Abstract
HER2/neu oncogene is frequently overexpressed in various types of cancer, and the (PI3K)-Akt signaling pathway is often activated in HER2-overexpressing cancer cells. CSN6, subunit 6 of the COP9 signalosome complex, is pivotal in regulating MDM2 to destabilize p53, but its upstream regulators remain unclear. Here we show that the HER2-Akt axis is linked to CSN6 regulation, and that Akt is a positive regulator of CSN6. Ectopic expression of Akt can increase the expression of CSN6; accordingly, Akt inhibition leads to CSN6 destabilization. Mechanistic studies show that Akt causes CSN6 phosphorylation at Ser 60, which, in turn, reduces ubiquitin-mediated protein degradation of CSN6. Significantly, Akt’s positive impact on CSN6 elevation translates into p53 degradation, potentiating transformational activity and increasing DNA damage. Akt inhibition can attenuate these defects caused by CSN6. These data suggest that Akt is an important positive regulator of CSN6, and that activation of Akt in many types of cancer could lead to abnormal elevation of CSN6 and result in downregulated p53 and increased DNA damage, which promotes cancer cell growth.
Keywords: Akt, CSN6, cell cycle, HER2, p53
Introduction
Amplification or overexpression of the HER2/neu oncogene (also named human EGF receptor type 2, ErbB2) is frequently found in various types of cancer, including breast, ovarian, lung, gastric and oral cancers.1 Importantly, HER2 overexpression has been associated with poor survival in patients with breast cancer.1 The phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway is often activated in HER2-overexpressing cancer cells.2 HER2 can also activate Akt to phosphorylate p21 at threonine 145, resulting in the cytoplasmic location of p21, which prevents p21’s growth inhibitory activity.3 Importantly, Akt activity is elevated in several types of human malignancies, including ovarian, breast, lung and thyroid cancers.4 The kinase activity of Akt is constitutively activated in human cancer as a result of dysregulation of its regulators, including the tumor suppressor phosphatase and tensin homolog (PTEN)5 and the amplification of the catalytic subunit of PI3K.6 Although Akt is known to phosphorylate and regulate several important substrates, many of its substrates remain to be identified.
The COP9 signalosome (CSN) protein complex is implicated in cancer cell signaling. For example, it is shown that CSN is involved in degrading proteins such as p27,7 c-Jun,8 p539,10 and 14-3-3σ11 via the ubiquitin-proteasome pathway. CSN is an evolutionarily conserved multiprotein complex that has been found in both plants and animals. It is a protein complex, which consists of eight subunits (CSN1 to CSN8), first characterized as a repressor of plant photomorphogenesis. While the exact biochemical function of the CSN in animal cells remains to be characterized, the complex or its individual subunits have been implicated in a wide variety of regulatory processes, including cell cycle control, signal transduction, transcriptional activation and possibly tumorigenesis (reviewed in refs. 12 and 13). Notably, the eight subunits of the COP9 signalosome are each paralogous to one of the eight subunits that form the lid complex (19S) of the 26S proteasome.14,15 The lid complex is thought to recognize ubiquitinated substrates and then funnel them into the proteolytic core complex for degradation. Because of the homology between the COP9 signalosome and the 19S lid complex, the COP9 signalosome has been postulated to play a role in protein degradation. However, the molecular signals in regulating CSN activity are largely unknown. It is known that HER2-Akt is critical in regulating p53 activity through MDM2.16 Also, we have identified CSN6 of the COP9 complex as being pivotal in regulating MDM2 to destabilize p53.10 However, it is not clear whether the HER2-Akt axis has any link to CSN6 regulation.
In this study, we demonstrate that Akt and CSN6 interact and further indicate that Akt is required in positively regulating CSN6 via the ubiquitination pathway. Significantly, we characterized the role of Akt phosphorylation in stabilizing CSN6, which has an impact on cell tranformation, p53 stabilization and DNA damage. Our studies provide another important insight into the activity of Akt.
Results
HER2-Akt signaling positively regulates CSN6 protein stability
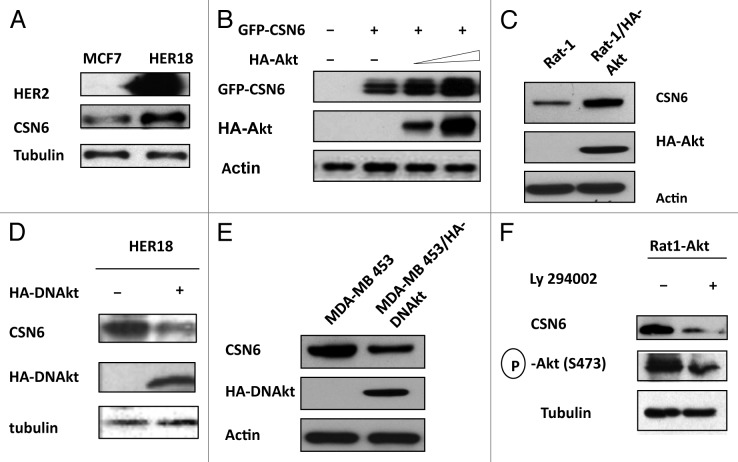
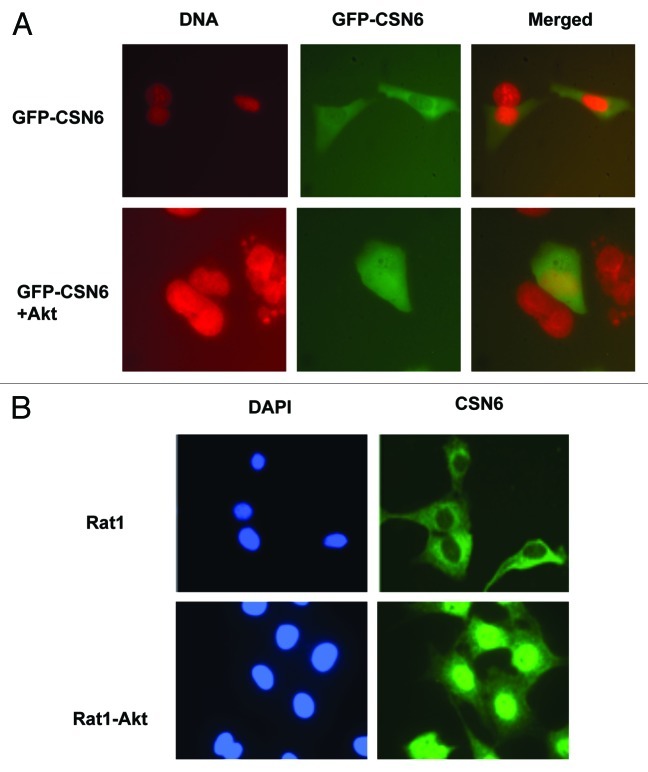
