Abstract
Aims: Coiled coil domain containing protein 116 (CCDC116) is a product of the gene coiled coil domain containing 116 located on human chromosome 22. Its function has not yet been established. The present study focuses on the expression of this protein in human pancreatic islets and in the endocrine pancreatic tumors (EPTs). Methods and Results: Expression of the protein was evaluated by immunohistochemistry in endocrine pancreas from six patients and in various EPTs from 51 patients. In pancreatic islets, virtually all insulin, approx. 75% of the somatostatin, and approx. 60% of the pancreatic polypeptide (PP) cells were immunoreactive for the CCDC116 protein whereas glucagon, ghrelin and the exocrine cells were not. All insulinomas, gastrinomas, non-functioning sporadic tumors and the hereditary multihormonal EPTs were immunoreactive with variable relative incidence. Two of the three somatostatinomas, and one of the three ACTH-secreting tumors also expressed CCDC116. Conclusions: The CCDC116 protein is expressed in all islet cell types except the glucagon and ghrelin cells. Most of the EPTs also contained CCDC116 protein. These findings suggest that this protein may play some role for the above mentioned endocrine cells and tumors. Its function has to be investigated in future studies.
Keywords: CCDC116, immunohistochemistry, co-localization, pancreatic islets, endocrine pancreatic tumors
Introduction
The pancreatic islets contain five distinct types of hormone producing cells that have also been characterized ultrastructurally.1-3 Endocrine pancreatic tumors (EPTs) are uncommon neoplasms that may appear sporadically or may be hereditary. EPTs are divided into two groups, functioning and non-functioning, based on the presence or absence of a specific hormone-related clinical syndrome.4
Chromosome 22 is the second smallest human chromosome and contains over 500 genes and about 49 million bases. In that chromosome (cytogenic band 22q11.21||C), a protein product of the gene coiled coil domain containing 116 (CCDC116, alias FLJ36046) termed “coiled coil domain containing 116” (CCDC116) (SwissProt accession nr. Q8IYX3) has been described. CCDC116 protein has 613 amino acids with an estimated molecular weight of 68 kDa. The Swedish Human Proteome Resource (HPR) program has screened a variety of human tissues using tissue microarrays immunostained with CCDC116 polyclonal antibody.5 These screening results showed that this protein was expressed in the human pancreatic islets, as well as cells in the gastrointestinal and respiratory tract (www.proteinatlas.org). Malignant ileal neuroendocrine tumors (midgut carcinoids) have been described to contain CCDC116 immunoreactivity, but a detailed distribution pattern of the protein in different neuroendocrine cell types and neuroendocrine tumors has not been reported. The function of this protein is unknown, but in general many coiled coils are known to play key roles in directing and controlling protein-protein interactions involved in transcription, membrane fusion, and chemotaxis etc.6-8
The aim of this study was to investigate the expression of the CCDC116 protein in the human pancreatic islets in relationship to their different cell types, as well as, its occurrence in different EPTs.
Results
Immunohistochemistry
(A) Pancreatic islets. CCDC116-IR cells occurred in all islets and usually in the majority of parenchymal cells. No exocrine cells were “positive.” The immunoreactivity appeared in the cytoplasm (Figs. 1, 2 and 3). The co-localization studies using double immunofluorescence revealed that virtually all insulin-, as well as, approx. 75% of SS-, and approx. 60% of the PP-IR cells expressed the CCDC116 protein. Ghrelin- and glucagon-IR cells were non-IR.
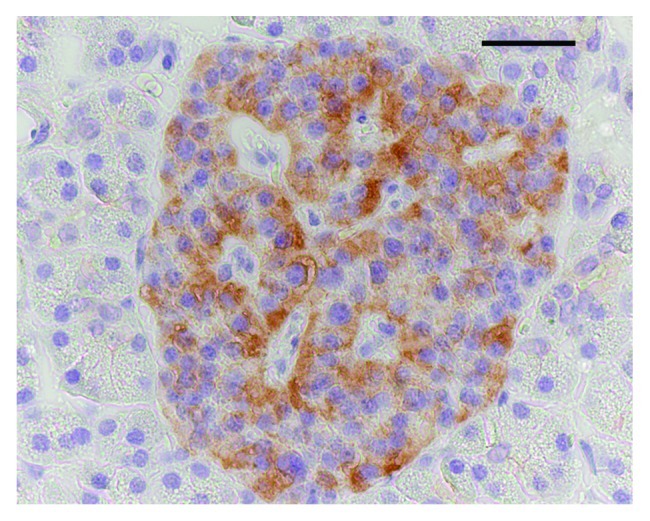
Figure 1. A pancreatic islet immunostained for the CCDC116 protein. The vast majority of the endocrine cells displayed cytoplasmatic immunoreactivity. Bar = 50 μm.
