Abstract
There is a relationship between various cellular stress factors and aging. In earlier studies, we demonstrated that overexpression of the D-GADD45 gene increases the life span of Drosophila melanogaster. In this study, we investigate the relationship between D-GADD45 activity and resistance to oxidative, genotoxic and thermal stresses as well as starvation. In most cases, flies with constitutive and conditional D-GADD45 overexpression in the nervous system were more stress-resistant than ones without overexpression. At the same time, most of the studied stress factors increased D-GADD45 expression in the wild-type strain. The lifespan-extending effect of D-GADD45 overexpression was also retained after exposure to chronic and acute gamma-irradiation, with doses of 40 сGy and 30 Gy, respectively. However, knocking out D-GADD45 resulted in a significant reduction in lifespan, lack of radiation hormesis and radioadaptive response. A dramatic decrease in the spontaneous level of D-GADD45 expression was observed in the nervous system as age progressed, which may be one of the causes of the age-related deterioration of organismal stress resistance. Thus, D-GADD45 expression is activated by most of the studied stress factors, and D-GADD45 overexpression resulted in an increase of stress resistance.
Keywords: GADD45, longevity, stress resistance
Introduction
Proteins of the GADD45 (growth-arrest and DNA-damage inducible 45) family take part in gene expression regulation, cell cycle arrest, DNA repair and apoptosis.1 In mammals, their expression is induced under conditions of stress, including oxidative and genotoxic stresses.2 GADD45 interacts with proteins involved in DNA excision repair3 and also with proteins that are involved in the repair of double-stranded breaks in DNA.4 In Drosophila melanogaster, only one homolog of the GADD45 gene is known—D-GADD455—and its function is less studied. However, we have previously shown an increase in the lifespan of D. melanogaster (here referred to as Drosophila) as a result of D-GADD45 overexpression.6
One of the probable mechanisms explaining the increase in longevity due to the overexpression of GADD45 is that it confers resistance to stresses. There is much evidence for the relationship between stress resistance and longevity. As organisms age, there is a decrease in stress resistance.7 The overexpression of genes that improve stress resistance increases the lifespan.8,9 Furthermore, animals with mutations that lead to longevity are characterized by an increased resistance to oxidative and temperature stress.10 However, short-lived mutants of model organisms have decreased resistance to stress factors.11 Gene activity contributing to the acceleration of the aging process also leads to endogenous stress (e.g., p66).12 Selection of animals on the basis of resistance to one stress factor increases resistance to other stress factors and leads to an increase in longevity.13 Species that have different life expectancies differ in stress resistance, especially to oxidative stress (for example, rodents and birds or bats).14 It is reported that the long-lived naked mole rat (Heterocephalus glaber) has an increased resistance to a broad spectrum of cytotoxins, including heat shock, heavy metals, genotoxicants and xenobiotics.15 In humans we see the same patterns; for example, human centenarians do not differ in having to follow a healthy lifestyle.16 Their extraordinary longevity can be explained by increased resistance to stress.
Mild stress results in an increase in lifespan.17 The mechanisms involved in increasing lifespan include the induction of protective systems, such as antioxidant defense,18 repair of DNA,19 synthesis of heat shock proteins,20 activation of the immune system21 and selection of weakened cells.22 The aim of this work is to demonstrate the relationship between the induction of D-GADD45 in Drosophila, and resistance to oxidative stress (paraquat), genotoxic stress (gamma-irradiation), heat shock (35°С) and starvation.