It is known that HER2-Akt signaling can negatively regulate p53 activity.16 In addition, we have demonstarated that CSN6 also has a negative impact on p53.10 It is therefore possible that a link exists between HER2-Akt and CSN6, which affects p53 activity. We first examined if HER2, a receptor tyrosine kinase involved in Akt activation, has a biological impact on CSN6. We determined the expression level of CSN6 in isogenic cell lines with different HER2 statuses: HER2-overexpressing cells (HER18, MCF7 overexpressed with HER2) and non-HER2-overexpressing cells (MCF7). The results showed that CSN6 protein levels are higher in HER2-overexpressing cells than in non-HER2-overexpressing cells (Fig. 1A). Because HER2/neu can activate the PI3K/Akt pathway, we then explored whether the Akt signaling pathway is involved in HER2-mediated CSN6 upregulation. CSN6 levels were increased when cells were transfected with Akt in a dose-dependent manner (Fig. 1B). We also employed other isogenic cell lines with different Akt statuses [Rat1, and Rat1 cells that overexpress constitutively active Akt (Rat1-HA-Akt)] for the assay, and found that CSN6 level is high in Rat1-HA-Akt cells (Fig. 1C). Also, expression of dominant-negative Akt (DN-Akt, Akt K179M) in HER18 cells led to downregulation of CSN6 (Fig. 1D). Similar results have been observed in MDA-MB-453 cells (a cell line with HER2/neu overexpression): when transfected with DN-Akt that antagonizes the activity of Akt, the expression of CSN6 is greatly reduced compared with parental cells (Fig. 1E). Interestingly, inhibiting Akt activity by LY294002 (a PI3K inhibitor) leads to CSN6 reduction in Rat1-Akt cells (Fig. 1F). As nuclear exporting seems to be a common scheme in protein degradation, we then determined whether Akt signaling affects CSN6 subcellular localization. Cells were transfected with GFP-CSN6 in the presence or absence of Akt. GFP-CSN6 seemed to be more abundant in the cytoplasm, while cotransfection with AKT promoted more nuclear localization of CSN6 (Fig. 2A). Similar results have been observed in Rat1-Akt cells. While CSN6 is located in the cytoplasm in Rat1 cells, CSN6 is relocated from the cytoplasm to the nucleus in Rat1-Akt cells (Fig. 2B). Together, these results suggest that HER2-Akt activity regulates CSN6 stability and subcellular localization.
Figure 1. HER2-Akt signaling regulates Aurora B stability. (A) HER2 overexpression positively regulates the steady-state expression of CSN6. Indicated equal amounts of cell lysates were immunoblotted with indicated antibodies. (B) Akt positively regulates the steady-state expression of CSN6. 293T cells were co-transfected with indicated plasmids and increasing amounts of Akt. Equal amounts of cell lysates were immunoblotted with indicated antibodies. (C) Akt-overexpressing cells have increased steady-state expression of CSN6. Indicated amounts of cell lysates were immunoblotted with indicated antibodies. (D) Antagonizing Akt leads to destabilization of CSN6 in HER2-overexpressing cells. Indicated amounts of cell lysates were immunoblotted with indicated antibodies. (E) Dmominant Akt reduces the expression levels of CSN6. Indicated amounts of cell lysates were immunoblotted with indicated antibodies. (F) Inhibitor of PI3K-Akt pathway causes destabilization of CSN6. Rat1-Akt cells were treated with LY294002 for 6 h before harvesting. Equal amounts of cell lysates were immunoblotted with indicated antibodies.
Figure 2. Akt induces CSN6 nuclear import. (A) CSN6 nuclear localization is induced in the presence of Akt. U2OS cells were transfected with indicated plasmids. Nucleus was stained with Hoechst 33342 and pseudo colored with red. (B) CSN6 is localized at the nucleus at Akt-overexpressing cells. Indicated cells were stained with Anti-Csn6 followed with FITC-conjugated secondary Ab. Nucleus was stained with DAPI.
Akt regulates CSN6 protein stability through reducing the ubiquitination level of CSN6
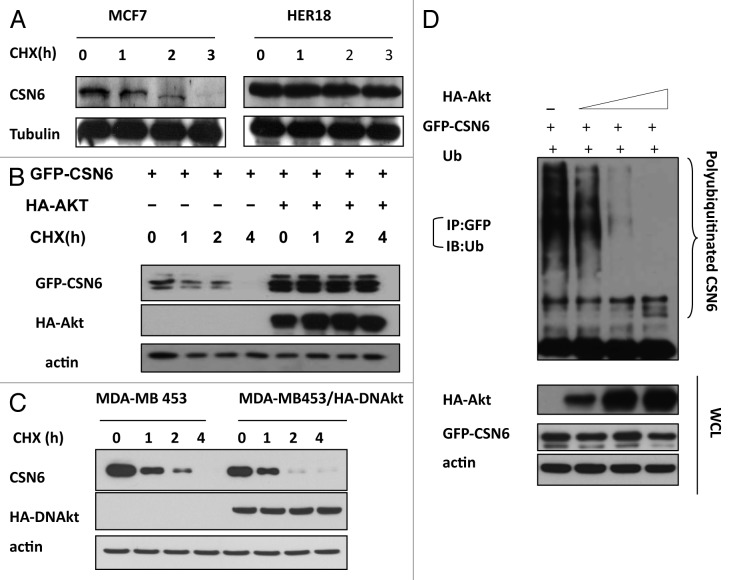
To further investigate the impact of HER2 and Akt on CSN6 stability, we examined the turnover of CSN6 in the presence of the de novo protein synthesis inhibitor cycloheximide (CHX) in MCF7 and HER18, and found that HER2-overexpressing HER18 cells have a decelerated turnover rate of endogenous CSN6 (Fig. 3A). To dissect the role of Akt in this regulation, we cotransfected Akt with CSN6 and performed the CHX experiments. The data showed that CSN6 has a slower turnover rate in the presence of Akt (Fig. 3B). Consistently, MDA-MB-453 cells stably transfected with DN-Akt showed an increased CSN6 turnover rate when compared with parental cells (Fig. 3C). Furthermore, we found that Akt decreased the ubiquitination level of CSN6 in a dose-dependent manner (Fig. 3D). These results suggest that HER2-Akt activity positively regulates CSN6 stability via decreasing the levels of CSN6 ubiquitination, thereby reducing CSN6’s turnover rate.