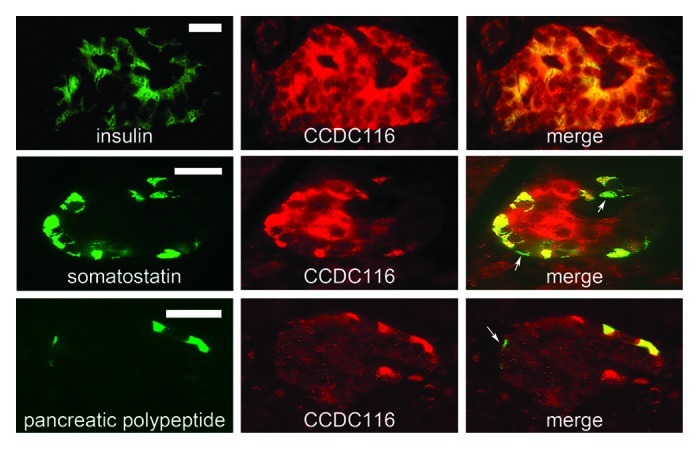
Figure 2. Human pancreatic tissue double immunostained for insulin (green), and the CCDC116 protein (red) (upper panel); somatostatin (green), and the CCDC116 protein (red) (middle panel), pancreatic polypeptide (green), and the CCDC116 protein (red) (lower panel). Co-localization (yellow) depicts that all the insulin-IR cells express the CCDC116 protein. All the somatostatin-IR cells except two (arrows) express the CCDC116 protein. In the lower panel, one of the pancreatic polypeptide expressing cells (arrow) does not express CCDC 116. Bars = 20 μm.
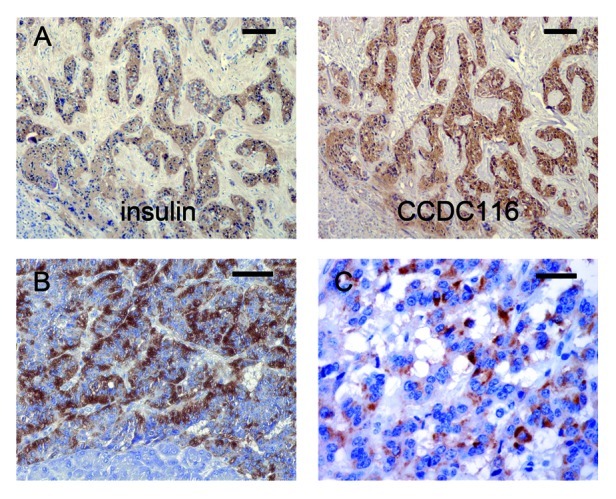
Figure 3. Expression of the CCDC116 protein in EPTs. (A) Consecutive sections of an insulinoma immunostained for insulin and the CCDC116 protein. Virtually all of the tumor cells express the two proteins. (B) A gastrinoma and (C) a non-functioning endocrine pancreatic tumor are immunostained for CCDC116. In both tumors, a fraction of the tumor cell population shows CCDC116 immunoreactivity. Bar for (A) = 100, (B) = 50 µm, and (C) = 25 µm.