Results
Expression levels of the D-GADD45 gene in flies with different genotypes
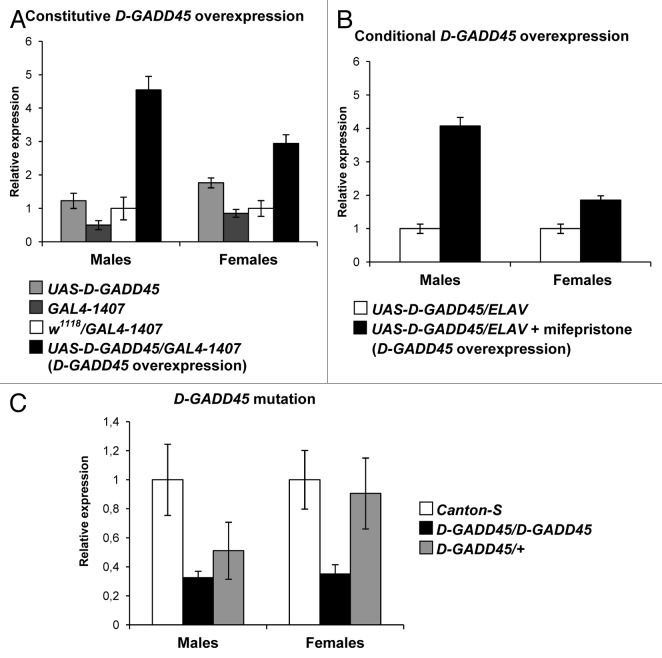
To confirm constitutive D-GADD45 overexpression, we compared expression levels of the D-GADD45 gene in the nervous tissue of 5-d-old males and females with the UAS-D-GADD45/GAL4–1407 genotype (flies with expected D-GADD45 overexpression in the nervous system) and with the UAS-D-GADD45 and GAL4–1407 genotypes. In addition, we measured D-GADD45 expression level in the F1 progeny of crosses between w1118 wild-type females and GAL4–1407 males to avoid the heterosis effect (w1118/GAL4–1407 flies). Analysis of the relative D-GADD45 expression values revealed 3–9-fold higher expression in UAS-D-GADD45/GAL4–1407 males and 2–3-fold higher expression in females in comparison with flies without overexpression (Fig. 1).
Figure 1. Expression levels of the D-GADD45 gene in Drosophila with different genotypes. (A) Expression levels in the nervous tissue of Drosophila flies with the UAS-D-GADD45/GAL4–1407 genotype (flies with expected constitutive D-GADD45 overexpression), the UAS-D-GADD45 and GAL4–1407 parental genotypes, and the w1118/GAL4–1407 genotype. (B) Expression levels in the nervous tissue of flies with the UAS-D-GADD45/ELAV genotype kept on a medium with mifepristone (flies with expected conditional D-GADD45 overexpression) and those not treated with mifepristone. (C) Ubiquitous expression in flies of the wild-type Canton-S strain, and flies with the homo- and heterozygous mutation in the D-GADD45 gene (D-GADD45/D-GADD45 and D-GADD45/+ flies, respectively).
To detect the conditional D-GADD45 overexpression effect, F1 progeny of crosses between UAS-D-GADD45 females and ELAV males were kept on a medium with mifepristone (inductor of the ELAV driver). Comparison of the relative D-GADD45 expression values in the nervous tissue of flies with the UAS-D-GADD45/ELAV genotype not treated with mifepristone exhibited a 4-fold higher expression in males and 2-fold higher expression in females when compared with wild-type flies (Fig. 1).
To analyze D-GADD45 expression levels in flies with mutation in D-GADD45, 5-d-old Drosophila male and female with D-GADD45/D-GADD45 (homozygous mutants) genotypes and D-GADD45/+ (heterozygous mutants) were examined and compared with wild-type Canton-S flies as a control. We found a 3-fold decrease in D-GADD45 expression both in males and females with homozygous insertion mutations in the gene D-GADD45, and a 2-fold decrease in heterozygous mutants (Fig. 1).
The impact of D-GADD45 overexpression in the nervous system on resistance to oxidative stress, hyperthermia and starvation
It was recently demonstrated that overexpression of the D-GADD45 gene in the nervous system leads to a significant increase in the lifespan of Drosophila without a corresponding decrease in fecundity or locomotor activity. This is apparently due to more efficient recognition and repair of DNA damage.6 In most cases reported in the literature, an increase in lifespan in model organisms is accompanied by an increase in stress resistance.10,11,13 We hypothesized that the overexpression of the D-GADD45 gene in the nervous system would lead to an increase in resistance to stress in addition to its previously characterized role in increasing lifespan. For this reason, we examined the impact of D-GADD45 overexpression on the survival of Drosophila during and after exposure to various stress factors—oxidative stress (20 mM paraquat), hyperthermia (35°C) and starvation.