Figure 3. HER2-Akt signaling reduces CSN6 turnover via decreasing CSN6 ubiquitination. (A) CSN6 turnover rate is decreased in HER2-overexpressing cells. Indicated cells were treated with cycloheximide (CHX) (100 µg/ml) for the indicated times. Cell lysates were immunoblotted with indicated antibodies. (B) Akt dereases the turnover of CSN6. Indicated 293T cells were transfected with GFP-CSN6 with or without Akt and then treated with cycloheximide (CHX) (100 µg/ml) for the indicated times. (C) CSN6 turnover rate is increased in Akt activity deficient cells. Indicated cells were treated with cycloheximide (CHX) (100 µg/ml) for the indicated times. Cell lysates were immunoblotted with indicated antibodies. (D) Akt stabilizes CSN6 by decreasing CSN6 ubiquitination. 293T cells were transfected with the indicated plasmids. The proteasome inhibitor MG132 was added 6 h before harvesting cells. The ubiquitinated amount of CSN6 is analyzed by immunoprecipitated (IP) with anti-GFP followed by immunoblotting (IB) with anti-ubi.
Akt interacts with and phosphorylates CSN6
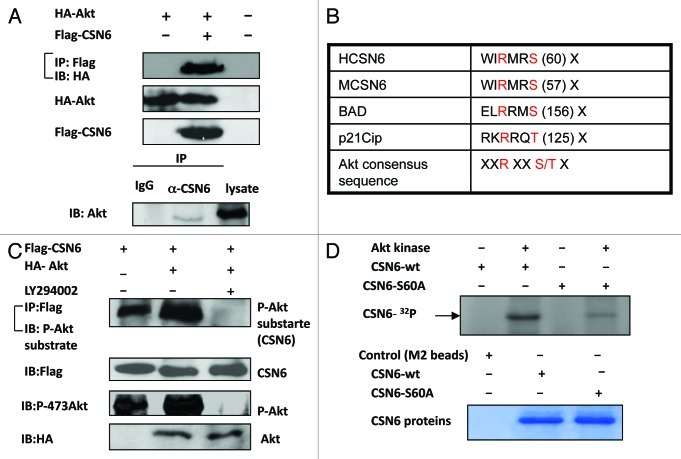
Although Akt is involved in the stabilization of CSN6, the interaction between Akt and CSN6 needs to be characterized. To investigate this possible interaction, we analyzed cell lysates cotransfected with Akt and CSN6. Indeed, Akt was able to associate with CSN6 as assayed by Co-IP (Fig. 4A). Endogenous interaction between Akt and CSN6 is also confirmed (Fig. 4A). As an Akt-associated protein, it is possible that CSN6 is a substrate of Akt. To further investigate if Akt can mediate phosphorylation of CSN6, we first scanned its sequence using the NetPhos algorithm (www.cbs.dtu.dk/services/NetPhos/) and identified a potential Akt phosphorylation site (55 WIRMRS 60), which is conserved among CSN6 proteins from different species and shares the conserved core Akt phosphorylation site of Bad (134RGRSRS139) and p21 (140RKRRQT146) (Fig. 4B). We then cotransfected Flag-CSN6 and Ha-Akt in the cells. Flag-tagged CSN6 was immunoprecipitated from cell lysates, and CSN6-containing immunoprecipitated complexes were examined for CSN6 phosphorylation using anti-phosphorylated Akt substrate antibody. The results showed that CSN6 was strongly phosphorylated in the presence of Akt, as CSN6 was recognized by Akt phosphorylation substrate antibody in the presence of constitutive Akt when compared with control (Fig. 4C). This notion that CSN6 is an Akt substrate is also supported by the finding that the PI3K inhibitor LY294002 diminished the phosphorylation status of CSN6 (Fig. 4C). We have thus mutated the potential serine phosphorylation site to alanine (S60A), and found that the phosphorylation of this mutant by Akt was diminished in an in vitro kinase assay, suggesting that S60 is indeed the Akt phosphorylation site on CSN6 (Fig. 4D).
Figure 4. Akt interacts with and phosphorylates CSN6. (A) Interaction of Akt with CSN6. Equal amounts of 293T cell lysates transfected with indicated plasmids were immunoprecipitated (IP) with anti-Flag and immunoblotted (IB) with anti-HA. Equal amounts of U2OS cell lysates were also immunoprecipitated with anti-CSN6 and immunoblotted with anti-Akt. (B) Consensus phosphorylation sequence for Akt phosphorylation sites in CSN6. (C) CSN6 is a phosphorylated target of Akt. 293T cells were transfected with the indicated expression vectors in the presence or absence of LY294002. Equal amounts of cell lysates were immunoprecipitated (IP) with anti-Flag antibody and immunoblotted with anti-phosphorylated Akt substrates antibody. (D) Akt phosporylates CSN6 in vitro. Baculovirus-produced active recombinant Akt1 was incubated with indicated equal amounts of bacterially produced and purified recombinant CSN6 and mutant CSN6 (S60A) protein for kinase activity. 32P autoradiography demonstrated the phosphorylated CSN6 and Coomassie blue-stained SDS-PAGE gel of CSN6 proteins showed comparable amounts of wild-type CSN6 and mutant CSN6 (S60A).
