Abstract
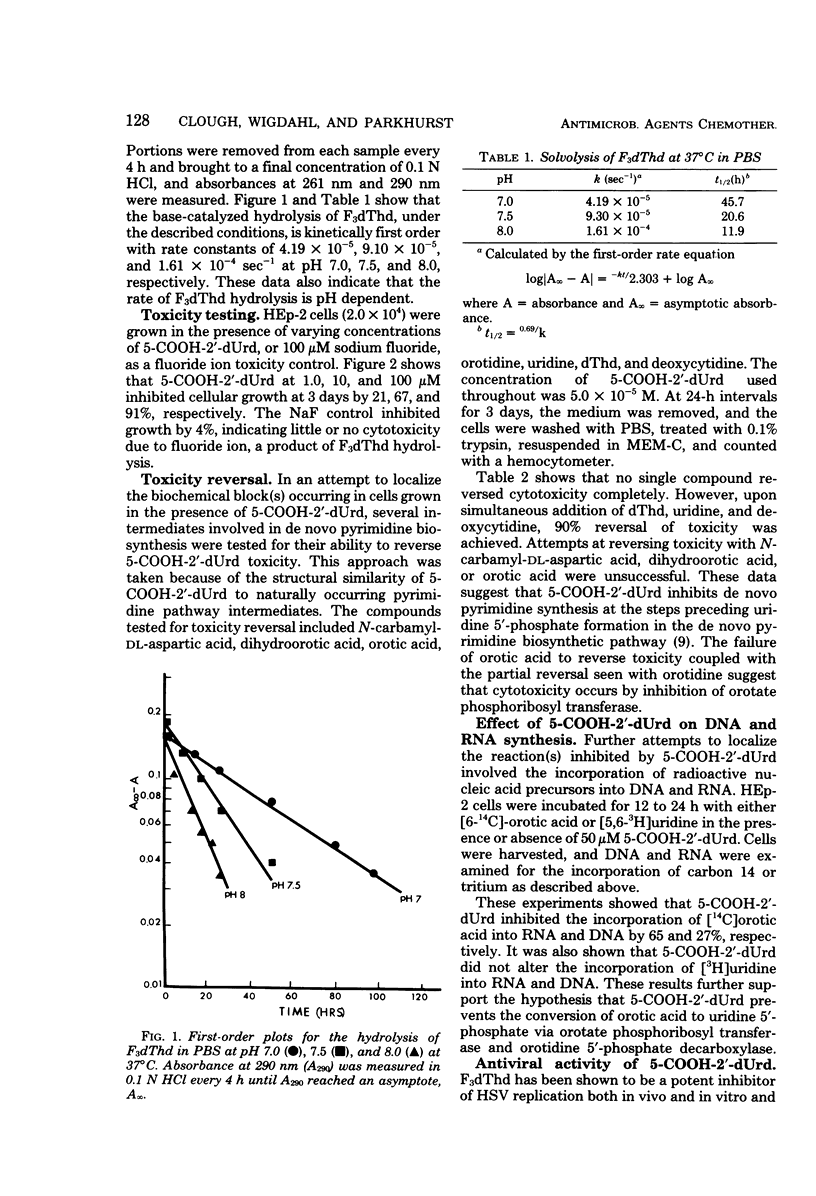
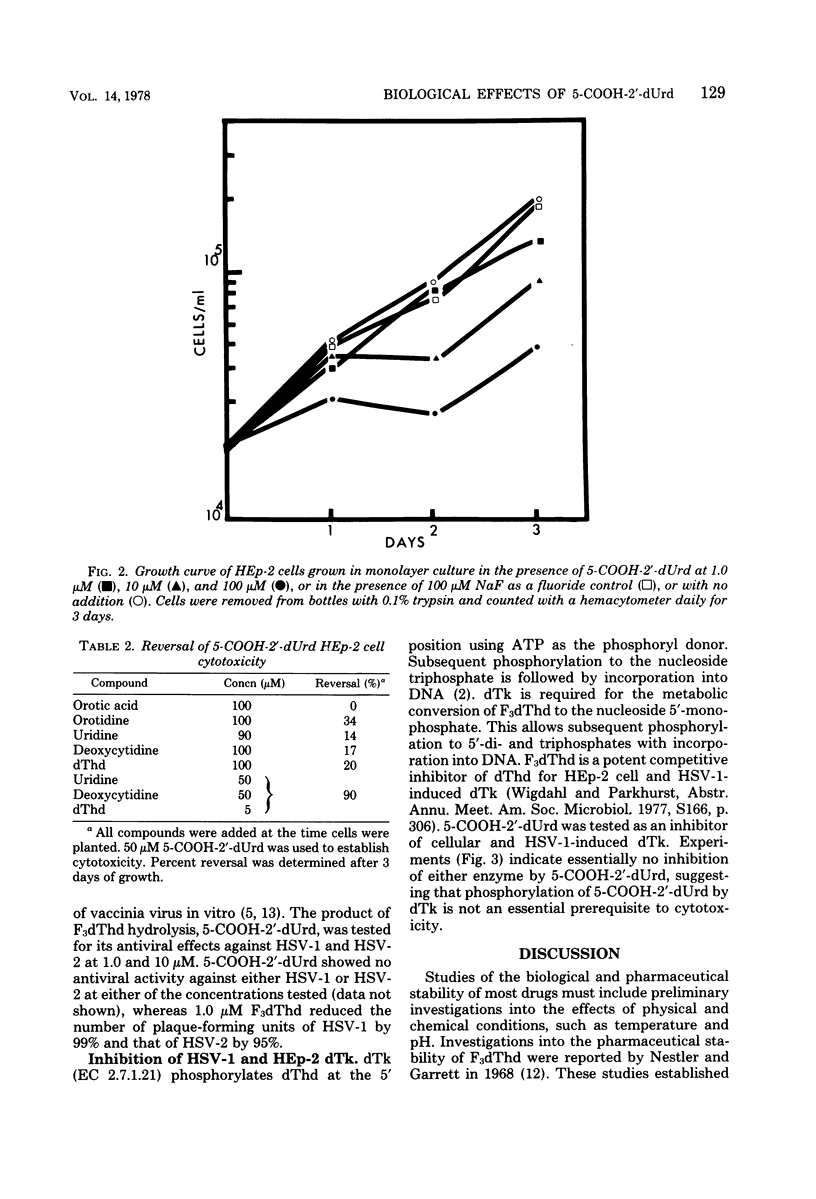
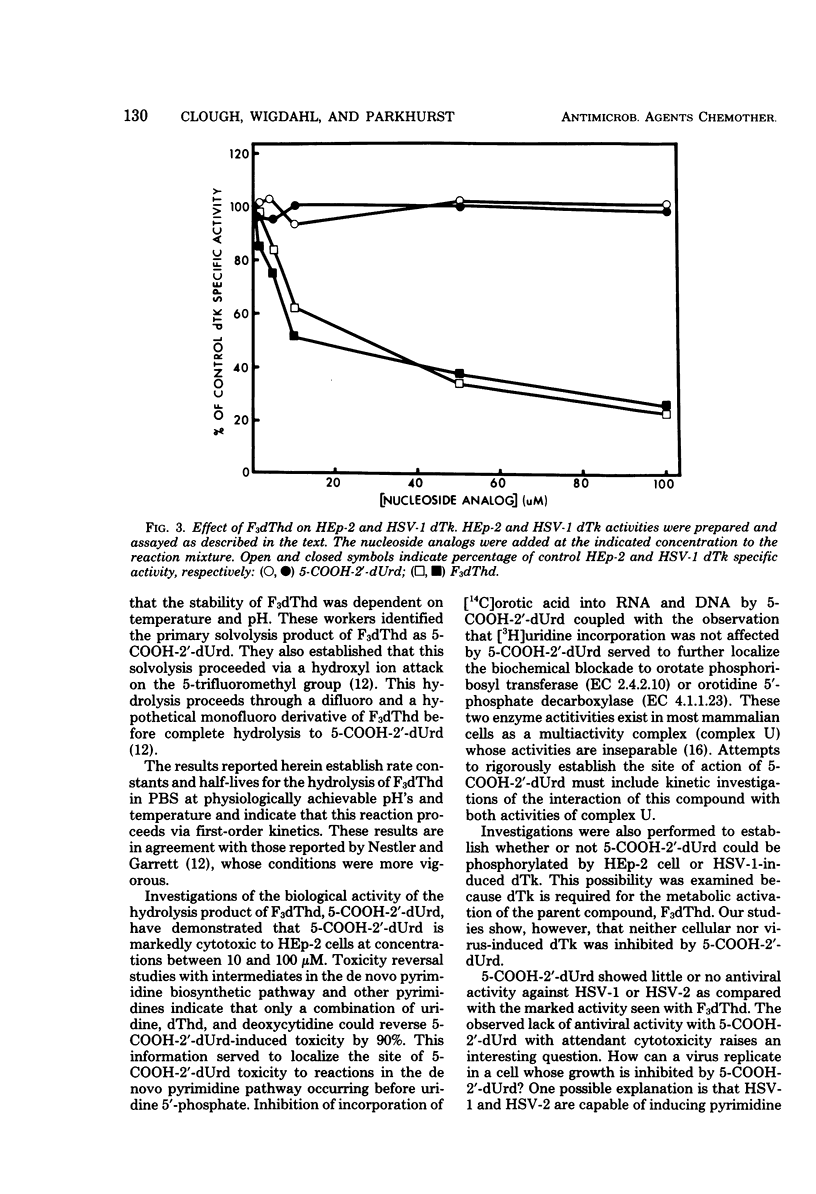
5-Carboxy-2′-deoxyuridine (5-COOH-2′-dUrd) is a product of the base-catalyzed hydrolysis of 5-trifluoromethyl-2′-deoxyuridine. Hydrolysis of 5-trifluoromethyl-2′-deoxyuridine to 5-COOH-2′-dUrd in phosphate-buffered saline was kinetically first order and was pH dependent. At 37°C and pH 7.0, 7.5, and 8.0, hydrolysis occurred with rate constants of 4.19 × 10−5, 9.30 × 10−5, and 1.61 × 10−4 s−1, respectively, with corresponding half-lives of 45.7, 20.6, and 11.9 h. 5-COOH-2′-dUrd inhibited growth of HEp-2 cells by 21, 67, and 91% at 1.0, 10, and 100 μM, with no antiviral activity against herpes simplex virus type 1 or herpes simplex virus type 2 at 1.0 or 10 μM. Partial reversal of cytotoxicity in HEp-2 cells was achieved with orotidine, uridine, deoxythymidine, or deoxycytidine, whereas complete reversal of cytotoxic effects was achieved with simultaneous addition of deoxythymidine, deoxycytidine, and uridine. 5-COOH-2′-dUrd at 50 μM inhibited incorporation of [14C]orotate into RNA and DNA by 65 and 27%, respectively. 5-COOH-2′-dUrd had no effect on the incorporation of [3H]uridine into DNA or RNA. Because of the structural similarities to deoxythymidine, 5-COOH-2′-dUrd was tested as an inhibitor of deoxythymidine kinase. 