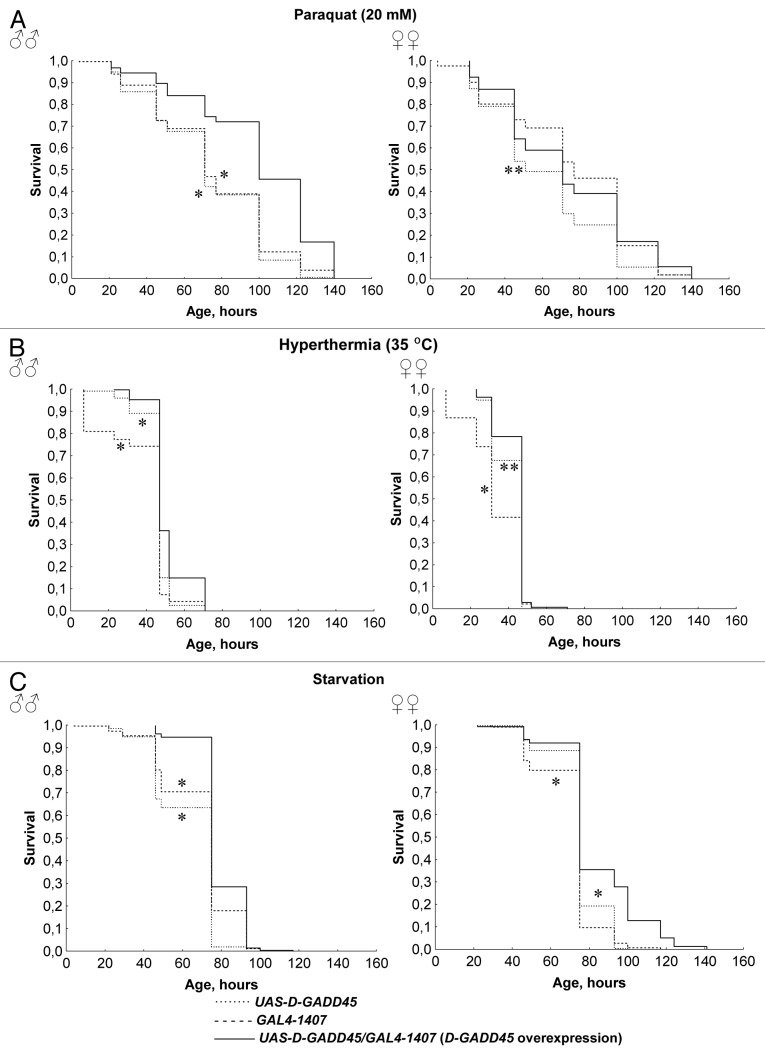
Male Drosophila with constitutive overexpression of D-GADD45 in the nervous system had higher resistance to superoxide-inducer paraquat, hyperthermia and starvation compared with males with UAS-D-GADD45 and GAL4–1407 parental genotypes (p < 0.001) (Fig. 2). The percentage of dead flies after 48 h under stresses increased by 2.3–8.2 times for flies constitutively overexpressing D-GADD45 (p < 0.05). In females, similar changes in survival rates were found, but it was significantly lower (p < 0.05) (Fig. 2). Differences in the percentage of dead flies between females with constitutive D-GADD45 overexpression in the nervous system and females without overexpression were 1.3–2.7 times (p < 0.05). The exception is that the effects in females with constitutive overexpression of the D-GADD45 gene were insignificant when compared with females of the GAL4–1407 strain under oxidative stress conditions.
Figure 2. The influence of constitutive D-GADD45 overexpression in the nervous system on stress resistance. (A–C) Surval curves of Drosophila males and females with constitutive D-GADD45 overexpression in the nervous system and with the UAS-D-GADD45 and GAL4–1407 parental genotypes under oxidative stress (20 mM paraquat) (A), hyperthermia (35°C) (B) and starvation (C). Significant differences in survival are marked by * - p < 0.001, ** - p < 0.05 (Kolmogorov-Smirnov test), compared with flies with D-GADD45 overexpression.
