Abstract
Background
The genetic background of Basal Cell Carcinoma (BCC) has been studied extensively, while its epigenetic makeup has received comparatively little attention. Epigenetic alterations such as promoter hypermethylation silence tumor suppressor genes (TSG) in several malignancies.
Objective
We sought to analyze the promoter methylation status of ten putative (tumor suppressor) genes that are associated with Sonic Hedgehog (SHH), WNT signaling and (hair follicle) tumors in a large series of 112 BCC and 124 healthy control samples by methylation-specific PCR.
Results
Gene promoters of SHH (P = 0.016), adenomatous polyposis coli (APC) (P = 0.003), secreted frizzled-related protein 5 (SFRP5) (P = 0.004) and Ras association domain family 1A (RASSF1A) (P = 0.023) showed significantly more methylation in BCC versus normal skin. mRNA levels of these four genes were reduced for APC and SFRP5 in BCC (n = 6) vs normal skin (n = 6). Down regulation of SHH, APC and RASSF1A could be confirmed on protein level as well (P<0.001 for all genes) by immunohistochemical staining. Increased canonical WNT activity was visualized by β-catenin staining, showing nuclear β-catenin in only 28/101 (27.7%) of BCC. Absence of nuclear β-catenin in some samples may be due to high levels of membranous E-cadherin (in 94.1% of the samples).
Conclusions
We provide evidence that promoter hypermethylation of key players within the SHH and WNT pathways is frequent in BCC, consistent with their known constitutive activation in BCC. Epigenetic gene silencing putatively contributes to BCC tumorigenesis, indicating new venues for treatment.
Introduction
Basal cell carcinoma (BCC) accounts for 75% of all skin cancers and is the most frequent malignancy in Caucasians. Its incidence is rising by 3–8% each year [1], resulting in an average lifetime risk for Caucasians of developing BCC of 30% [2], [3]. BCC rarely metastasize [4], but when left untreated they may cause extensive local tissue destruction [4], [5]. Surgical excision is the current standard treatment, with average costs in the Netherlands of €900 per procedure, amounting to a total of €45 million by 2015 [6], [7]. The inevitably rising workload can be expected to stress the health care system even further. Hence, BCC is becoming a serious health problem. There is a clear need for a simple and cost-efficient medical treatment. In order to develop one, a thorough understanding of BCC pathobiology will be required and the past few years have witnessed considerable progress in this respect.
Inappropriate activity of the Sonic Hedgehog (SHH) pathway due to mutations of its component genes is firmly implicated in BCC pathogenesis. Inactivating mutations of Patched-1 (PTCH1) are the most common (30–60%) in sporadic BCC [8]–[11], followed by mutations (10–20%) in Smoothened (SMO) or Suppressor of Fused (SU(FU)) [11]–[16]. Even though mutations of the SHH target gene, Glioma-associated oncogene homolog-1 (GLI1), are uncommon, approximately 90% of sporadic BCC overexpress GLI1, which contributes to tumor growth [17]. In addition, Yang and colleagues proved the wingless-type MMTV integration site family (WNT) pathway to be essential in tumorigenic response to deregulated SHH signaling, suggesting crosstalk between the SHH and canonical WNT pathways in BCC [18]. Non-canonical WNT signaling, however, might also be active in the context of repressed canonical WNT signaling in BCC, as recently reported by Pourreyron et al. [19]. Thus, both WNT signaling pathways might act in a mutually antagonistic fashion in driving BCC growth. In all, the genetic aspects of BCC development seem to be well defined. However, most if not all cancers are characterized by epigenetic alterations in addition to genetic changes [20]. Epigenetic modifications are heritable changes in deoxyribonucleic acid (DNA) structure other than alterations in the DNA sequence, and when pathologically altered are capable of driving malignant tumor development and progression. To date, DNA hypermethylation is the best-characterized epigenetic mechanism, comprising the addition of a methyl group to a cytosine base adjacent to a guanine base (the CG dinucleotide) [20]. CG-rich areas (CpG islands) located in the promoter region of many genes become hypermethylated in numerous malignancies [21] leading to gene silencing [22]. Promoter hypermethylation can precede genetic mutations and genomic instability in tumor development, and may thus not only be crucial for carcinogenesis, but also represent a potential therapeutic target [23]. Indeed, DNA demethylating agents, such as 5-azacytidine (Vidaza®, Celgene) and 5-aza-2′-deoxycytidine (Dacogen™, MGI Pharma) can lead to reactivation of silenced genes [24]. Thus, it would be of interest to determine the contribution of promoter hypermethylation to BCC pathogenesis. To date, a very restricted number of studies have addressed this question in a limited number of samples [25]–[30]. Therefore, we decided to assess the promoter CpG island methylation status of nine tumor suppressor genes (TSGs). Patched Homolog 1 (PTCH1) is the SHH receptor and is the most commonly mutated tumor suppressor in BCC, whereas adenomatous polyposis coli (APC), secreted frizzled-related protein 1 (SFRP1), SFRP2, SFRP4, and SFRP5 are all negative regulators of the canonical WNT pathway. Sustained signaling through the canonical WNT pathway contributes to the development of colorectal cancer as well as basal cell carcinoma [18], [19], [31]. Since BCC are considered as hair follicle tumors, we additionally analyzed the cylindromatosis (CYLD) and tuberous sclerosis-1 (TSC1) genes, which both are TSGs known to be involved in classic hair follicle tumor syndromes [32], [33]. Additionally, TSC complex proteins are crucial negative regulators of mTOR activity, which is implicated in tumor growth [34]. Ras association domain family 1A (RASSF1A) is a well-known TSG, promoter methylation of which has been described as an early and frequent event in several malignancies including basal cell carcinoma [28], [35]. Finally we examined SHH, since we hypothesized that downstream activation of its signaling pathway would permit it to become inactivated through methylation.