(B) Endocrine pancreatic tumors.Functioning tumors.CCDC116 immunoreactivity was seen in all of the 12 insulinomas examined. In seven of the cases, the relative incidence of CCDC116-IR cells was equal to the insulin-IR tumor cells (varied between 15–90% of tumor cells). In five cases, CCDC116 immunoreactivity was limited only to a fraction of the insulin-IR tumor cells, and the colocalization varied from solitary cells up to 50% of the insulin-IR tumor cells. All seven gastrinomas expressed CCDC116 immunoreactivity. In six of these cases, the relative incidence of gastrin and CCDC116 expressing cells was equal (varied between 10–90% of tumor cells); in one case about 50% of the gastrin-IR tumor cells were CCDC116-IR. In two out of three SSomas and in one out of three of the ACTH producing tumors, the majority of the hormone-IR cells displayed immunoreactivity for the CCDC116 protein. In these cases the relative incidence of CCDC116 was 5, 50 and 50% of the tumor cells, respectively. The remaining cases, as well as, the glucagonomas and the VIPomas were negative for CCDC116.
Non-functioning tumors. All of the non-functioning PP-IR tumors expressed the CCDC116 protein in variable number of the PP-IR cells. In two cases around 50% of the PP-IR tumor cells were IR for CCDC116, whereas in the remaining cases only a minority (5, 10 and 10%, respectively). In all of the non-functioning hereditary multi-hormonal tumors, CCDC116-IR cells were detected in variable number of tumor cells (between 2–90% of tumor cells). In these tumors, it was difficult to draw conclusion about the possible co-expression of the CCDC116 protein and the other hormones, due to the multi-hormonal expression pattern.
The non-functioning calcitonin producing tumors, including the tumors that were non-IR for the islet and ectopic hormones, were all non-IR for the CCDC116 protein.
Tests for specificity of the anti-CCDC116 protein antibody
No immunoreactivity was seen after the omission of the primary antiserum in question, or its replacement by non-immune serum in single imunohistochemistry. In double immunostaining, the omission of one of the primary antibodies, or its replacement by non-immune serum gave an immunostaining pattern corresponding to that obtained with the remaining primary antibody. After the omission of both antisera or their simultaneous replacement by non-immune serum, the controls were non-IR.
Discussion
The availability of a high affinity anti-CCDC116 protein antibody developed by the Swedish HPR program has opened the possibility to identify this protein in different tissues and tumors. The present study is descriptive and it confirms that CCDC116-IR cells occur in human endocrine pancreas, but not in the exocrine cells (www.proteinatlas.org). Virtually all the insulin cells and also the majority of SS- and PP-cells displayed the protein, whereas glucagon- and ghrelin-cells were non-IR. In the EPTs the CCDC116 immunoreactivity occurred in all insulinomas, gastrinomas, PP-IR tumors and multihormonal hereditary tumors, as well as, in the majority of SSomas, but only in one of the three ACTH secreting tumor. The relative incidence of the CCDC116-IR cells in the tumors varied.
The insulin and the glucagon cells are the two most abundant cell types in the pancreatic islets, and it is interesting that the CCDC116 protein is expressed in virtually all insulin, but not in the glucagon cells. This protein seems, therefore, not to have a general function in all of the endocrine cell types and its possible effect appears to be cell type related. The importance of CCDC116 is unknown and an essential functional role for insulin release seems less likely, since in the normal islets, only a fraction of the insulin cells is “endocrine active” and involved in the secretion process.9,10 The majority of the SS- and PP-cells also expressed the CCDC116 protein. Thus, the relative abundance of the CCDC116-IR cells helps identify two populations of the SS- and the PP-cells in the adult human islets. Alternatively, it is possible that the two subpopulations of SS- and PP-cells represent different functional stages of these cells. Taking into consideration that coiled coils play key roles in protein-protein interactions involved in transcription and membrane fusion a similar role for the CCDC116 in insulin-, SS- and PP-cells has to be pursued.6-8
The ratio of CCDC116/respective hormone-IR tumor cells was not consistent with that in the endocrine pancreas. This suggests that the tumor cells that are “negative” for the CCDC116 protein may derive from the population of endocrine cells that do not express this protein. Gastrin and ACTH are ectopic hormones in the pancreas. Interestingly, all gastrinomas and a minority of ACTH secreting tumors were “positive” for the CCDC116 protein. It is possible that the CCDC116 protein is also expressed by the gastrin cells/gastrinomas of the gastrointestinal tract and by ACTH cells/ACTH producing tumors of the pituitary playing a role in their function. This question needs to be addressed in future studies.
