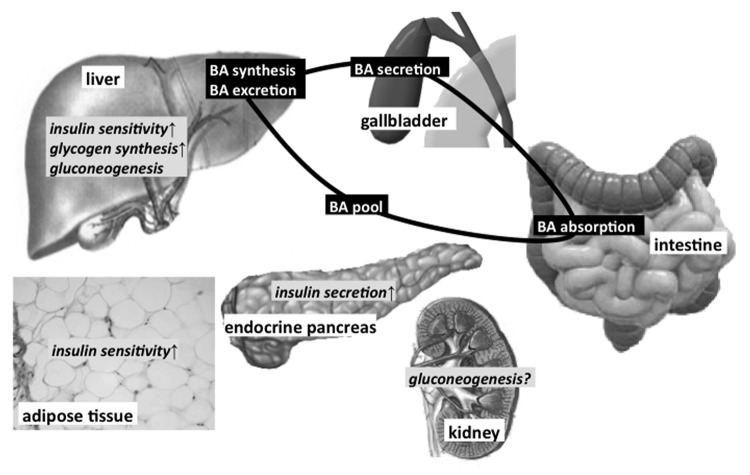
As mentioned above, FXR is involved in several signaling pathways that regulate glucose homeostasis. The significance of FXR for the control of blood glucose concentration is ascertained by the glucose-intolerant phenotype of FXR-KO mice which is associated with insulin resistance as well as higher levels of circulating triglycerides, cholesterol and FFAs.10,12,14 Since tissue-specific KOs of FXR are lacking so far it is hardly possible to assess the contribution of each FXR-expressing tissue to whole body glucose metabolism. Figure 2 summarizes the known effects of FXR activation in different organs involved in the maintenance of glucose homeostasis.
Figure 2. Effects of FXR activation on glucose metabolism in various organs
Hyperinsulinemic-euglycemic clamp experiments revealed that in the presence of elevated plasma insulin concentrations reflecting the postprandial state glucose disposal rate of FXR-KO mice is reduced without any effect on glucose production14 suggesting that FXR ablation induces peripheral insulin resistance but does not affect gluconeogenesis. Impaired insulin signaling in FXR-deficient mice is assumed to result from changes in signaling pathways in white adipose tissue, skeletal muscle and liver.12,14
With respect to the influence of FXR on blood glucose regulation during fasting, current data are inconsistent. Depending on the period of food deprivation and the age of the test animals, persistent or transient hypoglycemia, no change in plasma glucose concentration or even hyperglycemia have been reported10,12,14
As there is no doubt that FXR-KO clearly impairs glycemic control, drugs acting as FXR agonists have been suggested to constitute a novel strategy for the treatment of metabolic diseases, such as obesity, glucose intolerance or T2DM.1 However, at present this approach is challenged by several studies questioning the positive effect of FXR activation under conditions of overnutrition (see below). BAs that are well established endogenous FXR agonists, semi-synthetic derivatives or non-steroidal compounds have been tested for their effects on weight gain and glycemic control in various mouse models. In agreement with the hypothesis that FXR-regulated pathways improve glucose handling, the synthetic agonist GW4064 which in vitro has been clearly shown to interact with regulation of glucose and lipid metabolism via FXR lowers blood glucose concentration and reduces triglyceride and cholesterol levels in lean mice.12
Recent data show that FXR-mediated regulation of body weight and glucose homeostasis, respectively, critically depends on the nutritional status of the organism. The beneficial effect of FXR activation described above is lost under conditions of diet-induced metabolic disorder. In mice treated with high-fat diet supplemented with GW4064 weight gain is not reduced but even more pronounced than in control mice fed with high-fat diet only.24 In addition, GW4064 treatment further impairs diet-induced glucose intolerance and reduces insulin sensitivity. Changes are accompanied by a diminished BA pool of liver and intestine and by altered bile composition. Further analysis showed that GW4064 decreases energy expenditure in animals with diet-induced obesity.24 In this context, it is worth mentioning that FXR-KO mice develop an increase in BA pool size, which is associated with an elevated fraction of cholate.23 As cholic acid has been shown to counteract the harmful effect of high-fat diet in wild-type (WT)24 mice, the increased