We have demonstrated significant hypermethylation affecting several of the selected genes in BCC, supporting the involvement of epigenetic aberrations in the most common skin cancer.
Materials and Methods
Primary Tissue Specimens
The methylation status of ten selected genes was examined in 112 sporadic BCC samples (107 formalin-fixed paraffin-embedded (FFPE), five fresh frozen (FF)) diagnosed at the Department of Dermatology, MUMC+. All BCC samples were obtained from the Maastricht Pathology Tissue Collection (MPTC). Distinct histological subtypes included were: superficial (sBCC) (n = 37), nodular (nBCC) (n = 53), and infiltrative (iBCC) (n = 22). Thirty of these samples were used for immunohistochemical analysis of the expression of SHH, APC and RASSF1A. β-catenin and E-cadherin were assessed on respectively 101 (30 sBCC, 40 nBCC, 31 iBCC) and 59 BCC (17 sBCC, 27 nBCC, 15 iBCC) independent samples. Patients included 58 men (mean age 68.1 years, SD ±11.0) and 54 women (mean age 66.2 years, SD ±15.1) A total of 124 healthy control tissue samples (71 FFPE, 53 FF) was collected at autopsies and matched with the 112 BCC patients with regard to age and gender (Table 1). Relatives gave their written consent for usage of the skin tissues. For 31 of these controls, no data were available concerning age and gender. Collection, storage and use of all tissues and patient data were performed in agreement with the “Code for Proper Secondary Use of Human Tissue in the Netherlands”. All of the used samples and corresponding data were de-linked and anonymized. Usage of both BCC and control tissue samples was approved by the MPTCscientific committee (MPTC 2009-05).
Table 1. Sample characteristics.
| Tumor (n = 112) | Normal skin (n = 124) | |
| Sex | ||
| Male, n (%) | 58 (51.3) | 50 (53.8) |
| Female. n (%) | 54 (48.2) | 43 (46.2) |
| Unknown, n (%) | 0 (0) | 31 (25.0) |
| Age, years mean | ||
| Overall | 67.2 (±13.1 SD) | 68.1 (±14.0 SD) |
| Man | 68.1 (±11.0 SD) | 67.9 (±12.2 SD) |
| Women | 66.2 (±15.1 SD) | 68.4 (±16.0 SD) |
| Tissue Source | ||
| FFPE, n (%) | 107 (95.5) | 71 (57.3) |
| FF, n (%) | 5 (4.5) | 53 (42.7) |
| BCC subtypes, n (%) | ||
| Superficial | 37 (32.7%) | |
| Nodular | 53 (46.9%) | |
| Infiltrative | 22 (19.6%) |
FFPE = Formalin-fixed, paraffin-embedded, FF = Fresh- frozen.
DNA Isolation
From each paraffin tissue block and FF sample, a 4 µm section was cut and stained with haematoxylin & eosin (H&E) as a part of the routine intake procedure. All H&E sections were reviewed by a dermato-pathologist (AM or VW) to confirm diagnosis and histological subtype. After deparaffinization of five sections (20 µm) of each FFPE sample, tumor tissue was macroscopically scraped and collected in 1.5 ml tubes. The sections of the FF samples were directly collected into 1.5 ml tubes. Genomic DNA was extracted from FFPE tissue sections by using a Qiagen microkit for DNA isolation (Qiagen, Venlo, The Netherlands) following the manufacturer’s directions.
Bisulfite Modification of Genomic DNA, Methylation-specific PCR and Bisulfite Genomic Sequencing
Promoter CpG island methylation was determined by bisulfite genomic sequencing (BGS) for SHH, and by MSP for PTCH1, SHH, APC, SFRP1, SFRP2¸ SFRP4, SFRP5, CYLD, TSC1, and RASSF1A. Sodium bisulfite modification was performed on 500 ng of genomic DNA isolated from the tissue sections by use of an EZ DNA methylation kit (Zymo Research Co, Orange, CA, USA) according to the manufacturer’s instructions. The modified DNA was eluted to 50 ng/µl in H20 and stored at −80°C.
Nested methylation specific polymerase chain reaction (MSP) was performed on bisulfite-modified genomic DNA with primers specific for methylated DNA and unmethylated DNA as previously described [36]–[38]. Nested MSP is exquisitely suitable for methylation analysis of FFPE DNA since it is highly sensitive. Primer sequences and PCR conditions are listed in Table S1. To assess reproducibility of the nested MSP approach, MSP reactions were performed in duplicate starting from DNA amplification with flanking polymerase chain reaction (PCR) primers. Non-concordant MSP results were analyzed a third time and concordance in two out of three assays was accepted as end result. The overall reproducibility for the MSP data was 90.3%.
For BGS analysis, one µl of PCR product from bisulfite-modified genomic DNA was cloned into TOP10 bacteria using a TOPO-TA cloning kit (Invitrogen, Breda, The Netherlands). Bacteria were cultured at 37°C overnight. DNA was extracted from at least ten independent bacterial clones and sequenced using an automated DNA sequencer (Applied Biosystems, Foster City, CA, USA). Sequencing data was analyzed using Sequence Scanner v1.0 software (Applied Biosystems). Mean values were calculated and represented in pie-chart figures per CpG.
RNA Isolation, Reverse Transcription and Quantitative Reverse Transcription PCR (RT-PCR)
Total ribonucleic acid (RNA) from FF samples containing more than 75% BCC cells (n = 6) as evaluated from H&E stained sections and FF normal skin samples (n = 6) was isolated using the standard procedure for TRIzol® RNA extraction (Invitrogen) and stored at −80°C. Complementary DNA (cDNA) synthesis was performed using the iScript™ cDNA Synthesis kit (Biorad, Veenendaal, The Netherlands) according to the manufacturer’s instructions. Quantitative RT-PCR on 30 ng cDNA was carried out using primer sets for APC, RASSF1A, SFRP5, and SHH. Primer sequences and PCR conditions are listed in Table S1. Messenger RNA (mRNA) for SHH and RASSF1A could not be detected. APC and SFRP5 expression levels for each BCC sample were normalized to the housekeeping gene Cyclophylin A and average expression levels in normal skin tissues (n = 6) by 2−ΔΔCt parameter [39]. To assure accuracy, all reactions were performed in triplicate.