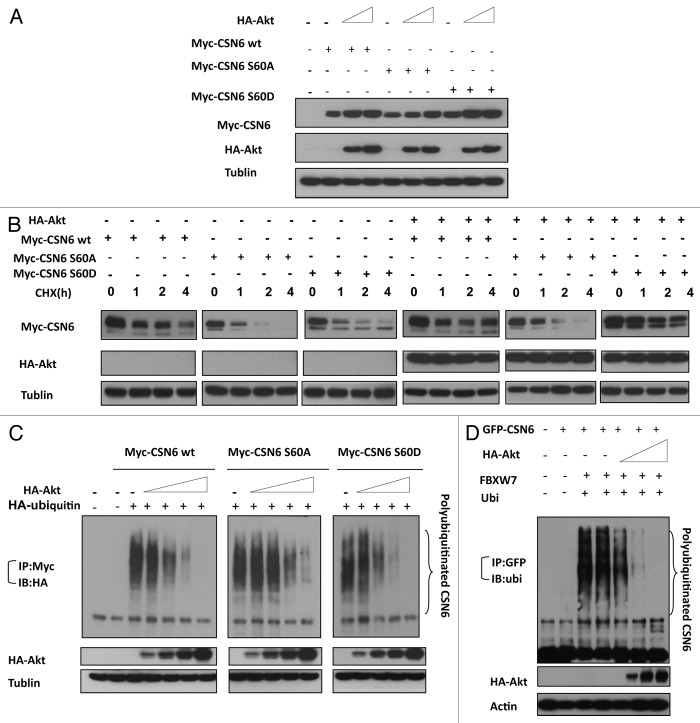
CSN6 S60 phosphorylation is essential for Akt-mediated CSN6 stabilization
To determine whether the Akt-mediated phosphorylation affects CSN6 stability, we constructed S60A and S60D mutants of CSN6 and examined the steady-state expression of these CSN6 mutants. Akt increased the steady-state expression of wt CSN6 and CSN6S60D (phosphorylation mimetic) mutant in a dose-dependent manner, while the steady-state expression of CSN6S60A mutant has a diminished response to Akt’s positive impact (Fig. 5A). We then examined the turnover rate of these CSN6 mutants. We compared their turnover rate to wt CSN6 in the presence of CHX. The CSN6S60A mutant has a faster turnover rate than wt CSN6 regardless of the presence or absence of constitutive Akt (Fig. 5B). As expected, the CSN6S60D mutant seemed to have a slower turnover rate than wt CSN6 (Fig. 5B). We further investigated the ubiquitination process, and found that Akt, as expected, reduced the level of wt CSN6 ubiquitination in a dose-dependent manner (Fig. 5C). Interestingly, the ubiquitination level of CSN6S60A mutant is less responsive to Akt’s impact when compared with wt CSN6 and CSN6S60D. We then showed that Akt reduced the ubiquitination levels of CSN6 in the presence of FBXW7, an important component of an E3 ubiquitin ligase (Fig. 5D). These results indicate that Akt-mediated phosphorylation is critical in reducing the turnover rate and ubiquitination level of CSN6, thereby increasing steady-state expression of CSN6.
Figure 5. CSN6 phosphorylation mutant S60A has different stability. (A) The steady-state expression of phosphorylation mutant CSN6S60A. 293T cells were co-transfected with indicated plasmids and increasing amounts of Akt. Equal amounts of cell lysates were immunoblotted with indicated antibodies. (B) Turnover rate of CSN6 phosphorylation mutant S60A is accelerated. Indicated wt and CSN6 mutant tranfected cells were treated with cycloheximide (CHX) (100 µg/ml) for the indicated times. Cell lysates were immunoblotted with indicated antibodies. (C) Akt upregulates CSN6 by decreasing CSN6 ubiquitination. 293T cells were transfected with the indicated plasmids. The proteasome inhibitor MG132 was added 6 h before harvesting cells. The ubiquitinated amount of CSN6 is analyzed by immunoprecipitated (IP) with anti-myc followed by immunoblotting (IB) with anti-HA. (D) Akt reduces CSN6 ubiquitination in the presence of FBXW7. 293T cells were transfected with the indicated plasmids. The proteasome inhibitor MG132 was added 6 h before harvesting cells. The ubiquitinated amount of CSN6 is analyzed by immunoprecipitated (IP) with anti-GFP followed by immunoblotting (IB) with anti-ubi.
CSN6 S60 phosphorylation leades to efficient destabilization of p53
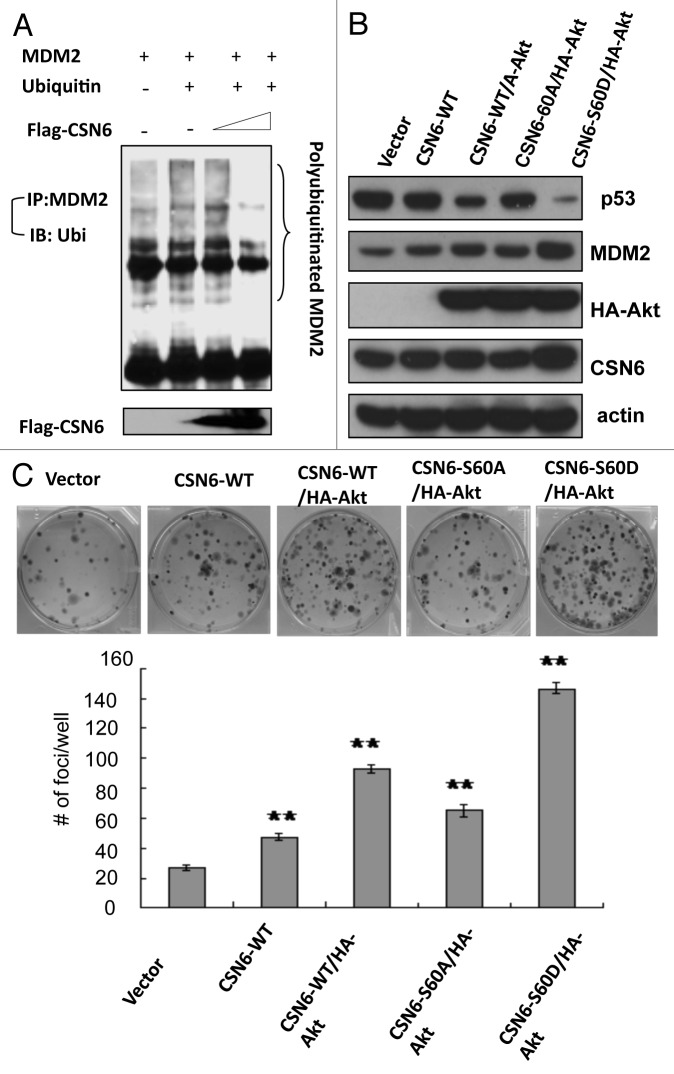
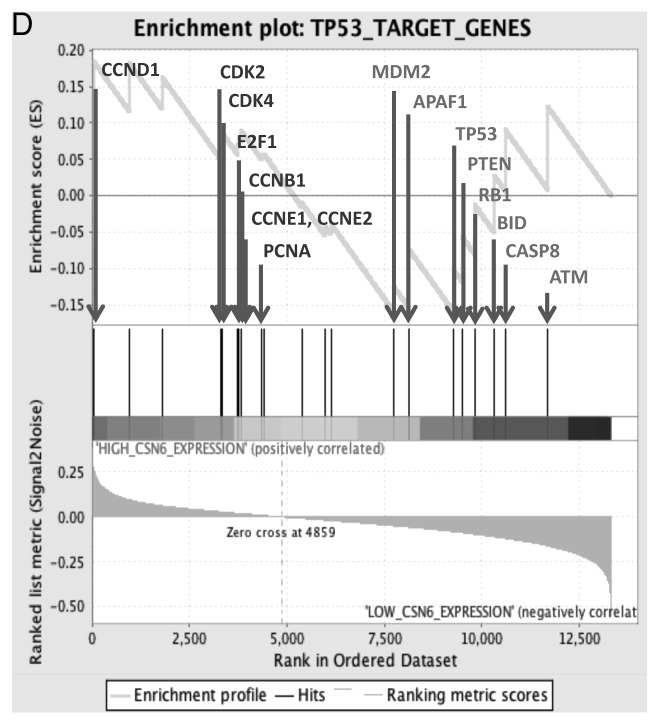
CSN6 has been demonstrated to regulate the MDM2-p53 axis.10 Here we showed that CSN6 expression led to reduced ubiquitination of MDM2 (Fig. 6A), suggesting that CSN6 can cause stabilization of MDM2 and subsequently decrease p53 stabilization. Since CSN6 decreases the stability of p53, we reasoned that the Akt-CSN6 axis would have a role in p53 regulation. We show that indeed Akt overexpression facilitates CSN6’s impact in decreasing p53 (Fig. 6B), and during such a process, MDM2 levels are elevated (Fig. 6B). Interestingly, the impact of the CSN6S60A mutant in causing