amount of this BA in FXR-deficient mice may contribute to the protective effects. Feeding of cholic acid prevents the negative effects of high-fat diet and neutralizes the unfavorable action of GW4064 on glucose homeostasis and body weight. Interestingly, compared with mice receiving standard chow BA pool size is twice as high after two months feeding of cholic acid alone or in combination with GW4064 suggesting that alterations in pool size and composition play a central role for the observed differences between specific FXR activation and BA supplementation under conditions of high-fat diet. Besides an increase in circulating BAs per se that may weaken the effects of GW4064 by competitive interaction with receptor binding, one must keep in mind that BAs not only target FXR but are also activators of TGR5. Thereby alterations in bile composition as well as increased pool size may potentiate several TGR5-regulated cascades that improve glucose handling, e.g., secretion of GLP1 in the intestine20 or cAMP-dependent activation of deiodinase 2 in skeletal muscle or brown adipose tissue.25
The striking differences observed with respect to FXR activation in lean and obese mice may be explained by the fact that diet-induced obesity per se has been shown to alter transcriptional signaling of FXR. Lee and colleagues26 addressed this point by mapping genome-wide binding sites of agonist-activated FXR in the liver. The data reveal that FXR binding sites are significantly reduced in mice receiving high-fat diet. In addition, the binding pattern of GW4064-induced activation of FXR is altered depending on the diet suggesting activation of different genes in lean vs. obese mice. It is very likely that similar variations in FXR-mediated regulation of gene transcription exist among genetic models for obesity and diabetes. For example, Zhang and colleagues12 report that GW4064 induces PEPCK, which is the rate limiting enzyme for gluconeogenesis, in the liver of control mice but represses this enzyme in hyperglycemic db/db mice. In addition, the composition of the BA pool that changes in response to food supply24 and after genetic manipulation27 is a very important endogenous modulator of gene transcription. At present, there are only two studies reporting beneficial effects of FXR activation in obese diabetic mice: one of them describes a positive influence of GW4064 after short-term administration (5 d) in db/db mice. In this study GW4064 increases hepatic glycogen storage by mimicking the effect of insulin on GSK3beta, IRS1 and IRS2 proteins and Akt phosphorylation. As a consequence, administration of GW4064 reduces several plasma parameters that are elevated in db/db mice compared with their lean littermates, e.g., concentration of glucose, FFAs, triglycerides and cholesterol.12 Recently, another non-steroidal component specifically activating FXR was designed and tested in mice with diet-induced obesity. The diet-induced increase in body weight and the rise in blood glucose and insulin concentrations are significantly reduced after 4-weeks treatment with this novel FXR agonist.28
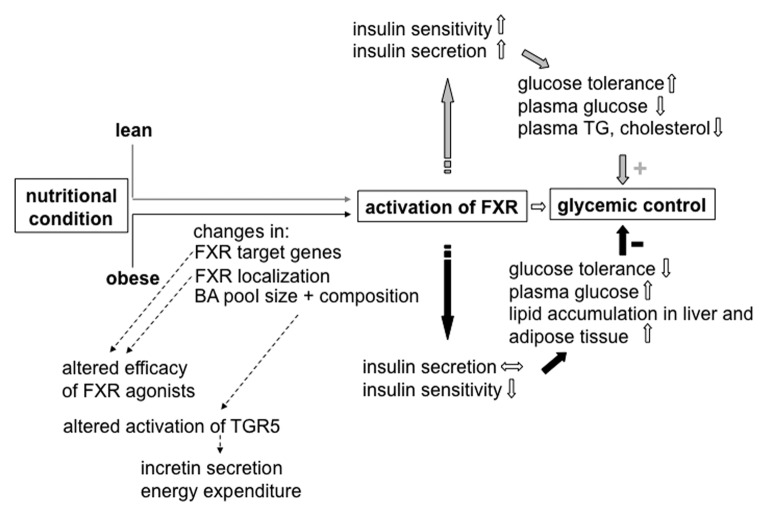
In summary, current data show that regulation of whole body glucose homeostasis by FXR critically depends on the initial metabolic situation (see Fig. 3). Important parameters in this scenario seem to be (1) the composition of the BA pool that determines the level of activation of different target receptors and (2) variations in FXR transcriptional activity that depend on the degree of metabolic excursion.