Immunohistochemistry
For SHH, APC and RASSF1A analysis, FFPE sections (4 µm) were deparaffinized in xylene, rehydrated and incubated in 0.3% hydrogen peroxide (H2O2) in methanol for 30 minutes to inactivate endogenous peroxidase activity. Antigen retrieval was performed by microwave treatment at 90 W for 10 minutes in 10 mM citrate buffer (pH 6) (APC and SHH) or Envision Flex target retrieval solution high pH (Dako, Heverlee, Belgium) (RASSF1A). Next, non-specific protein binding was blocked using 3% bovine-serum-albumin (BSA). Subsequently, the sections were incubated for 1 h at room temperature with primary antibodies listed in table S2. A horseradish peroxidase (HRP)-conjugated second antibody, either PowerVision+ (ImmunoVision Technology, Brisbane, CA, USA) (APC and SHH) or Envision detection system (Dako) (RASSF1A) was applied for 30 minutes. Bound antibody was visualized by using 3,3-diaminobenzidine (DAB) for 10 minutes. Tissue was counterstained with haematoxylin, dehydrated and sealed with coverslips. Phosphate-buffered saline (PBS) was used throughout for washing steps.
For β-catenin and E-cadherin analysis, sections were pre-treated in a pre-treatment module using EnVision FLEX Target Retrieval Solution, High pH (Dako). Staining was performed on a Dako autostainer system using the Dako Envision Flex kit (K8002) for secondary detection. Sections were counterstained with Gill II haematoxylin, dehydrated and sealed with coverslips. For all antibodies, tissue known to be strongly expressing respective protein was included as positive control (Table S2). Negative controls (omission of the primary antibody) were included in all experiments.
Interpretation of Staining
A specialized dermato-pathologist of the Department of Pathology, MUMC+ (VW) and an experienced resident of the Department of Dermatology, MUMC+ (TB), examined sections independently. Any discrepancy between the observers was discussed and resolved by consensus. β-catenin staining was assessed with respect to localization (membranous, cytoplasmic, and nuclear). For the other antibodies, the percentage positive tumor cells were determined by assessing ten randomly chosen high-power-fields (magnification 200×) per slide and the average of both observers’ values was used for analysis. Additionally, the intensity of E-cadherin was considered with respect to an internal positive control ranging from 0 (no staining) to 1 (weak), 2 (moderate), and 3 (strong) staining.
Statistics
Statistical analyses were carried out using SPSS version 18.0 software (SPSS, Chicago, IL, USA). Discrete data were analyzed using a Chi-square or Fisher’s exact test, where the independent-samples T-test was used for continuous variables. The correlation between two discrete variables was evaluated by the Cohen’s kappa [40]. To evaluate the effect of methylation on the probability of the presence of tumor, multivariate binary logistic regression analyses were performed with presence or absence of tumor as dependent variable. The two way random effect model with absolute agreement intra-class correlation coefficient (ICC) was used as inter-rater reliability analysis to determine consistency among raters [41]. An ICC ≥0.75 indicates excellent reproducibility [42]. All reported P values are two-sided, and P values ≤0.05 were considered statistically significant.
Results
Methylation of SHH and WNT Pathway Components in BCC
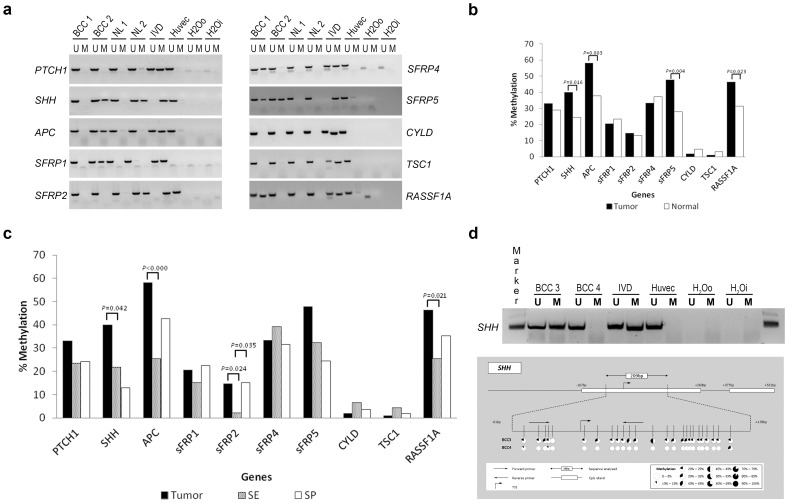
A total of nine candidate TSGs and one oncogene was examined in a series of primary BCC (n = 112) of three different subtypes and normal skin (n = 124). Characteristics of the tumor and normal skin samples are listed in Table 1. Primers located in promoter CpG islands were previously determined [36]–[38]. Our data showed that promoters of four genes were significantly more frequently methylated in BCC tissue versus normal skin: SHH (44/100 (40.0%) vs. 30/122 (24.6%), P = 0.016), APC (64/110 (58.2%) vs. 47/124 (37.9%), P = 0.003), SFRP5 (52/109 (47.7%) vs. 28/100 (28.0%), P = 0.004), and RASSF1A (52/112 (46.4%) vs. 39/124 (31.3%), P = 0.023. The correlation between methylation in SHH and SFRP5 was moderate (kappa 0.68 (95% CI 0.50–0.78)); no further correlations between genes could be detected. After mutual correction of the two genes SHH and SFRP5 by multivariate logistic regression, odds ratios for individual genes were still larger than 1 (O.R. 1.42 (P = 0.304) and 2.01 (P = 0.032) respectively), suggesting that methylation of SFRP5 is significantly associated with the occurrence of BCC. Notably, of the four significant differentially methylated genes, nBCC harbored more frequent methylation for APC and RASSF1A, whereas SHH and SFRP5 were more frequently methylated in iBCC. Nevertheless, none of these differences were significant because of the low sample numbers. Moreover, although SFRP4 overall was not significantly hypermethylated in BCC, its methylation frequencies did vary according to the subtype (P = 0.010 for sBCC vs nBCC plus iBCC, P = 0.011 sBCC vs nBCC) (Figure S1). No significant differential methylation could be detected for PTCH1, SFRP1, SFRP2, SFRP4, CYLD, and TSC1 (Figure 1A and 1B). When we compared sun-exposed (SE) (n = 47) with sun-protected (SP) (n = 54) normal skin tissue, only methylation of SFRP2 was found to be significantly different, with more frequent methylation in SP skin (SE 2.2% vs. SP 15.1%, P = 0.035) (Figure 1C). Gender or age-associated methylation patterns were not identified.