In summary, the differential expression of the CCDC116 protein in the endocrine pancreatic cells and the respective tumors indicates that it may have cell type specific functions and further studies are needed to elucidate its role.
Materials and Methods
Normal tissues and controls
Pancreatic tissue specimens were obtained from six patients operated for pancreatic adenocarcinoma. The tissues originated from macro- and micro-scopically normal pancreatic tissue, and were located at least 3 cm away from the neoplasm. Pancreatic islets in the surrounding of the tumor were used as internal immunostaining controls.
Ethical approval
The study was performed with permission from the local ethics committee.
Patients and tumors
Tissue specimens from 51 patients with primary EPTs were collected from the Laboratory of Pathology at the Uppsala University Hospital in Sweden. The tumors were either sporadic (S) or hereditary (H) (Multiple Endocrine Neoplasia 1). Based on clinical criteria they were classified as functioning and non-functioning. The functioning tumors consisted of: insulinomas (n = 12; S:H ratio 8:4), gastrinomas (n = 6; S:H ratio 4:2), glucagonomas (n = 6; all sporadic), somatostatinomas (SSomas) (n = 3; all sporadic), VIPomas (n = 2; S:H ratio 1:1), and ACTH-producing tumors causing ectopic Cushing syndrome (n = 3; all sporadic). The non-functioning tumors were: immunoreactive (IR) for pancreatic polypeptide (PP) (n = 5; S:H ratio 3:2), and calcitonin (n = 3; all sporadic), as well as, tumors non-IR for the islet and ectopic (calcitonin, gastrin, ACTH) hormones (n = 5; all sporadic). The patients with the non-functioning tumors did not reveal elevated concentration of islet- or ectopic-hormones in their plasma/serum. Six hereditary multihormonal tumors were included in the study. They expressed the following hormone combinations: Two of these tumors showed insulin-, PP-, and SS-IR cells, three insulin- and SS-IR cells, and one insulin-, PP-, SS-, and gastrin-IR cells.
Anti-CCDC116 protein antibody
A polyclonal rabbit IgG antibody from Atlas Antibodies AB, Stockholm, Sweden (product no. HPA00853) was used. The method for generating this antibody has been described before.11 The amino acid sequence of the complete immunogen (HIS6-ABD-PrEST) is:
H H H H H H S G L V P R G S H M A S L A E A K V L A N R E L D K Y G V S D Y H K N L I N N A K T V E G V K D L Q A Q V V E S A K K A R I S E A T D G L S D F L K S Q T P A E D T V K S I E L A E A K V L A N R E L D K Y G V S D Y Y K N L I N N A K T V E G V K A L I D E I L A A L P G T F A H Y M D P N S S S V D K L A A A Q Q A A S L V I R K Y E F E K D L S K Q L G F F S F P I T H V L R D L S L G L K K V K G S R I H L S S E T H R S C L L R K L E E S K R A R Q A S R L S T S H C S T E T P S V Q Q E P A T H T A Q D Q A T E P C R S L Y T N L P A S R Q L S P L E P K L Y M S A C T G M G S S P P K S K D M* D N E G R D K A E I
The underlined amino acid sequence corresponds to that of the recombinant protein epitope signature tag (PrEST) for the CCDC116 protein, which was linked to the HIS6, and the serum albumin binding domain (residues 146–266) of the streptococcal protein G (shown in bold).12 HIS6-ABD-PrEST was expressed as a fusion protein to enable purification in one step under denaturating condition on immobilized metal affinity chromatography matrix via the HIS6 part.11 The antibody was affinity purified by using the PrEST as the affinity ligand.11 This antibody detects the protein product of the canonical isoform of the CCDC116 gene (i.e., the 68 kDa CCDC116 protein).