Figure 3. Crosstalk between nutritional condition, BA pool and FXR-regulated pathways. Y arrows indicate the influence of BAs and FXR activation, respectively, in the lean organism, black arrows indicate changes caused by over-nutrition
Recent data suggest that FXR interacts with insulin secretion of the β cells by an influence on KATP channel activity not mediated by transcription (see Section 3).
3. Role of FXR in β Cells
It has been shown by others4,7 and our own group5 that FXR is present in human and murine β cells and insulin-secreting tumor β cell lines. FXR expression in β cells has been visualized on mRNA and protein level. However, the localization is still disputed. Popescu and coworkers report that FXR in β cells is, in contrast to liver, primarily localized in the extranuclear space in lean mice. However, it is assumed to be translocated to the nucleus under conditions of metabolic stress, e.g., insulin resistance or obesity.7 Renga, et al. describe an opposite observation, i.e., that FXR is commonly more abundant in the nucleus than in the cytoplasm.4 However, since quantification of FXR distribution under distinct conditions is missing in both studies the question of FXR localization in β cells remains open. We showed increased FXR staining after treatment of the islets with the FXR agonist GW4064.5 The fact that FXR is detectable in β cells raises the question whether FXR in islets contributes to the regulation of whole-body glucose homeostasis. So far this issue has been addressed by only three groups, including our own.
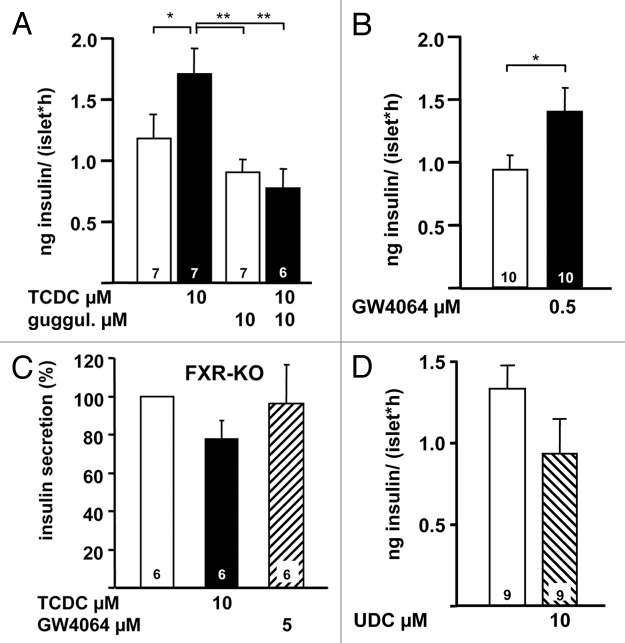
All three papers consistently demonstrate that FXR activation ameliorates insulin secretion. The ligand 6E-CDC increases insulin secretion at high glucose in βTC6 cells.4 The authors also show that in vivo treatment of NOD mice with 6E-CDC prevents hyperglycemia and glycosuria and that this protective effect is accompanied by an increase in plasma insulin concentration and a decrease in plasma glucose concentration. We find a significant augmentation of glucose-induced insulin secretion with taurochenodeoxycholic acid (TCDC) in primary mouse β cells5 (Fig. 4A, left white bar: control in 15 mM glucose; left black bar: augmentation of secretion by addition of TCDC). The stimulatory effect of TCDC is suppressed by the FXR antagonist guggulsterone (Fig. 4A, right bars) and mimicked by the FXR agonist GW4064 (Fig. 4B). The stimulatory effects of both FXR agonists on insulin secretion are blunted in islets from FXR-KO mice (Fig. 4C). Popescu and coworkers do not observe direct effects of GW4064 or chenodeoxycholic acid (CDC) on the secretory capacity in human β cells but show that these compounds can prevent the decrease in the secretory capacity induced by the combination of high glucose and palmitate.7 They also describe a reduction of insulin content and glucose-induced insulin secretion in FXR-KO mice compared with WT mice. Taken together these experimental data suggest a role for FXR in insulin secretion and thus a contribution of β cell FXR to regulation of whole body glucose homeostasis. Interesting in this context, we do not observe an augmentation of insulin secretion with a BA that has almost no affinity to FXR,29 the ursodeoxycholic acid (UDC) (see Fig. 4D).