p53 downregulation seems compromised when compared with wt CSN6 and CSN6S60D (Fig. 6B). We previously indicated that CSN6 can potentiate the transformational activity of cancer cells.10 We sought to further examine the effect of Akt in CSN6-potentiated cell transformation. We found that A549 cells with cotransfected Akt and CSN6 showed an increase of cell foci when compared with CSN6 transfected cells (Fig. 6C). Interestingly, the transformational capability of the CSN6S60A mutant is less responsive to Akt’s postitive impact when compared with wt CSN6 and CSN6S60D (Fig. 6C). In addition, our Gene Set Enrichment Analysis (Broad Institute) data showed a positive correlation between CSN6 expression and the levels of p53 suppressed genes involved in cell cycle, such as cyclin D, CDK4, E2F1 and cyclin B (Fig. 6D), confirming CSN6’s negative impact on p53 activity.
Figure 6A-C. CSN6 S60D mutant efficiently induces p53 degradation and enhances transformation. (A) CSN6 reduces the levels of MDM2 ubiquitination. 293T cells were transfected with the indicated plasmids. The proteasome inhibitor MG132 was added 6 h before harvesting cells. The ubiquitinated amount of MDM2 is analyzed by immunoprecipitated (IP) with anti-MDM2 followed by immunoblotting (IB) with anti-ubi. (B) CSN6 S60D mutant efficiently degrades p53. A549 cells were transfected with indicated plasmids and equal amounts of cell lysates were were immunoblotted with indicated antibodies. (C) Akt enhances CSN6-mediated cell transformation. Cells were transfected with indicated plasmids, and foci-formation assay was performed.
Figure 6D. (D) Enrichment score graph and Ranked list metric graph showing the upregulation of p53-suppressed target genes in CSN6-highly expressing patients (Gene Set Enrichment Analysis, Broad Institute, MIT. Cohort: GSE-20194 consisting of 255 untreated stage I–stage III breast cancer patients at MD Anderson Cancer Center. Blue, p53-suppressed target genes; red, p53-activated target genes.
Impacts of Akt-CSN6 axis on ROS production and DNA damage
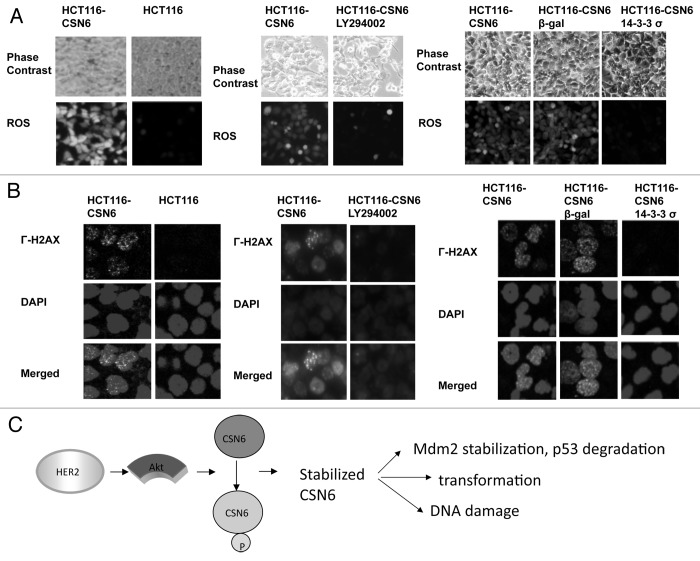
p53 has been characterized as a genome guardian.17 Since CSN6 overexpression leads to decreasing amounts of p53, we sought to determine the impact of the Akt-CSN6 axis on DNA damage/chromosomal instability. We first analyzed the level of reactive oxygen species (ROS), which is known to cause DNA damage. We showed that CSN6-overexpressing HCT116 cells had a higher level of ROS than HCT116 parental cells (Fig. 7A). We then investigated the involvement of PI3K-Akt signaling, and found that the production of ROS in CSN6-overexpressing HCT116 cells was attenuated by PI3K inhibitor LY294002, which blocks PI3K-Akt activity (Fig. 7A). Similar results were observed in cells treated with Ad-14-3-3 σ (using an adenovirus delivery), which can inhibit the activity of Akt18 (Fig. 7A). To examine the functional link between ROS and DNA damage as a result of CSN6 overexpression, we then analyzed the γ-H2AX foci formation (a marker of double-strand DNA breaks) in cells. We detected higher numbers of γ-H2AX foci in CSN6-overexpressing than in HCT116 cells (Fig. 7B). Significantly, LY294002 decreased numbers of γ-H2AX foci caused by CSN6 overexpression (Fig. 7B). Similar results were obtained by the expression of Ad-14-3-3σ (Fig. 7B). i.e., 14-3-3σ-mediated inhibition of Akt leads to hindering CSN6-potentiated γ-H2AX foci formation (Fig. 7B).