Figure 1. Methylation analysis in BCC and normal skin of ten candidate genes.
A. Illustration of MSP results of ten candidate genes resolved on a 2% agarose gel. BCC: Basal cell carcinoma; NL, normal; IVD, In Vitro Methylated-DNA; Huvec, Human Umbilical Vein Endothelial Cells; U, unmethylated; M, methylated; H20o, water control outside PCR; H20i, water control inside PCR. B. Illustration of methylation frequencies of ten candidate genes in BCC (n = 112) and normal skin samples (n = 124). P-values represent the difference between percentage methylation in BCC an normal skin. C. Illustration of methylation frequencies of ten candidate genes in BCC, sun-exposed (SE) normal skin and sun-protected (SP) normal skin. P-values represent both the difference between percentage methylation in BCC and SE and SP. D. SHH sequence data of bisulfite treated genomic DNA from patients. Upper part shows the SHH promoter region starting 1000 base pairs (bp) upstream of the transcription start site (TSS) to 1000 bp downstream. White boxes indicate putative CpG islands (EMBOSS, http://emboss.sourceforge.net). The 256 bp region sequenced stretches from −51 bp from the TSS to +409 bp. Indicated with arrows are the forward and reverse methylation specific PCR (MSP) primers. Vertical bars represent CpG dinucleotides and pie -charts represent the percentage of methylated CpG sites (percentage over at leased 10 sequenced clones).
As MSP primers of APC, SFRP5, and RASSF1A had been previously established and validated [38], [43], [44], we only validated our MSP results of the fourth differentially methylated gene, SHH, by BGS, which is considered the gold standard [45]. Figure 1D depicts the BGS results of one methylated and one unmethylated SHH sample as measured by MSP, and BGS could confirm MSP results.
Expression of SHH, APC, SFRP5, and RASSF1A is Reduced in BCC
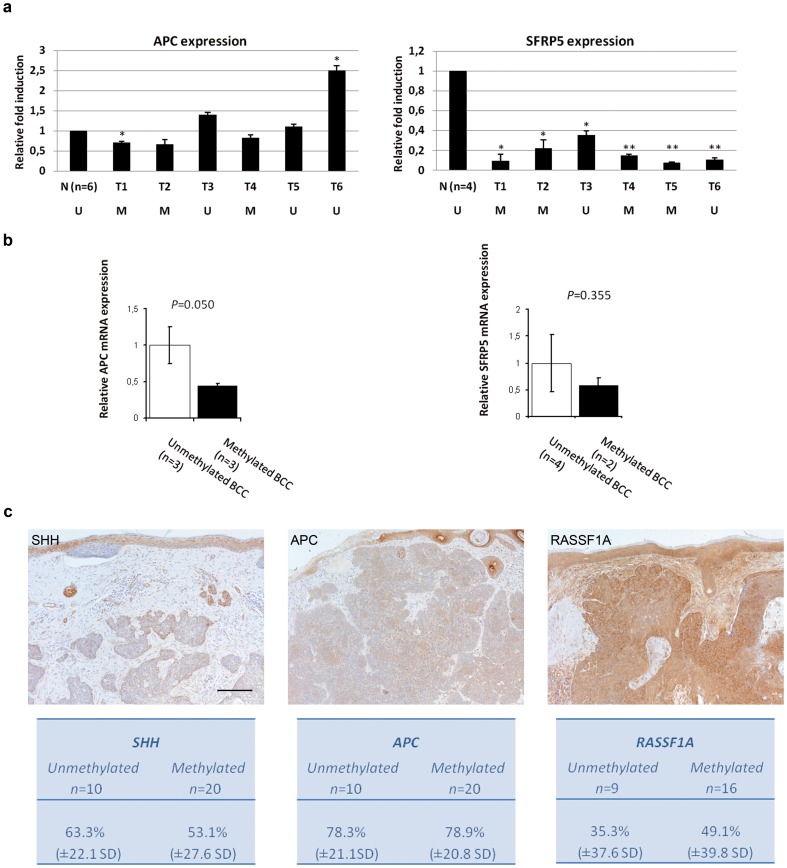
To assess whether the methylation status impacts expression of the differential methylated genes, we performed quantitative real-time PCR on primary BCC (n = 6) and normal skin samples (n = 6). Whereas we could not detect SHH and RASSF1A mRNA in BCC and normal skin samples (data not shown), we did observe that SFRP5 mRNA expression level was significantly reduced in BCC versus normal skin (P = 0.019). APC mRNA expression level was not altered (P = 0.937) in the samples examined (Figure 2A). Nevertheless, BCC with methylated APC showed borderline significant reduction mRNA level compared with BCC in which APC was unmethylated (P = 0.050). Correlation of mRNA expression and methylation was not observed for SFRP5 (P = 0.355) (Figure 2B)¸ probably due to the fact that SFRP5 expression levels in all BCC were very low.