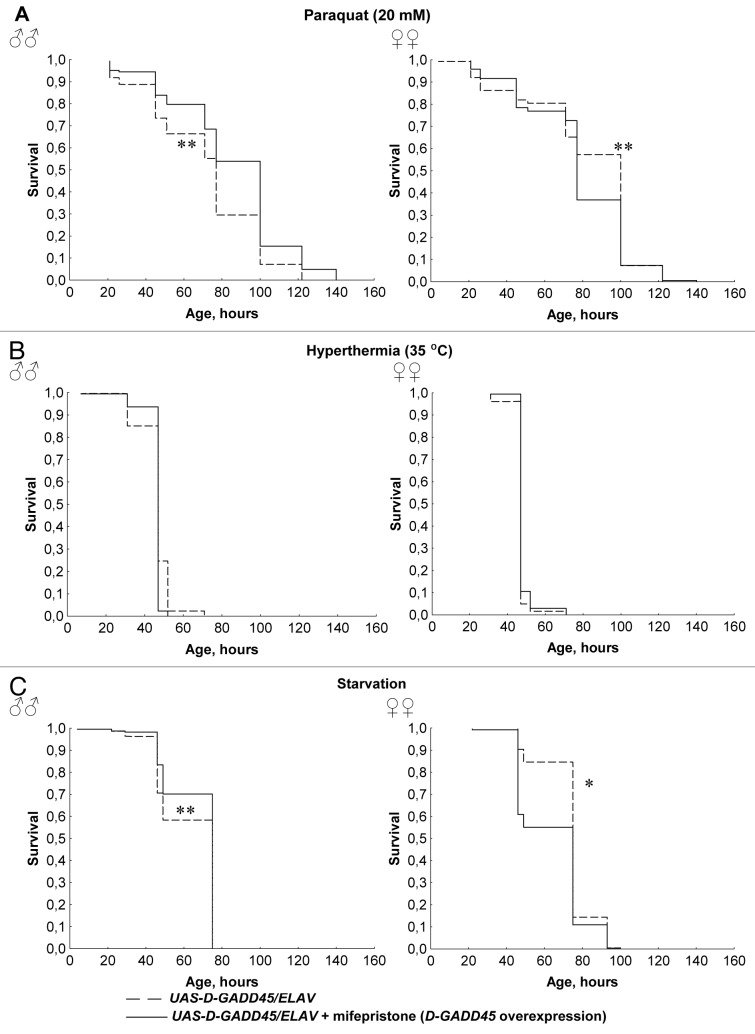
In order to avoid the possible influence of heterosis and differences in genetic background, we examined the effect of conditional (mifepristone-inducible) D-GADD45 overexpression in the nervous system of Drosophila under oxidative stress, thermal stress and starvation. Males with the UAS-D-GADD45/ELAV genotype treated with mifepristone (conditional D-GADD45 overexpression in the nervous system) had higher survival rates compared with those kept without mifepristone induction (p < 0.05) during increased levels of oxidative stress and starvation (Fig. 3). In males with conditional D-GADD45 overexpression, the percentage of dead flies after 48 h of oxidative stress and starvation increased by 1.6–1.8 times compared with males without overexpression (p < 0.05). Females, however, responded differently; they had a higher resistance to paraquat and lower survival rates under starvation conditions (p < 0.05). Conditional overexpression of the D-GADD45 gene did not lead to significant alterations in resistance to hyperthermia in either males or females (Fig. 3).
Figure 3. The influence of conditional D-GADD45 overexpression in the nervous system on stress resistance. (A–C) Surval curves of Drosophila males and females with conditional D-GADD45 overexpression in the nervous system and without overexpression under oxidative stress (20 mM paraquat) (A), hyperthermia (35°C) (B) and starvation (C). Significant differences in survival are marked by * - p < 0.001, ** - p < 0.05 (Kolmogorov-Smirnov test).
Thus, in the majority of the experimental conditions examined here, constitutive and conditional overexpression of D-GADD45 in the nervous system resulted in an increase in resistance to the superoxide inducer paraquat, hyperthermia and starvation in males, but led to lower or negative alterations in females. The dissimilarity in the response to stress factors between males and females is explained by the different levels of D-GADD45 activity. In males, 3–9-fold D-GADD45 overexpression led to more efficient repair of cellular structure damage and caused an increase in resistance to oxidative stress, hyperthermia and starvation. In females, 2–3-fold overexpression of D-GADD45 could be insufficient for the increase in stress resistance. Furthermore, it is possible that greater energy costs are associated with the generation of D-GADD45 gene products and lead to a decrease in viability in females with D-GADD45 overexpression.