Figure 7. Impacts of Akt-CSN6 axis on ROS production and DNA damage. (A) ROS production (green fluorescence) was detected by DCFDA and fluorescence microscopy in indicated cells. Phase-contrast images and merged images of the same microscopic fields are shown. (B) The distribution of γ-H2AX foci (green) was analyzed in indicated cells by immunofluorescent microscopy with anti-γ-H2AX and Alexa Fluor 488-conjugated secondary antibodies. DNA was counterstained with DAPI (blue). (C) Model of the impact of the Akt-CSN6 axis in regulating p53, cell transformation and DNA damage.
Discussion
The COP9 complex or its individual subunits have been implicated in a wide variety of regulatory processes, including cell cycle control, signal transduction, transcriptional activation and tumorigenesis (reviewed in refs. 12 and 13). As a COP9 complex subunit, CSN6 is found to be overexpressed in many types of cancers.13 Here, we discover a critical role for the HER2-Akt axis in controlling CSN6 homeostasis by regulating its ubiquitin-proteasomal degradation. Our results provide insights into the consequence of activation of the HER2-Akt axis in affecting CSN6 expression during carcinogenesis and cancer progression.
Akt is highly active in many types of cancers. This activation of Akt signaling has made Akt an attractive target for therapeutic cancer drugs. However, although high activation of Akt is associated with advanced clinical stage and poor prognosis in several cancers, the impact of aberrant overactivation of Akt remains unclear. Our studies showing that activation of Akt leads to accumulation of CSN6 provide critical insights into Akt-overexpression in cancer, as Akt can mediate poshorylation and stabilization of CSN6 to enhance p53 downregulation and insitigate DNA damage (Figs. 6 and 7). We have found that CSN6 is regulated by HER2/Akt signaling (Fig. 1). These results suggest that CSN6 plays a critical role in HER2/Akt signaling. However, the regulation and biological function of CSN6 are still largely unknown. Structurally, CSN6 contains a MPN (Mpr1p and Pad1p N terminal) domain, which is found in the N terminus of yeast Mpr1 and Pad1 proteins.19-21 The biological function of the MPN domain remains to be determined, although it consists of polar residues that resemble the active site residues of metalloproteases22 and is involved in a proteasome-associated deneddylation activity.23 Our data show that there is an Akt phosphorylation site on S60 of CSN6. This site is locatesd in the early portion of MPN domain (aa 43–181). Whether this phosphorylation event participates in any activity of MPN domain remains to be investigated. It is noteworthy that cells treated with antisense CSN6 were growth-inhibited,24 suggesting that CSN6 plays an important role in cell proliferation. Interestingly, CSN6 also interacts with human immunodeficiency virus type-1 (HIV-1) viral protein R (Vpr), which, in turn, regulates CSN6’s subcellular localization to the nuclear membrane.24 Expression of Vpr results in blocking of cell proliferation and causes an accumulation of cells in the G2/M phase of the cell cycle.24,25 Thus, the subcellular localization of CSN6 is critical in cell cycle regulation. However, it is not clear whether Vpr–mediated CSN6 subcellular localization involves Akt. We have shown that Akt enhances nuclear localization of CSN6 (Fig. 3). Presumably, this nuclear import will be more efficient in affecting its major targets such as MDM2 and p53 in the nucleus. It is important to point out that Akt can phosphorylate MDM2 at Ser166 and Ser168, thereby enhancing nuclear import of MDM2.16 Thus Akt’s impact on both CSN6 and MDM2 will potentiate CSN6-mediated MDM2 stabilization (Fig. 6) and subsequent p53 degradation.10 It is conceivable that the impact of the Akt-CSN6 axis on p53 will affect the genome integrity. Significantly, CSN6 overexpression results in ROS production and γ-H2AX foci (Fig. 7). On the other hand, Akt inhibition by LY294002 can correct such a phenotype caused by CSN6 overexpression (Fig. 7), supposedly a manifestation of downregulation of CSN6 caused by Akt inhibition. Another of our observations, that expression of 14-3-3 σ, which causes inhibition and downregulation of Akt,18,26 leads to reduction of ROS production and γ-H2AX foci in CSN6-overexpressing cells (Fig. 7), also provides insight into the role of Akt-CSN6 axis in DNA damage.
The SCF (SKP1–CUL1–F-box protein) ubiquitin ligases are mammalian cullin RING ubiquitin ligases, and the F-box protein provides the substrate targeting specificity of the complex.27 Sixty-nine F-box proteins have been identified in humans. FBXW7 (F-box and WD repeat domain-containing 7) is one of the F-box proteins that functions as a substrate-recognition subunit of SCF complexes.28 Our data showed that Akt reduced ubiquitination of CSN6 in a dose-dependent manner (Fig. 5C). Another interesting observation is that Akt mediates reduction of CSN6 ubiquitination in the presence of FBXW7 (Fig. 5D). Whether FBXW7 is involveld in CSN6 ubiquitination remains to be investigated. It is important to point out that the known proteins interacting with FBXW7, including cyclin E, Myc, c-Jun and Aurora A and others all play important roles in tumorigenesis.29-32 CSN6 is amplified in many types of cancer,13 and it is reasonable to picture that FBXW7, a tumor suppressor,28 plays a role in controlling the level of CSN6. It is possible that CSN6 is a potential FBXW7 target.
