The mammalian Hippo pathway plays pivotal roles in regulating organ size, stem cell pluripotency and tumorigenesis. In this pathway, the kinase Lats1/2, in complex with their regulatory subunit Mob, inhibit YAP and TAZ by a direct phosphorylation. YAP and TAZ are two main downstream effectors of the Hippo pathway, and they function as transcription co-activators to promote cell proliferation and inhibit apoptosis.1 A number of modulators of the Hippo pathway have been identified via extensive genetic and biochemical analysis; however, the identity of the diffusible/extracellular signals and cell surface receptors regulating the mammalian Hippo pathway remains elusive.1
We have recently reported that the Hippo pathway interacts with G-protein-coupled receptor (GPCR) signaling.2 The activity of Lats1/2 kinases and YAP/TAZ are robustly regulated by to GPCRs and their extracellular ligands. GPCR signaling can either activate or inhibit YAP/TAZ depending on which classes of downstream heterotrimeric G-protein are coupled with. Gα12/13-, Gαq/11- or Gαi/o-coupled signals, such as lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), repress Lats1/2 activity, leading to dephosphorylation and activation of YAP/TAZ. On the other hand, Gαs-coupled signals, such as epinephrine and glucagon, induce kinase activity of Lats1/2, leading to phosphorylation and inhibition of YAP/TAZ (Fig. 1). These hormones also regulate the nuclear and cytoplasmic translocation of YAP/TAZ in a manner correlating with phosphorylation. Indeed, YAP/TAZ activation is crucial in mediating gene expression, cell proliferation and cell migration induced by LPA. An independent study by Wu and colleagues has similarly demonstrated the role of LPA and S1P in YAP/TAZ regulation.3
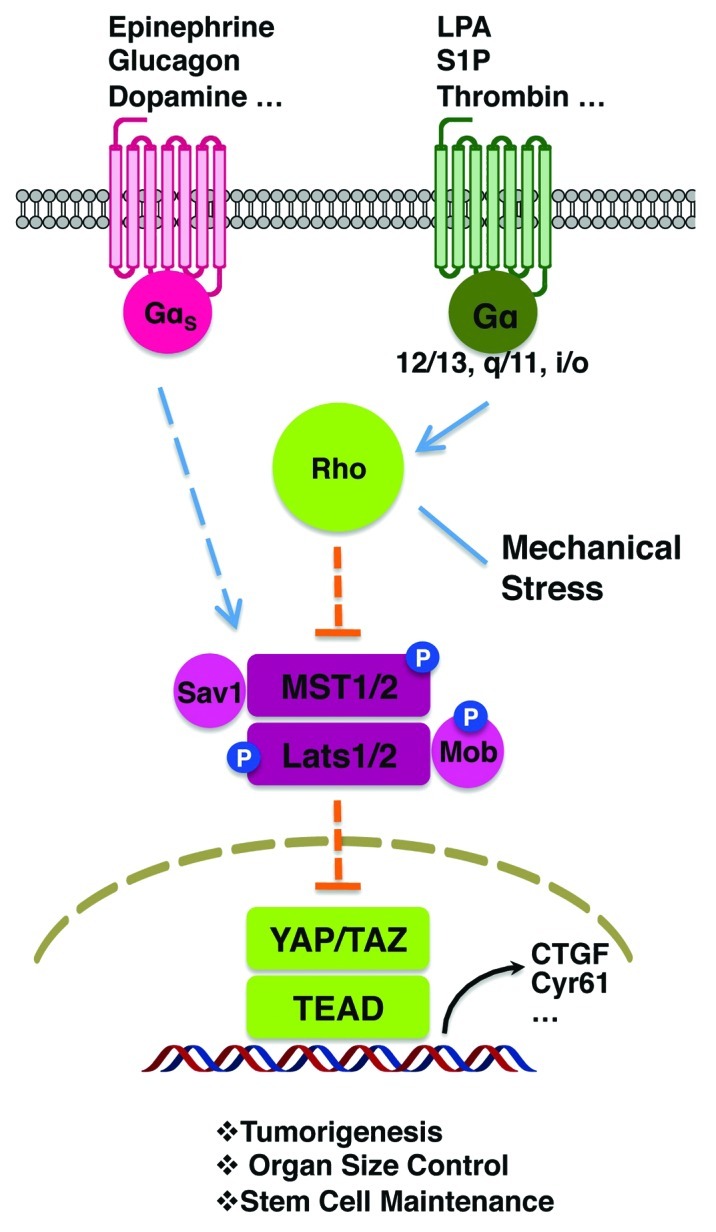
Figure 1. GPCR signaling regulates the Hippo pathway. Gα12/13-, Gαq/11- and Gαi/o-coupled receptors and ligands activate Rho GTPases, inhibit Lats1/2 and induce YAP/TAZ. Gαs-coupled receptors and ligands induce Lats1/2, leading to inhibition of YAP/TAZ activity. Mechanical cues may also modulate YAP/TAZ activity by regulating Rho GTPases. YAP/TAZ regulates a transcriptional program to control organ size, tumorigenesis and stem cell maintenance.