Figure 2. Expression of SHH, APC, SFRP5 and RASSF1A is reduced in BCC versus healthy skin control tissue.
A. Relative expression levels of APC and SFRP5 in BCC tissues as compared to the expression level in normal skin (n = 6) (2−ΔΔCt). All reactions were done in triplicates, standard error of the mean (SEM) is shown as error bars. Expression levels were normalized to Cyclophilin A. U, unmethylated sample; M, methylated sample. *p≤0.05, **p≤0.001. B. Relative mRNA expression in unmethylated versus methylated BCC samples for either APC or SFRP5. C. Microphotographs of selected samples of SHH, APC and RASSF1A. Bar = 200 µm.
To investigate if we could see an effect of DNA methylation on the protein level, we performed immunohistochemical protein staining for SHH, APC and RASSF1A in a random selection of nodular BCC for which the methylation status had also been determined. After extensive testing, we found that commercially available antibodies for SFRP5 were not suitable for immunohistochemistry. Using the hair follicle as internal control (set at 100% positivity), the expression of SHH, APC and RASSF1A in tumor cells was significantly lower (all p-values <0.001) (Figure 2A). The intra-class correlation coefficients (ICC) for the scores of all stainings were high, with 0.87 for SHH, 0.8 for APC and 0.83 for RASSF1A. However, expression levels of methylated samples were not further reduced when compared with unmethylated samples (Figure 2C). Lastly, for SFRP5 we considered the immunohistochemical data available at the ProteinAtlas website by way of independent comparison with our data. For SFRP5, ProteinAtlas has data for one antibody (HPA019840) in six BCC and six squamous cell carcinomas, showing strong cytoplasmic and membranous antibody staining and with strong intensity in >75% of the tumor cells as well as the overlying skin, which is in contrast with the low mRNA expression levels we found (Data S1). We think that the ProteinAtlas samples are affected by overstaining. As a result, it is not possible to conclude from these data whether or not sFRP5 expression in BCC is lower than in unaffected skin, as it is in our series. Furthermore, heterogeneity among immunohistochemical stainings is a frequent problem interfering with any assessment and can be due to several factors including the antibody of choice. To settle this matter, more comprehensive SFRP5 expression analyses in a larger series of BCC, using both immunohistochemistry and quantitative RT-PCR, would be desirable.
Low Levels of Nuclear ß-catenin Coincide with High Levels of E-cadherin in BCC
As our results suggest epigenetic involvement of the WNT pathway in BCC, we subsequently analyzed the expression of β-catenin as readout for WNT pathway activity. β-catenin expression was localized at cell membranes of normal epidermis and within the cytoplasm and nuclei of hair follicles, consistent with the known activity of canonical WNT signaling in this structure [18]. In the 101 BCC we examined, β-catenin was present only at cell membranes in 65 (64.4%) tumors, and localized at both the cell membrane and in the cytoplasm in 8 (7.9%) cases. Nuclear staining was predominantly located at the tumor periphery and only seen in 28 (27.7%) of the BCC samples without preference for one of the subtypes (Figure 3A). No correlation could be detected between the methylation status of APC or SFRP5 and the presence or localization of β-catenin. As it is known that the E-cadherin/β-catenin complex is important in both cell adhesion and canonical WNT signaling [46], we additionally analyzed the expression of E-cadherin, which is normally expressed throughout all layers of the epidermis, including hair follicles [47]. Expression of E-cadherin in 59 BCC was significantly lower compared with the normal epidermis (P = 0.001), but was rated high (94.1%) in the tumor cells. Likewise, intensity of staining was rated as strong (69.5%) in the BCC (Figure 3B). These findings were independent of tumor subtype and in agreement with the generally non-metastatic behavior of BCC [3]. The ICC for E-cadherin (0.91) again was high.
Figure 3. Low levels of nuclear ß-catenin coincide with high levels of E-cadherin in BCC.
A. Microphotographs of selected sample of ß-catenin, showing nuclear staining only at the periphery or the tumor. Bar = 200 µm. B. Microphotographs of selected sample of E-cadherin showing lowered expression of the tumor compared with the normal epidermis.
Discussion
To the best of our knowledge, this is the largest study to date addressing epigenetic changes in the most common human malignancy, BCC. We provide data which suggest that the SHH and WNT pathways may be epigenetically involved in BCC pathobiology, since both networks are affected by aberrant promoter methylation of SHH, APC and SFRP5. Aberrant methylation and associated gene silencing of APC and SFRP5 may contribute to the pathogenesis of BCC by impairing negative regulation of WNT pathway activity. In addition, we detected differential methylation of RASSF1A, a well-known tumor suppressor modulating a broad range of cellular functions that are essential for normal cell growth. RASSF1A expression is lost in high frequency by promoter methylation in a wide variety of human tumors, in fact, it is one of the best-characterized tumor suppressor genes in UV-induced squamous cell carcinoma of the skin [48], [49] and methylation of its promoter had been previously demonstrated in BCC [28].