5-COOH-2′-dUrd was neither a substrate nor an inhibitor of herpes simplex virus type 1 induced deoxythymidine kinase or HEp-2 cell deoxythymidine kinase. Based on these observations, the metabolic block induced by 5-COOH-2′-dUrd has been localized to the de novo pyrimidine biosynthetic pathway between orotate phosphoribosyl transferase and orotidine 5′-phosphate decarboxylase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J., POTTER V. R. Nucleic acid metabolism in regenerating rat liver. VI. Soluble enzymes which convert thymidine to thymidine phosphates and DNA. Cancer Res. 1959 Jun;19(5):561–565. [PubMed] [Google Scholar]
- BREITMAN T. R. The feedback inhibition of thymidine kinase. Biochim Biophys Acta. 1963 Jan 8;67:153–155. doi: 10.1016/0006-3002(63)91807-9. [DOI] [PubMed] [Google Scholar]
- Bittlingmaier K., Schneider D., Falke D. Thymidine transport in herpesvirus hominis type 1 and 2 infected BHK 21 cells. J Gen Virol. 1977 Apr;35(1):159–173. doi: 10.1099/0022-1317-35-1-159. [DOI] [PubMed] [Google Scholar]
- Bresnick E., Williams S. S. Effects of 5-trifluoromethyldeoxyuridine upon deoxythymidine kinase. Biochem Pharmacol. 1967 Mar;16(3):503–507. doi: 10.1016/0006-2952(67)90097-4. [DOI] [PubMed] [Google Scholar]