How upstream GPCR signaling is connected to the Hippo pathway is not fully understood at this stage. Nevertheless, several components have been implicated in signaling from GPCR to Lats1/2 regulation. Actin cytoskeleton rearrangement has been shown to regulate YAP/TAZ activity; therefore, Rho GTPases and actin filaments may function as a bridge between G-protein signals and Hippo pathway kinases.2,4-8 The phosphorylation and in vitro kinase activity of MST1/2 are not significantly regulated by GPCR signaling; it is likely that MST1/2 phosphorylation is not a direct target of GPCR signaling.2 However, the phosphorylation status of Lats1/2 (which is responsive to MST1/2 kinase activity) is sensitive to different GPCR ligands, suggesting that MST1/2 or another similar kinase are involved in the regulation of Lats1/2 by GPCR signaling.2
Our study suggests that a diverse diffusible/extracellular signals can fine-tune the activity of the Hippo pathway. More recently, we have found that thrombin, which activates protease-activated receptors (PARs), also stimulates YAP/TAZ activity via Gα12/13 and Rho GTPases (Fig. 1).9 Over 40 GPCRs have been tested in our study; the majority display strong activity to either activate or inhibit YAP/TAZ.2 Furthermore, all active Gα proteins can modulate the phosphorylation of YAP/TAZ with varying degrees of potency. These results indicate that the Hippo-YAP pathway is likely to be regulated by a large numbers of GPCRs and their cognate ligands, firmly placing this pathway downstream of GPCR signaling. It would be not surprising to see a long list of signals that exert their biological regulation via modulating the Hippo-YAP pathway.
Many GPCR ligands, such as LPA, S1P and Thrombin, have been shown to induce tumorigenesis and cancer metastasis.10 The Hippo pathway kinases MST1/2 and Lats1/2 are tumor suppressors, whereas YAP and TAZ are considered oncoproteins.1 The identification of LPA, S1P and thrombin as YAP/TAZ activators suggests a role of YAP/TAZ in mediating the oncogenic effect of these tumor promoters. In addition, elevated expression of GPCRs and activating mutations of GPCR and G-proteins are sporadically present in human cancers; meanwhile, high YAP/TAZ expression and nuclear localization are observed in a number of human cancers.1 In the future, it will be important to investigate the function of YAP/TAZ in cancer development caused by dysregulated GPCR signaling.
The Hippo pathway also plays important roles in stem cell biology and organ size control. Our results suggest that GPCR signaling might regulate stem cell functions and even organ size via YAP/TAZ. The function of GPCR in stem cell pluripotency and differentiation has been under extensive research.11 However, the role of GPCRs in organ size control is largely unknown. The expression of GPCR is subjected to spatial and temporal regulation, and any given organ may express many GPCRs; therefore, the Hippo pathway and in turn organ size might be tightly regulated by multiple GPCRs together with their ligands. GPCRs coupled to same Gα may have redundant functions, and knockout of a single GPCR may not reveal significant effect on the Hippo pathway output. It would be interesting to express Gα12/13-, Gαq/11- or Gαi/o-coupled receptors in liver to test their effect on organ size control.
In nervous system, many hormones or neurotransmitters are GPCR ligands, such as dopamine, epinephrine, norepinephrine, serotonin and oxytoxin, and these hormones are important in regulating animal behaviors. We have shown that some neuronal hormones, such as dopamine receptor agonist and epinephrine, can activate Lats1/2 and inhibit YAP/TAZ.2 The function of the Hippo pathway in neural circuitry is unknown; however, NDR1/2, homologs of Lats1/2, are crucial for neuronal functions.12 It is possible that different neuronal hormones also modulate NDR1/2 kinase activity, and Lats1/2 and YAP/TAZ may also play roles in neuroendocrine system and even animal behaviors.
GPCR is the largest family of cell surface receptors that have diverse functions and serve as therapeutic targets. The research on the Hippo pathway is relatively new but has quickly expanded in recent years. The connection between GPCR signaling and the Hippo pathway would provide new insights for both fields.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/22322
References
- 1.Zhao B, et al. Genes Dev. 2010;24:862–74. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu FX, et al. Cell. 2012;150:780–91. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller E, et al. Chem Biol. 2012;19:955–62. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Zhao B, et al. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sansores-Garcia L, et al. EMBO J. 2011;30:2325–35. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauskolb C, et al. PLoS Biol. 2011;9:e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández BG, et al. Development. 2011;138:2337–46. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 8.Dupont S, et al. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 9.Mo JS, et al. Genes Dev. 2012;29 In press. [Google Scholar]
- 10.Dorsam RT. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 11.Callihan P, et al. Pharmacol Ther. 2011;129:290–306. doi: 10.1016/j.pharmthera.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Emoto K. J Biochem. 2011;150:133–41. doi: 10.1093/jb/mvr080. [DOI] [PubMed] [Google Scholar]