It is indicated that Akt enhances MDM2 nuclear localization and facilitates p53 degradation. Also, CSN6 can stabilize MDM2 and subsequently enhance MDM2-mediated p53 degradation. Here, our study of Akt-mediated CSN6 stabilization serves as a new novel way for reinforcing MDM2’s negative impact on p53. Our mechanistic studies of Akt-mediated CSN6 phosphorylation and stabilization explain how activation of Akt activity can lead to stabilized CSN6, thereby causing p53 downregulation, DNA damage and cell transformation in our proposed model (Fig. 7C). Clearly, the Akt-CSN6 link will be an important molecular target for rational cancer therapy. Targeting the CSN6 may be a useful therapeutic strategy for cancer intervention in Akt-overexpressing cancer.
Materials and Methods
Cell lines and reagents
293T (human embryonic kidney cell line), A549 (Human lung adenocarcinoma cell line), Rat1 (Rat embryo fibroblast cell line) and MDA-MB 453 (Human breast cancer cell line) were obtained from the ATCC. Rat1-Akt cells stably transfected Akt and MDA-MB 453-DNAkt cells stably transfected DNAkt (Domain Negative Akt) have been described previously.18 Cycloheximide was obtained from Sigma. MG132 is obtained from Bortezomib. Antibodies: CSN6 (BIOMOL international), Flag (M2 monoclonal antibody, Sigma), tubulin (Sigma), Hemagglutinin (HA, 12CA5, Roche), ubiquitin (Zymed Laboratories, Inc.), MDM2 (SMP14, Santa Crutz), p53 (DO1, Oncogene Science), GFP (SC-9996; Santa Cruz). Flag-CSN6, GFP-CSN6, Flag-FBXW7, Ad-β-gal and Ad-14–3-3 σwere previously described.10,32-35 CSN6 (S60A) and CSN6 (S60D) mutants were constructed using a site-directed mutagenesis technique (Stratagene).
Turnover assay
The cells were transfected with indicated plasmids and incubated in the 37°C with 5% (vol/vol) CO2 for 24 h. Then cycloheximide was added into the media at 60 μg/ml of final concentration. The cells were harvested at the indicated time points after CHX treament. The protein levels were analyzed by immunoblotting.
Ubiquitination assay
The 293T cells were cotransfected with indicated plasmids. At 24 h post-transfection, cells were treated with 50 μg/ml of MG132 for 6 h. The CSN6 proteins were immunoprecipitated by the anti-myc or anti-GFP antibody. The protein complexes were then resolved by SDS-polyacrylamide gel and probed with anti-ubiquitin or Anti-HA to observe the ubiquitinated CSN6.
Immunofluorescence
Cells were grown on sterile coverslip in 6-well plate overnight. The cells were fixed in 100% methanol at -20°C for 3 min. Cells were probed with rabbit anti-γ-H2AX antibody (Trevigen) or anti-CSN6. Alexa Fluor 488 goat anti-rabbit antibody (Molecular Probe) was added to detect γ-H2AX or CSN6. DAPI was applied to stain the nuclei. Immunofluorescence was observed by using a confocal microscope (Olympus IX81). For ROS detection, cells were treated with 2’-7’- dichloro fluoresin diacetate (DCFDA) (5μg/ml, Molecular Probes) and observed under fluorescent microscope.
In vitro kinase assays
Recombinant CSN6 substrates were produced by BL-21 E. coli bacteria. Briefly, The bacterial cells were transformed with the pET-Flag-CSN6 (wild type) and pET-Flag-CSN6 (S60A) plasmid and grown in 5 mL LB media overnight and in 500 ml LB media for 5h. One mM isopropyl β-D-1 thiogalactopyranoside (IPTG) (Fisher) was added in culture media for 3 h. Then the cells were lysed with NETN buffer (20 mM TRIS-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% [vol/vol] Nonidet P-40 plus protease-phosphatase inhibitor mixture) and sonicated.36 The CSN6 proteins were pulled down by M2 beads and eluted by Flag peptide. Recombinant Akt kinase (Cell Signaling) was incubated with immunopurified CSN6 (wild type) and CSN6 (S60A) substrates in 1 × kinase buffer [80 mM Mops (Sigma), 7.5 mM MgCl2 (Fisher), pH 7.0] with cold ATP and γ32 ATP (Perkin-Elmer) at 30°C for 15 min. Kinase reactions were analyzed by SDS/PAGE as described before. After the SDS/PAGE gel was dried, the radioactivity image was visualized using a phosphoimager cassette (Molecular Dynamics) and a Typhoon Trio variable mode imager.
Foci formation assay
500 cells were plated in each well of 6-well plates for each type of transfected cells. Medium was changed every three days over 14 d of foci formation. At the end of the period, cell monolayer was stained in crystal violet solution (0.5% crystal violet, 20% methanol) and then destained by wash with water. Foci were then counted and photographed.11
Acknowledgments
This work was supported by grants in part from the National Institutes of Health (NIH) (R01CA089266), Directed Medical Research Programs (DOD SIDA BC062166 to S.J.Y. and M.H.L.) and Susan G. Komen Breast Cancer Foundation (KG081048). The University of Texas MD Anderson Cancer Center is supported by NIH core grant CA16672. We thank Michael Mcguire and Jessica Cromheecke for editing.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/22413
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Lee MH, Yang HY. Negative regulators of cyclin-dependent kinases and their roles in cancers. Cell Mol Life Sci. 2001;58:1907–22. doi: 10.1007/PL00000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–52. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–91. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001;2:760–8. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 7.Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–5. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- 8.Naumann M, Bech-Otschir D, Huang X, Ferrell K, Dubiel W. COP9 signalosome-directed c-Jun activation/stabilization is independent of JNK. J Biol Chem. 1999;274:35297–300. doi: 10.1074/jbc.274.50.35297. [DOI] [PubMed] [Google Scholar]