- Clough D. W., Parkhurst J. R. Experimental herpes simplex virus type 1 encephalitis: treatment with 5-trifluoromethyl-2'-deoxyuridine. Antimicrob Agents Chemother. 1977 Feb;11(2):307–311. doi: 10.1128/aac.11.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIDELBERGER C., PARSONS D. G., REMY D. C. SYNTHESES OF 5-TRIFLUOROMETHYLURACIL AND 5-TRIFLUOROMETHYL-2'-DEOXYURIDINE. J Med Chem. 1964 Jan;7:1–5. doi: 10.1021/jm00331a001. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Huffman J. H., Sidwell R. W., Khare G. P., Witkowski J. T., Allen L. B., Robins R. K. In vitro effect of 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole, ICN 1229) on deoxyribonucleic acid and ribonucleic acid viruses. Antimicrob Agents Chemother. 1973 Feb;3(2):235–241. doi: 10.1128/aac.3.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBERMAN I., KORNBERG A., SIMMS E. S. Enzymatic synthesis of pyrimidine nucleotides; orotidine-5'-phosphate and uridine-5'-phosphate. J Biol Chem. 1955 Jul;215(1):403–451. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin S. S., Munyon W. Expression of the viral thymidine kinase gene in herpes simplex virus-transformed L cells. J Virol. 1974 Nov;14(5):1199–1208. doi: 10.1128/jvi.14.5.1199-1208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler H. J., Garrett E. R. Prediction of stability in pharmaceutical preparations. XV. Kinetics of hydrolysis of 5-trifluoromethyl-2'-deoxyuridine. J Pharm Sci. 1968 Jul;57(7):1117–1125. doi: 10.1002/jps.2600570706. [DOI] [PubMed] [Google Scholar]