Figure 4. (A) Stimulation of glucose-induced insulin secretion (15 mM glucose) by TCDC (left bars) and suppression of the stimulatory effect of TCDC by addition of the FXR antagonist guggulsterone (right bars). (B) Effect of GW4064 on glucose-stimulated insulin secretion. (C) Ineffectiveness of TCDC and GW6064 in glucose-stimulated islets of FXR-KO mice. Data obtained with islets from FXR-KO mice are expressed as % of secretion in 15 mM glucose. (D) UDC does not increase glucose-induced insulin secretion. n is given within each bar. *p ≤ 0.05, **p ≤ 0.01. Image adapted with permission from Düfer M, Hörth K, Wagner R, Schittenhelm B, Prowald S, Wagner TF, et al. Bile acids acutely stimulate insulin secretion of mouse β-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes 2012; 61:1479-89; PMID: 22492528; 10.2337/db11-0815.
At present the knowledge about the signaling pathways by which FXR activation leads to insulin secretion is scarce. Genomic and non-genomic pathways seem to be involved. Popescu, et al. show that some transcription factors that are involved in regulation of β cell function are reduced in islet cells of FXR-KO mice.7 On the other hand they demonstrate that FXR agonists including CDC and GW4064 upregulate the FXR target gene FGF-19 pointing to transcriptional effects of FXR activation in β cells. However, the physiological significance of the latter finding remains unclear. Although it is known that members of the FGF family affect β cell glucose sensing and maintenance of glucose homeostasis,30 the role of FGF-19 has not been studied in this context so far. The group of Fiorucci4 suggests that genomic and non-genomic effects contribute to the FXR-induced activation of insulin secretion. Their data evidence that the transcriptional effects are mediated by upregulation of krueppel-like factor 11 (KLF11) after FXR stimulation. KLF11 is a glucose-dependent transcription factor involved in the regulation of the insulin gene.31 Interestingly, mutations in the KLF11 gene predispose to T2DM. We have shown very recently, that FXR activation interferes with several parameters of β cell stimulus-secretion coupling.5 TCDC rapidly (within min) increases the cytosolic Ca2+ concentration, depolarizes the plasma membrane potential and enhances electrical activity. These effects are due to inhibition of KATP current (Fig. 1A). However, TCDC does not directly affect KATP channels because the effect is only observed in cells with intact metabolism and not in excised patches. The inhibition of KATP current is essential for the TCDC-induced augmentation of insulin secretion because this inhibition is blunted in islet cells from SUR1-KO mice. These mice lack functional KATP channels due to genetic deletion of the SUR1 subunit of the channels.32 The rapid onset of the effects of TCDC suggests a non-genomic action of FXR activation on KATP channels. However, the link between FXR stimulation and KATP channel closure is still unclear and has to be identified in future studies.