Routine staining and immunohistochemistry
All the tissue samples were fixed in 10% buffered neutral formalin and routinely processed to paraffin wax. Consecutive sections, about 4 µm thick, were attached to positively charged glass slides (Superfrost® Plus; Menzel Gläser). Haematoxylin-eosin was used as routine stain. The immunohistochemical staining was based on the avidin biotin complex technique (Vectastain ABC PK-6100). Table 1 summarizes the primary antibodies used (incubation overnight at room temperature). The secondary biotinylated polyclonal antibodies were: goat anti-rabbit (Vector Laboratories, BA-1000, dilution 1:100), goat anti-chicken (Vector Laboratories, BA-9010, 1:100), and horse anti-mouse (Vector Laboratories, BA-2000, 1:100) (incubation 30 min at room temperature). Before immunostaining, the sections were microwave-treated for 2 × 5 min at 750 W using Tris buffer saline, pH 8.0, as retrieval solution. Endogenous horseradish peroxidase activity was quenched by incubating the sections with 0.3% hydrogen peroxide (incubation 30 min at room temperature). Before application of the primary antibody, the sections were incubated (30 min at room temperature) with non-immune serum from the animal species producing the secondary antibodies, at a dilution of 1:10. Diaminobenzidine was used as chromogen. The sections were mounted in Pertex (Histolab Products AB , art. nr. 00814) medium for light microscopy.
Table 1. List of antibodies used in immunohistochemistry.
|
Antibody raised to |
Code |
Source |
Dilution |
Species |
| ACTH |
56 |
Novocastra, Newcastle upon Tyne, UK |
1:800 |
Rabbit |
| Calcitonin |
A0576 |
DakoCytomation, Glostrup, Denmark |
1:10000 |
Rabbit |
| CCDC 116 |
HPA00853 |
Atlas Antibodies AB, Stockholm, Sweden |
1:1600; 1:200 (IF) |
Rabbit |
| Gastrin |
A0568 |
DakoCytomation |
1:12000 |
Rabbit |
| Ghrelin |
Y-031–44 |
Phoenix Pharmaceuticals, INC., Belmont, CA, USA |
1:8000; 1:800 (IF) |
Chicken |
| Glucagon |
7361157 |
Novo BioLabs, NovoCloneTM, Bagsvaerd, Denmark |
1:24000; 1:2400 (IF) |
Mouse |
| Insulin |
|
BioGenex Laboratories, San Ramon, CA, USA |
1:16000; 1:1600 (IF) |
Mouse |
| Pancreatic polypeptide |
ab37285 |
Abcam, Cambridge, UK |
1:50000; 1:10000 (IF) |
Mouse |
| Somatostatin-18 |
7360088 |
Novo BioLabs, NovoCloneTM, Bagsvaerd, Denmark |
1:16000; 1:1600 (IF) |
Mouse |
| VIP | AB982 | Millipore, Temecula, CA, USA | 1: 6000 | Rabbit |
IF, immunofluorescence
Double immunofluorescence in pancreatic islets
Co-localization studies of the islet hormones and the CCDC116 protein were performed using immunofluorescence technique with the primary antibodies vs. the islet hormones mentioned above. For double immunofluorescence staining, the sections were microwave treated as mentioned, and then incubated overnight with a cocktail of two primary antibodies at 4°C. Before application of the antibody cocktail, the sections were incubated (30 min at 4°C) with a mixture of non-immune sera from the animal species producing the secondary antibodies, diluted 1:10. The secondary antibodies (incubation time 30 min, at room temperature) used were: tetramethyl rhodamine isothiocyanate (TRITC)-conjugated donkey anti-rabbit (Jackson ImmunoResearch Laboratories, 711-025-152, 1:100); fluorescein isothiocyanate (FITC)-conjugated donkey anti-chicken (Jackson Labs, 703-095-155, 1:200); and FITC-conjugated donkey anti-mouse (Jackson Labs, 715-095-150, 1:100). TRITC chromogen gives rise to red fluorescence, and FITC to green. Colocalization gives rise to yellow color. The sections were mounted in Vectashield medium for fluorescence (Vector Laboratories, H-1000).