- Parkhurst J. R., Danenberg P. V., Heidelberger C. Growth inhibition of cells in cultures and of vaccinia virus infected HeLa cells by derivatives of trifluorothymidine. Chemotherapy. 1976;22(3-4):221–231. doi: 10.1159/000221929. [DOI] [PubMed] [Google Scholar]
- Reyes P., Heidelberger C. Fluorinated pyrimidines. XXVI. Mammalian thymidylate synthetase: its mechanism of action and inhibition by fluorinated nucleotides. Mol Pharmacol. 1965 Jul;1(1):14–30. [PubMed] [Google Scholar]
- Tone H., Heidelberger C. Fluorinated pyrimidines. XLIV. Interaction of 5-trifluoromethyl-2'-deoxyuridine 5'-triphosphate with deoxyribonucleic acid polymerases. Mol Pharmacol. 1973 Nov;9(6):783–791. [PubMed] [Google Scholar]
- Traut T. W., Jones M. E. Inhibitors of orotate phosphoribosyl-transferase and orotidine-5'-phosphate decarboxylase from mouse Ehrlich ascites cells: a procedure for analyzing the inhibition of a multi-enzyme complex. Biochem Pharmacol. 1977 Dec 1;26(23):2291–2296. doi: 10.1016/0006-2952(77)90293-3. [DOI] [PubMed] [Google Scholar]
- Umeda M., Heidelberger C. Comparative studies of fluorinated pyrimidines with various cell lines. Cancer Res. 1968 Dec;28(12):2529–2538. [PubMed] [Google Scholar]
- Wellings P. C., Awdry P. N., Bors F. H., Jones B. R., Brown D. C., Kaufman H. E. Clinical evaluation of trifluorothymidine in the treatment of herpes simplex corneal ulcers. Am J Ophthalmol. 1972 Jun;73(6):932–942. doi: 10.1016/0002-9394(72)90463-1. [DOI] [PubMed] [Google Scholar]