- 9.Bech-Otschir D, Kraft R, Huang X, Henklein P, Kapelari B, Pollmann C, et al. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 2001;20:1630–9. doi: 10.1093/emboj/20.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao R, Yeung SC, Chen J, Iwakuma T, Su CH, Chen B, et al. Subunit 6 of the COP9 signalosome promotes tumorigenesis in mice through stabilization of MDM2 and is upregulated in human cancers. J Clin Invest. 2011;121:851–65. doi: 10.1172/JCI44111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi HH, Gully C, Su CH, Velazquez-Torres G, Chou PC, Tseng C, et al. COP9 signalosome subunit 6 stabilizes COP1, which functions as an E3 ubiquitin ligase for 14-3-3σ. Oncogene. 2011;30:4791–801. doi: 10.1038/onc.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei N, Deng XW. The COP9 signalosome. Annu Rev Cell Dev Biol. 2003;19:261–86. doi: 10.1146/annurev.cellbio.19.111301.112449. [DOI] [PubMed] [Google Scholar]
- 13.Lee MH, Zhao R, Phan L, Yeung SJ. Roles of COP9 signalosome in cancers. Cell Cycle. 2011;10:3057–66. doi: 10.4161/cc.10.18.17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karniol B, Chamovitz DA. The COP9 signalosome: from light signaling to general developmental regulation and back. Curr Opin Plant Biol. 2000;3:387–93. doi: 10.1016/S1369-5266(00)00101-1. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Deng XW. The COP9 signalosome: an alternative lid for the 26S proteasome? Trends Cell Biol. 2003;13:507–9. doi: 10.1016/j.tcb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 17.Lee MH, Lozano G. Regulation of the p53-MDM2 pathway by 14-3-3 sigma and other proteins. Semin Cancer Biol. 2006;16:225–34. doi: 10.1016/j.semcancer.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Wen YY, Zhao R, Lin YL, Fournier K, Yang HY, et al. DNA damage-induced protein 14-3-3 sigma inhibits protein kinase B/Akt activation and suppresses Akt-activated cancer. Cancer Res. 2006;66:3096–105. doi: 10.1158/0008-5472.CAN-05-3620. [DOI] [PubMed] [Google Scholar]
- 19.Rinaldi T, Bolotin-Fukuhara M, Frontali L. A Saccharomyces cerevisiae gene essential for viability has been conserved in evolution. Gene. 1995;160:135–6. doi: 10.1016/0378-1119(95)00212-O. [DOI] [PubMed] [Google Scholar]
- 20.Penney M, Wilkinson C, Wallace M, Javerzat JP, Ferrell K, Seeger M, et al. The Pad1+ gene encodes a subunit of the 26 S proteasome in fission yeast. J Biol Chem. 1998;273:23938–45. doi: 10.1074/jbc.273.37.23938. [DOI] [PubMed] [Google Scholar]
- 21.Wei N, Deng XW. Making sense of the COP9 signalosome. A regulatory protein complex conserved from Arabidopsis to human. Trends Genet. 1999;15:98–103. doi: 10.1016/S0168-9525(98)01670-9. [DOI] [PubMed] [Google Scholar]
- 22.Maytal-Kivity V, Reis N, Hofmann K, Glickman MH. MPN+, a putative catalytic motif found in a subset of MPN domain proteins from eukaryotes and prokaryotes, is critical for Rpn11 function. BMC Biochem. 2002;3:28. doi: 10.1186/1471-2091-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyapina S, Cope G, Shevchenko A, Serino G, Tsuge T, Zhou C, et al. Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science. 2001;292:1382–5. doi: 10.1126/science.1059780. [DOI] [PubMed] [Google Scholar]
- 24.Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Kao GD, Muschel RJ, et al. HIV-1 Vpr interacts with a human 34-kDa mov34 homologue, a cellular factor linked to the G2/M phase transition of the mammalian cell cycle. Proc Natl Acad Sci USA. 1998;95:3419–24. doi: 10.1073/pnas.95.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amini S, Saunders M, Kelley K, Khalili K, Sawaya BE. Interplay between HIV-1 Vpr and Sp1 modulates p21(WAF1) gene expression in human astrocytes. J Biol Chem. 2004;279:46046–56. doi: 10.1074/jbc.M403792200. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Zhang Y, Zhao R, Wen YY, Fournier K, Wu HB, et al. Negative cell cycle regulator 14-3-3sigma stabilizes p27 Kip1 by inhibiting the activity of PKB/Akt. Oncogene. 2006;25:4585–94. doi: 10.1038/sj.onc.1209481. [DOI] [PubMed] [Google Scholar]
- 27.Jin J, Ang XL, Shirogane T, Wade Harper J. Identification of substrates for F-box proteins. Methods Enzymol. 2005;399:287–309. doi: 10.1016/S0076-6879(05)99020-4. [DOI] [PubMed] [Google Scholar]
- 28.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 29.Onoyama I, Nakayama KI. Fbxw7 in cell cycle exit and stem cell maintenance: insight from gene-targeted mice. Cell Cycle. 2008;7:3307–13. doi: 10.4161/cc.7.21.6931. [DOI] [PubMed] [Google Scholar]
- 30.Minella AC, Clurman BE. Mechanisms of tumor suppression by the SCF(Fbw7) Cell Cycle. 2005;4:1356–9. doi: 10.4161/cc.4.10.2058. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Inuzuka H, Fukushima H, Wan L, Gao D, Shaik S, et al. Emerging roles of the FBW7 tumour suppressor in stem cell differentiation. EMBO Rep. 2012;13:36–43. doi: 10.1038/embor.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu CC, Yang TY, Yu CT, Phan L, Ivan C, Sood AK, et al. p53 negatively regulates Aurora A via both transcriptional and posttranslational regulation. Cell Cycle. 2012;11:3433–42. doi: 10.4161/cc.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su CH, Zhao R, Zhang F, Qu C, Chen B, Feng YH, et al. 14-3-3sigma exerts tumor-suppressor activity mediated by regulation of COP1 stability. Cancer Res. 2011;71:884–94. doi: 10.1158/0008-5472.CAN-10-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su CH, Zhao R, Velazquez-Torres G, Chen J, Gully C, Yeung SC, et al. Nuclear export regulation of COP1 by 14-3-3σ in response to DNA damage. Mol Cancer. 2010;9:243. doi: 10.1186/1476-4598-9-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang HY, Wen YY, Chen CH, Lozano G, Lee MH. 14-3-3 sigma positively regulates p53 and suppresses tumor growth. Mol Cell Biol. 2003;23:7096–107. doi: 10.1128/MCB.23.20.7096-7107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laronga C, Yang HY, Neal C, Lee MH. Association of the cyclin-dependent kinases and 14-3-3 sigma negatively regulates cell cycle progression. J Biol Chem. 2000;275:23106–12. doi: 10.1074/jbc.M905616199. [DOI] [PubMed] [Google Scholar]