Calculation of immunoreactive cells in pancreatic islets and in endocrine pancreatic tumors
(A) Double immunostained sections in pancreatic islets. The relative incidence of the neuroendocrine cells that co-expressed the CCDC116 protein and the respective islet hormone was calculated in ten randomly selected islets in each of the six pancreata (control tissues) that were included in this study. The double-immunostained islets were photographed with the 63× plan-apochromat objective, and the number of islet cells that coexpressed the hormone in question, and the CCDC116 protein, was counted in the merged pictures
(B) Single immunostained sections in endocrine pancreatic tumors. In consecutive immunostained sections for CCDC116 protein and respective hormone, the percentages of the IR cells were calculated by light-microscopy at a magnification of X400, using a square grid in one of the oculars. At least five randomly selected areas, and in smaller lesions, of the whole neoplastic tissue were examined.
Antibody specificity tests
Specificity tests were performed for the CCDC116 protein, insulin, glucagon, somatostatin (SS), PP and ghrelin antibodies, in conventional IHC analyses, and in double immunofluorescence staining. Control immunostainings included omission of the primary antisera and replacement of the primary antibody by non-immune serum at the same dilution as the primary antibody in question, and in the same diluents.
Acknowledgments
The authors thank Cristina Al-Khalili Szigyarto, Martina Banyay, Soraya Djerbi, Mohamed Eweida and Kenneth Wester for numerous discussions and help with the experiments in connection with the project. This work was supported by the Selander Foundation, Uppsala Läns Landsting and Karolinska Institutet.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/22416
References
- 1.Andralojc KM, Mercalli A, Nowak KW, Albarello L, Calcagno R, Luzi L, et al. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia. 2009;52:486–93. doi: 10.1007/s00125-008-1238-y. [DOI] [PubMed] [Google Scholar]
- 2.In´t Veld P, Marichal M. Microscopic anatomy of the human islet of Langerhans. Adv Exp Med Biol. 2010;654:1–19. doi: 10.1007/978-90-481-3271-3_1. [DOI] [PubMed] [Google Scholar]
- 3.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107:63–9. doi: 10.1016/S0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 4.Tsolakis AV, Janson ET. Endocrine pancreatic tumors: diagnosis and treatment. Expert Reviews of Endocrinology and Metabolism. 2008;3:187–205. doi: 10.1586/17446651.3.2.187. [DOI] [PubMed] [Google Scholar]
- 5.Berglund L, Björling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA, et al. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008;7:2019–27. doi: 10.1074/mcp.R800013-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Bromley EH, Channon K, Moutevelis E, Woolfson DN. Peptide and protein building blocks for synthetic biology: from programming biomolecules to self-organized biomolecular systems. ACS Chem Biol. 2008;3:38–50. doi: 10.1021/cb700249v. [DOI] [PubMed] [Google Scholar]
- 7.Lupas AN, Gruber M. The structure of α-helical coiled coils. Adv Protein Chem. 2005;70:37–78. doi: 10.1016/S0065-3233(05)70003-6. [DOI] [PubMed] [Google Scholar]
- 8.Parry DA, Fraser RD, Squire JM. Fifty years of coiled-coils and α-helical bundles: a close relationship between sequence and structure. J Struct Biol. 2008;163:258–69. doi: 10.1016/j.jsb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Clark A, Jones LC, de Koning E, Hansen BC, Matthews DR. Decreased insulin secretion in type 2 diabetes: a problem of cellular mass or function? Diabetes. 2001;50(Suppl 1):S169–71. doi: 10.2337/diabetes.50.2007.S169. [DOI] [PubMed] [Google Scholar]
- 10.Gylfe E, Grapengiesser E, Hellman B. Propagation of cytoplasmic Ca2+ oscillations in clusters of pancreatic beta-cells exposed to glucose. Cell Calcium. 1991;12:229–40. doi: 10.1016/0143-4160(91)90023-8. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson P, Paavilainen L, Larsson K, Odling J, Sundberg M, Andersson AC, et al. Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. Proteomics. 2005;5:4327–37. doi: 10.1002/pmic.200500072. [DOI] [PubMed] [Google Scholar]
- 12.Nygren PA, Eliasson M, Abrahmsén L, Uhlén M, Palmcrantz E. Analysis and use of the serum albumin binding domains of streptococcal protein G. J Mol Recognit. 1988;1:69–74. doi: 10.1002/jmr.300010204. [DOI] [PubMed] [Google Scholar]