Epigenetic alterations in BCC have previously been reported, but in limited sample series [26]–[30] and without providing further experimental evidence for relevance of the epigenetic changes for BCC carcinogenesis. Sathyanarayana et al. investigated promoter methylation of 12 genes in a series of 68 BCC samples and 58 non-malignant lesions (skin tags) and showed that laminin gamma 2 (LAMC2), cadherin 1 (CDH1) and RASSF1A were significantly more often methylated in BCC. These findings are in agreement with our results on RASSF1A methylation, even though we found higher methylation frequencies in both BCC and normal skin. A possible explanation for this is that we performed nested MSP, which is a very sensitive technique for methylation analysis on FFPE tissue, while Sathyanarayana et al. performed direct MSP, which is less sensitive. In addition, they investigated the effect of sun exposure on promoter methylation in skin tags and cancer, and found similar methylation frequencies for the genes examined. From this, it was concluded that promoter hypermethylation in general was more likely related to sun exposure rather than being tumor-specific. In our study, we could not detect comparable methylation patterns in sun-exposed skin and BCC for SHH APC, SFRP5 and RASSF1A. Moreover, normal SE and SP skin samples showed similar methylation patterns, suggesting that the methylation we observed is tumor-specific. It is of interest that methylation patterns in sun-exposed skin did not resemble those in BCC, as sun exposure is the main risk factor in the development of BCC [50]. Thus, our findings do not support a contribution of UVB exposure to the observed promoter hypermethylation of SHH, APC, SFRP5 and RASSF1A. The intriguing observation that nBCC and iBCC harbored more frequent methylation for these four genes compared with sBCC might indicate that DNA methylation contributes to the development or evolution of BCC. It is tempting to speculate that increased silencing of putative tumor suppressors correlates with increasingly invasive behavior, a notion that is supported by our finding of differential SFRP5 methylation in iBCC versus nBCC and sBCC. The increased SHH methylation is probably an epiphenomenon, as SHH is no longer required for SMOH activity in the majority of BCC. As an alternative explanation, SHH silencing might contribute through as yet unidentified pathways to tumorigenesis.
To confirm functionally relevant methylation, i.e., transcriptional silencing of affected genes, we performed gene expression analyses with RT-PCR on FF and immunohistochemical staining on FFPE samples with verified methylation status. Our mRNA expression analysis of APC and SFRP5 in patient and control samples confirmed a down regulation of these genes in samples harboring methylation. By immunohistochemistry, expression of SHH, APC and RASSF1A (all p-values <0.001) was lowered in BCC as compared to normal skin. We did not detect a direct correlation between methylation status and expression levels as visualized by immunohistochemistry. We think that this observation can be explained by the two-way detection method in immunohistochemical staining intensifying the original signal. Also, mRNA levels do not always correlate directly with protein levels [51].
These data together support our hypothesis of epigenetic involvement of the SHH and WNT pathways in BCC pathogenesis. Promoter hypermethylation-mediated silencing of negative regulators of WNT signaling is consistent with activity of this pathway, however available literature concerning activity of the WNT pathway in BCC is ambiguous. Therefore we assessed activation of canonical WNT signaling by determining β-catenin intracellular localization. β-catenin, a key WNT effector, is a membrane-bound protein which accumulates in the cytoplasm and subsequently translocates to the nucleus when activated [52]. In BCC, the literature concerning immunohistochemical analysis of β-catenin is ambiguous [19], [53]–[55]. El-Bahrawy et al. [53] showed nuclear staining in 55% (n = 56) of BCC, mostly at the periphery of the tumors, while only 23% of the 86 BCC examined by Saldanha et al. [54] had β-catenin positive nuclei. No correlation between β-catenin localization and histological subtype was seen in either study. Furthermore, in a paper recently published by Pourreyron et al. [19], nuclear β-catenin was absent in all examined BCC (n = 7) [19]. In our series, we demonstrated nuclear β-catenin in 28 of the 101 (27.7%) BCC examined, which is consistent with previous reports. It would be of interest in this context to examine Axin 2 expression levels in BCC as an additional marker for canonical WNT activity [19]. We reasoned that the lack of β-catenin nuclear localization might be due to E-cadherin co-expression, as high levels of E-cadherin can prevent nuclear translocation of β-catenin [46], [56]. Indeed our data show high levels of E-cadherin expression in BCC, with 94.1% of the cells being positive, although significantly lowered when compared to adjacent normal epidermis. Thus, the absence of nuclear β-catenin in many cases may be due to high E-cadherin levels, which would also be consistent with the general inability of BCC to metastasize [50].
It is of considerable interest that the epigenetic changes we found parallel the genetic changes driving BCC growth, in particular the silencing of known WNT pathway inhibitors. These observations support a biological relevance of gene silencing by promoter hypermethylation in BCC. Moreover, they are consistent with a model in which epigenetic changes help to drive BCC tumor growth through deregulation of the WNT pathway, upon initiation of growth by mutations affecting SHH signaling. Previous work has demonstrated that BCC growth requires WNT signaling [18]–[19]. WNT pathway effectors are among the target genes of SHH, providing a rationale for this positive feedback mechanism. Our findings uncover a new level of regulation; inactivation of WNT inhibitors may be equally important as increased activity of WNT effectors.
In conclusion, we provide evidence that promoter hypermethylation-mediated silencing of known and putative TSGs is present in BCC. The extent to which these epigenetic changes actively contribute to BCC development will be more fully charted in order to determine whether DNA demethylation could be a viable strategy for BCC treatment.
Supporting Information
Methylation analysis in three BCC subtypes. Illustration of ten candidate genes in superfical, nodular and invasive BCC (respectively sBCC, nBCC, iBCC). P-values represent the difference between percentage methylation in the various subtypes.
(DOCX)
Primer sequences and PCR conditions. Overview of all used primer sequences and PCR conditions.
(DOCX)
Antibody characteristics. Overview of the used antibodies for all immunohistochemical analysis performed.
(DOCX)
SFRP5 expression in squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) by immunohistochemistry. Overview of the considerations concerning the data available at ProteinAtlas for SFRP5.
(DOCX)
Acknowledgments
We are grateful to Kim Wouters and Roy Cloots for technical and Patty Nelemans for statistical support.