Perspectives
Recent papers establish FXR as a new player in the regulation of β cell function that may be essentially involved in the crosstalk between liver and islets of Langerhans. Signaling mechanisms in β cells downstream to FXR activation have to be ascertained in more detail. Similarly, more studies about genomic and non-genomic effects of FXR activation are required. FXR in β cells could constitute an interesting drug target for the treatment of T2DM because its activation increases insulin secretion in a glucose-dependent manner. However, with respect to the possible clinical use of BAs or FXR agonists the different effects of FXR activation and signaling mechanisms in lean and obese animals have to be completely understood.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/22383
References
- 1.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 2.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25:1419–25. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai SY, Xiong L, Wray CG, Ballatori N, Boyer JL. The farnesoid X receptor FXRalpha/NR1H4 acquired ligand specificity for bile salts late in vertebrate evolution. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1400–9. doi: 10.1152/ajpregu.00781.2006. [DOI] [PubMed] [Google Scholar]
- 4.Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802:363–72. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Düfer M, Hörth K, Wagner R, Schittenhelm B, Prowald S, Wagner TF, et al. Bile acids acutely stimulate insulin secretion of mouse β-cells via farnesoid X receptor activation and K(ATP) channel inhibition. . Diabetes. 2012;61:1479–89. doi: 10.2337/db11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–30. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 7.Popescu IR, Helleboid-Chapman A, Lucas A, Vandewalle B, Dumont J, Bouchaert E, et al. The nuclear receptor FXR is expressed in pancreatic beta-cells and protects human islets from lipotoxicity. FEBS Lett. 2010;584:2845–51. doi: 10.1016/j.febslet.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 8.Teodoro JS, Rolo AP, Palmeira CM. Hepatic FXR: key regulator of whole-body energy metabolism. Trends Endocrinol Metab. 2011;22:458–66. doi: 10.1016/j.tem.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J, et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279:23158–65. doi: 10.1074/jbc.M314322200. [DOI] [PubMed] [Google Scholar]
- 10.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–9. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, Christe ME, et al. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 2005;146:984–91. doi: 10.1210/en.2004-0965. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–11. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prawitt J, Caron S, Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diab Rep. 2011;11:160–6. doi: 10.1007/s11892-011-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–49. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 15.Duran-Sandoval D, Mautino G, Martin G, Percevault F, Barbier O, Fruchart JC, et al. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53:890–8. doi: 10.2337/diabetes.53.4.890. [DOI] [PubMed] [Google Scholar]
- 16.Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861–71. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X, Peter A, Fritsche J, Elcnerova M, Fritsche A, Häring HU, et al. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab. 2009;296:E384–93. doi: 10.1152/ajpendo.90748.2008. [DOI] [PubMed] [Google Scholar]
- 18.Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staels B, Kuipers F. Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs. 2007;67:1383–92. doi: 10.2165/00003495-200767100-00001. [DOI] [PubMed] [Google Scholar]
- 20.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafferty EP, Wylie AR, Hand KH, Elliott CE, Grieve DJ, Green BD. Investigating the effects of physiological bile acids on GLP-1 secretion and glucose tolerance in normal and GLP-1R(-/-) mice. Biol Chem. 2011;392:539–46. doi: 10.1515/bc.2011.050. [DOI] [PubMed] [Google Scholar]
- 22.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 23.Kok T, Hulzebos CV, Wolters H, Havinga R, Agellon LB, Stellaard F, et al. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem. 2003;278:41930–7. doi: 10.1074/jbc.M306309200. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M, Horai Y, Houten SM, Morimoto K, Sugizaki T, Arita E, et al. Lowering bile acid pool size with a synthetic FXR agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem. 2011;286:26913–20. doi: 10.1074/jbc.M111.248203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Seok S, Yu P, Kim K, Smith Z, Rivas-Astroza M, et al. Genomic analysis of hepatic farnesoid X receptor binding sites reveals altered binding in obesity and direct gene repression by farnesoid X receptor in mice. Hepatology. 2012;56:108–17. doi: 10.1002/hep.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrema H, Meissner M, van Dijk TH, Brufau G, Boverhof R, Oosterveer MH, et al. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology. 2010;51:806–16. doi: 10.1002/hep.23408. [DOI] [PubMed] [Google Scholar]
- 28.Bass JY, Caravella JA, Chen L, Creech KL, Deaton DN, Madauss KP, et al. Conformationally constrained farnesoid X receptor (FXR) agonists: heteroaryl replacements of the naphthalene. Bioorg Med Chem Lett. 2011;21:1206–13. doi: 10.1016/j.bmcl.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 29.Lew JL, Zhao A, Yu J, Huang L, De Pedro N, Peláez F, et al. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem. 2004;279:8856–61. doi: 10.1074/jbc.M306422200. [DOI] [PubMed] [Google Scholar]
- 30.Hart AW, Baeza N, Apelqvist A, Edlund H. Attenuation of FGF signalling in mouse beta-cells leads to diabetes. Nature. 2000;408:864–8. doi: 10.1038/35048589. [DOI] [PubMed] [Google Scholar]
- 31.Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, Dina C, Hamid YH, Joly E, et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005;102:4807–12. doi: 10.1073/pnas.0409177102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 2000;275:9270–7. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]