The constitutive photomorphogenesis 9 (COP9) signalosome (CSN) is an evolutionarily conserved multiprotein complex in plants and animals.1 This protein complex, consisting of eight subunits (CSN1 to CSN8), is homologous with the 19S regulatory lid complex of the 26S proteasome, which plays a central role in the recognition and efficient degradation of misfolded proteins.2 The diverse functions of the COP9 complex include regulation of several important intracellular pathways, such as the ubiquitin/proteasome system, DNA repair, cell cycle, apoptosis and tumorigenesis.3 However, detailed mechanisms through which COP9 and its subunits contribute toward tumor development remain unclear.
Previously, Lee’s laboratory demonstrated that CSN6, a subunit of the COP9 signalosome, interacts with and inhibits the degradation of an oncogene MDM2, leading to its stabilization (Fig. 1).4 MDM2 is a RING domain-containing E3 ubiquitin ligase, toward the tumor suppressor p53 and itself, thereby regulating the proteolytic turnover of these proteins.5 Overexpression of MDM2 is detected in approximately 30% of human cancers due to gene amplification and other unknown mechanisms.5 They found that gene amplification of CSN6 is also detected in a high percentage of human breast cancer tissues, and there is a positive correlation between copy numbers of CSN6 gene and breast tumor size.4 Importantly, CSN6 is concomitantly overexpressed with MDM2 in human breast cancer tissues, consistent with their observation that CSN6 stabilizes MDM2 by inhibiting its self-ubiquitination. Complete deletion of CSN6 in mice results in early embryonic lethality, which is partially rescued by concomitant deletion of p53.4 Mice heterozygous for CSN6 (CSN6+/−) exposed to high doses of γ-irradiation (IR) show decreased survival due to an increased p53 activity. However, exposure to lower doses delays the onset of tumor development, suggesting that reduced CSN6 levels result in an increase in the p53 activity and prevent tumorigenesis in vivo.4 Thus, CSN6 plays a crucial role in the MDM2-p53 pathway and tumor development. However, the regulatory mechanisms behind the activation of the CSN6-MDM2-p53 axis are unknown.
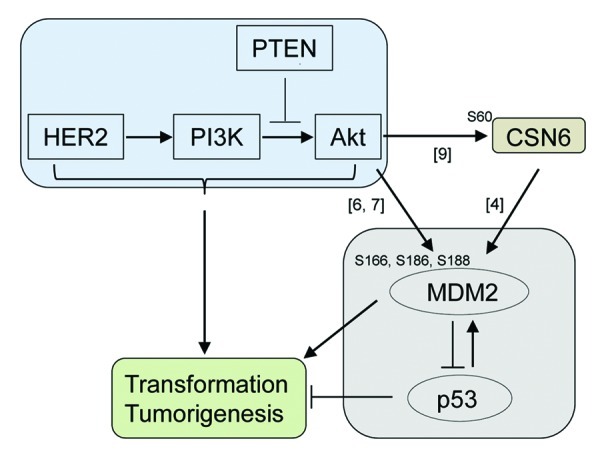
Figure 1. The HER2-Akt pathway affects the MDM2-p53 pathway by direct phosphorylation of MDM2 and via stabilization of MDM2 by CSN6. Numbers indicate the references cited.
The stability and activity of MDM2 are regulated by multiple means. One of the mechanisms is that the oncogenic HER2-Akt signaling phosphorylates and activates MDM2 (Fig. 1).6,7 The HER2 oncoprotein is overexpressed in approximately 30% of breast cancers and activates Akt.8 Although the phosphorylation of MDM2 at Ser166, Ser186 and Ser188 by Akt is involved in the subcellular localization and stabilization of MDM2,6,7 the exact mechanisms by which HER2-Akt signaling activates MDM2 remain unclear.
A recent paper from Lee’s laboratory illustrates a novel mechanism by which HER2-Akt signaling stabilizes MDM2 through CSN6 (Fig. 1).9 They demonstrate that Akt directly interacts with and phosphorylates CSN6 at Ser60, which inhibits the degradation of CSN6 by reducing its ubiquitination. Phosphorylated and stabilized CSN6, as previously established, increases the level of MDM2 and subsequently reduces p53 expression.4 Overexpression of Akt translocates CSN6 from the cytoplasm to the nucleus, which could facilitate its effects on MDM2.9 They also show that CSN6 cooperates with Akt in cellular transformation. Hence, this study has further strengthened the significance of the MDM2-p53 pathway on tumorigenesis through a novel link of Akt-mediated CSN6 activation. Thus, MDM2 can be activated by direct phosphorylation via HER2-Akt singling and indirect stabilization via the Akt-CSN6 axis (Fig. 1).
This study has opened new avenues for future exploration that might advance the aspect of using CSN6 as a therapeutic target. It is important to determine a positive clinical correlation between HER2 and CSN6 expression in human tumor samples. Studies using mouse models manipulated for both HER2 and CSN6 will further provide in vivo evidence for their roles in tumorigenesis. It would be interesting to learn whether or not the Akt-mediated phosphorylation of MDM2 and the Akt-mediated stabilization of CSN6 cooperate with each other toward the Akt-mediated MDM2 activation. Further investigations to understand the effects of CSN6 phosphorylation on tumorigenesis and COP9 function should also be performed. Taken together, this study opens a new field of oncogene-mediated tumorigenesis, thus significantly accelerating the development of a novel therapeutic strategy for various types of cancer.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/22606
References
- 1.Wei N, et al. Annu Rev Cell Dev Biol. 2003;19:261–86. doi: 10.1146/annurev.cellbio.19.111301.112449. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka T, et al. Mol Oncol. 2012;6:267–75. doi: 10.1016/j.molonc.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MH, et al. Cycle. 2011;10:3057–66. doi: 10.4161/cc.10.18.17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao R, et al. J Clin Invest. 2011;121:851–65. doi: 10.1172/JCI44111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, et al. FEBS Lett. 2012;586:1390–6. doi: 10.1016/j.febslet.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Zhou BP, et al. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 7.Feng J, et al. J Biol Chem. 2004;279:35510–7. doi: 10.1074/jbc.M404936200. [DOI] [PubMed] [Google Scholar]
- 8.Eroles P, et al. Cancer Treat Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Xue Y, et al. Cell Cycle. 2012;11:4181–90. doi: 10.4161/cc.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]