Funding Statement
This work was supported by the Dutch Cancer Society, grants to TB (KWF UM2009-4609) and MVS (KWF UM2009-4352). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Szeimies RM, Karrer S (2006) Towards a more specific therapy: targeting nonmelanoma skin cancer cells. Br J Dermatol 154 Suppl 116–21. [DOI] [PubMed] [Google Scholar]
- 2. Holterhues C, Vries E, Louwman MW, Koljenovic S, Nijsten T (2010) Incidence and trends of cutaneous malignancies in the Netherlands, 1989–2005. J Invest Dermatol 130: 1807–1812. [DOI] [PubMed] [Google Scholar]
- 3. Roewert-Huber J, Lange-Asschenfeldt B, Stockfleth E, Kerl H (2007) Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol 157 Suppl 247–51. [DOI] [PubMed] [Google Scholar]
- 4. von Domarus H, Stevens PJ (1984) Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol 10: 1043–1060. [DOI] [PubMed] [Google Scholar]
- 5. Tilli CM, Van Steensel MA, Krekels GA, Neumann HA, Ramaekers FC (2005) Molecular aetiology and pathogenesis of basal cell carcinoma. Br J Dermatol 152: 1108–1124. [DOI] [PubMed] [Google Scholar]
- 6. Mosterd K, Arits AH, Thissen MR, Kelleners-Smeets NW (2009) Histology-based treatment of basal cell carcinoma. Acta Derm Venereol 89: 454–458. [DOI] [PubMed] [Google Scholar]
- 7. Smeets NW, Krekels GA, Ostertag JU, Essers BA, Dirksen CD, et al. (2004) Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet 364: 1766–1772. [DOI] [PubMed] [Google Scholar]
- 8. Bonifas JM, Bare JW, Kerschmann RL, Master SP, Epstein EH Jr (1994) Parental origin of chromosome 9q22.3-q31 lost in basal cell carcinomas from basal cell nevus syndrome patients. Hum Mol Genet 3: 447–448. [DOI] [PubMed] [Google Scholar]
- 9. Gailani MR, Stahle-Backdahl M, Leffell DJ, Glynn M, Zaphiropoulos PG, et al. (1996) The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet 14: 78–81. [DOI] [PubMed] [Google Scholar]
- 10. Hahn H, Wojnowski L, Miller G, Zimmer A (1999) The patched signaling pathway in tumorigenesis and development: lessons from animal models. J Mol Med 77: 459–468. [DOI] [PubMed] [Google Scholar]
- 11. Reifenberger J, Wolter M, Knobbe CB, Kohler B, Schonicke A, et al. (2005) Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol 152: 43–51. [DOI] [PubMed] [Google Scholar]
- 12. Epstein EH (2008) Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer 8: 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reifenberger J, Wolter M, Weber RG, Megahed M, Ruzicka T, et al. (1998) Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res 58: 1798–1803. [PubMed] [Google Scholar]
- 14. Xie J, Murone M, Luoh SM, Ryan A, Gu Q, et al. (1998) Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391: 90–92. [DOI] [PubMed] [Google Scholar]
- 15. Zedan W, Robinson PA, Markham AF, High AS (2001) Expression of the Sonic Hedgehog receptor “PATCHED” in basal cell carcinomas and odontogenic keratocysts. J Pathol 194: 473–477. [DOI] [PubMed] [Google Scholar]
- 16. Donovan J (2009) Review of the hair follicle origin hypothesis for basal cell carcinoma. Dermatol Surg 35: 1311–1323. [DOI] [PubMed] [Google Scholar]
- 17. Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A (1997) Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature 389: 876–881. [DOI] [PubMed] [Google Scholar]
- 18. Yang SH, Andl T, Grachtchouk V, Wang A, Liu J, et al. (2008) Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/beta3-catenin signaling. Nat Genet 40: 1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pourreyron C, Reilly L, Proby C, Panteleyev A, Fleming C, et al. (2012) Wnt5a is strongly expressed at the leading edge in non-melanoma skin cancer, forming active gradients, while canonical Wnt signalling is repressed. PLoS One 7: e31827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herman JG, Baylin SB (2003) Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 349: 2042–2054. [DOI] [PubMed] [Google Scholar]
- 21. Schuebel KE, Chen W, Cope L, Glockner SC, Suzuki H, et al. (2007) Comparing the DNA hypermethylome with gene mutations in human colorectal cancer. PLoS Genet 3: 1709–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giacinti L, Vici P, Lopez M (2008) Epigenome: a new target in cancer therapy. Clin Ter 159: 347–360. [PubMed] [Google Scholar]
- 23. Hatziapostolou M, Iliopoulos D (2011) Epigenetic aberrations during oncogenesis. Cell Mol Life Sci 68: 1681–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Issa JP, Kantarjian HM (2009) Targeting DNA methylation. Clin Cancer Res 15: 3938–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abdulla FR, Feldman SR, Williford PM, Krowchuk D, Kaur M (2005) Tanning and skin cancer. Pediatr Dermatol 22: 501–512. [DOI] [PubMed] [Google Scholar]
- 26. Goldberg M, Rummelt C, Laerm A, Helmbold P, Holbach LM, et al. (2006) Epigenetic silencing contributes to frequent loss of the fragile histidine triad tumour suppressor in basal cell carcinomas. Br J Dermatol 155: 1154–1158. [DOI] [PubMed] [Google Scholar]
- 27. Heitzer E, Bambach I, Dandachi N, Horn M, Wolf P (2010) PTCH promoter methylation at low level in sporadic basal cell carcinoma analysed by three different approaches. Exp Dermatol 19: 926–928. [DOI] [PubMed] [Google Scholar]
- 28. Sathyanarayana UG, Moore AY, Li L, Padar A, Majmudar K, et al. (2007) Sun exposure related methylation in malignant and non-malignant skin lesions. Cancer Lett 245: 112–120. [DOI] [PubMed] [Google Scholar]
- 29. van Doorn R, Gruis NA, Willemze R, van der Velden PA, Tensen CP (2005) Aberrant DNA methylation in cutaneous malignancies. Semin Oncol 32: 479–487. [DOI] [PubMed] [Google Scholar]
- 30. Wu W, Zhang J, Yang H, Shao Y, Yu B (2011) Examination of AKAP12 promoter methylation in skin cancer using methylation-sensitive high-resolution melting analysis. Clin Exp Dermatol 36: 381–385. [DOI] [PubMed] [Google Scholar]
- 31. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee DA, Grossman ME, Schneiderman P, Celebi JT (2005) Genetics of skin appendage neoplasms and related syndromes. J Med Genet 42: 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borkowska J, Schwartz RA, Kotulska K, Jozwiak S (2011) Tuberous sclerosis complex: tumors and tumorigenesis. Int J Dermatol 50: 13–20. [DOI] [PubMed] [Google Scholar]
- 34. van Steensel MA, van Geel M, Badeloe S, Poblete-Gutierrez P, Frank J (2009) Molecular pathways involved in hair follicle tumor formation: all about mammalian target of rapamycin? Exp Dermatol 18: 185–191. [DOI] [PubMed] [Google Scholar]
- 35. Richter AM, Pfeifer GP, Dammann RH (2009) The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta 1796: 114–128. [DOI] [PubMed] [Google Scholar]
- 36. Derks S, Lentjes MH, Hellebrekers DM, de Bruine AP, Herman JG, et al. (2004) Methylation-specific PCR unraveled. Cell Oncol 26: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 93: 9821–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Engeland M, Roemen GM, Brink M, Pachen MM, Weijenberg MP, et al. (2002) K-ras mutations and RASSF1A promoter methylation in colorectal cancer. Oncogene 21: 3792–3795. [DOI] [PubMed] [Google Scholar]
- 39. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 40. Kundel HL, Polansky M (2003) Measurement of observer agreement. Radiology 228: 303–308. [DOI] [PubMed] [Google Scholar]
- 41. Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86: 420–428. [DOI] [PubMed] [Google Scholar]
- 42.Rosner B (2006) Fundamentals of biostatistics. Belmont: Duxbury.
- 43. Veeck J, Geisler C, Noetzel E, Alkaya S, Hartmann A, et al. (2008) Epigenetic inactivation of the secreted frizzled-related protein-5 (SFRP5) gene in human breast cancer is associated with unfavorable prognosis. Carcinogenesis 29: 991–998. [DOI] [PubMed] [Google Scholar]
- 44. House MG, Guo M, Iacobuzio-Donahue C, Herman JG (2003) Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis 24: 193–198. [DOI] [PubMed] [Google Scholar]
- 45. Chhibber A, Schroeder BG (2008) Single-molecule polymerase chain reaction reduces bias: application to DNA methylation analysis by bisulfite sequencing. Anal Biochem 377: 46–54. [DOI] [PubMed] [Google Scholar]
- 46. Tian X, Liu Z, Niu B, Zhang J, Tan TK, et al. (2011) E-cadherin/beta-catenin complex and the epithelial barrier. J Biomed Biotechnol 2011: 567305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bezdekova M, Brychtova S, Sedlakova E, Steigerova J, Hlobilkova A, et al. (2007) Immunohistochemical assessment of E-cadherin and beta-catenin in trichofolliculomas and trichoepitheliomas. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 151: 251–255. [DOI] [PubMed] [Google Scholar]
- 48. Donninger H, Vos MD, Clark GJ (2007) The RASSF1A tumor suppressor. J Cell Sci 120: 3163–3172. [DOI] [PubMed] [Google Scholar]
- 49. Nandakumar V, Vaid M, Tollefsbol TO, Katiyar SK (2011) Aberrant DNA hypermethylation patterns lead to transcriptional silencing of tumor suppressor genes in UVB-exposed skin and UVB-induced skin tumors of mice. Carcinogenesis 32: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Madan V, Lear JT, Szeimies RM (2010) Non-melanoma skin cancer. Lancet 375: 673–685. [DOI] [PubMed] [Google Scholar]
- 51. Gort EH, Suijkerbuijk KP, Roothaan SM, Raman V, Vooijs M, et al. (2008) Methylation of the TWIST1 promoter, TWIST1 mRNA levels, and immunohistochemical expression of TWIST1 in breast cancer. Cancer Epidemiol Biomarkers Prev 17: 3325–3330. [DOI] [PubMed] [Google Scholar]
- 52. MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. El-Bahrawy M, El-Masry N, Alison M, Poulsom R, Fallowfield M (2003) Expression of beta-catenin in basal cell carcinoma. Br J Dermatol 148: 964–970. [DOI] [PubMed] [Google Scholar]
- 54. Saldanha G, Ghura V, Potter L, Fletcher A (2004) Nuclear beta-catenin in basal cell carcinoma correlates with increased proliferation. Br J Dermatol 151: 157–164. [DOI] [PubMed] [Google Scholar]
- 55. Fukumaru K, Yoshii N, Kanzaki T, Kanekura T (2007) Immunohistochemical comparison of beta-catenin expression by human normal epidermis and epidermal tumors. J Dermatol 34: 746–753. [DOI] [PubMed] [Google Scholar]
- 56. Li L, Zhang Z, Li B, Gao F, Jonas JB (2011) E-cadherin and beta-catenin expression in sebaceous eyelid adenocarcinomas. Graefes Arch Clin Exp Ophthalmol 249: 1867–1873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methylation analysis in three BCC subtypes. Illustration of ten candidate genes in superfical, nodular and invasive BCC (respectively sBCC, nBCC, iBCC). P-values represent the difference between percentage methylation in the various subtypes.
(DOCX)
Primer sequences and PCR conditions. Overview of all used primer sequences and PCR conditions.
(DOCX)
Antibody characteristics. Overview of the used antibodies for all immunohistochemical analysis performed.
(DOCX)
SFRP5 expression in squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) by immunohistochemistry. Overview of the considerations concerning the data available at ProteinAtlas for SFRP5.
(DOCX)