Hormesis and radioadaptive response in D-GADD45 mutants
We studied the role of D-GADD45 in the formation of hormesis and the radioadaptive response. To do this, we examined the life expectancies of Drosophila flies of the wild-type Canton-S strain, as well as homo- and heterozygous strains with mutations in the D-GADD45 gene.
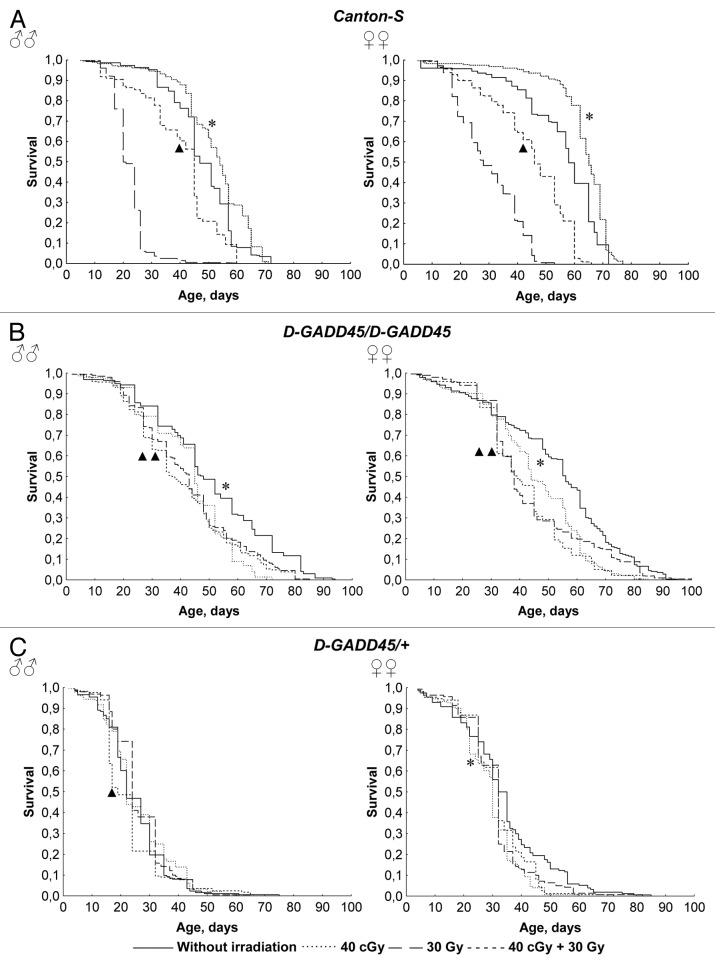
Analysis of survival curves and lifespan parameters showed that wild-type Canton-S flies exposed to low-dose chronic gamma-irradiation at the pre-imago stages develop hormesis (Fig. 4). The median lifespan of flies that underwent chronic exposure to 40 сGy of gamma-irradiation was 12–15% (p < 0.001) higher in comparison with flies that were not exposed to the radiation (Table 1). Moreover, wild-type flies developed a radiation-induced adaptive response. In contrast, acute exposure at a dose of 30 Gy decreased the median lifespan by 51–57% (p < 0.001); however, the consistent effects of gamma-irradiation at a dosage of 40 сGy and 30 Gy decreased the median lifespan by only 6–21% (p < 0.001). As a result, the negative effect of irradiation at a dose of 30 Gy was reduced by 1.6–2.3 times (p < 0.001), with the help of pre-irradiation in low doses. Similar changes have been identified during the analysis of other factors affecting lifespan. For example, in male Drosophila undergoing chronic exposure to 40 cGy gamma irradiation, there was an increase in mortality rate (here, 90% mortality is considered an index of maximum lifespan) by 12% (p < 0.05), but pre-irradiation at the same dosage reduced the negative effect of acute exposure to 30 Gy gamma-radiation by 1.6–2.2 times in males and females of the Canton-S strain (Table 1).
Figure 4. Survival of the wild-type Canton-S flies and flies with the D-GADD45 mutation under different irradiation conditions. (A–C) Survival curves of Drosophila males and females of the wild-type Canton-S strain (A), and flies with the homo- and heterozygous mutation in the D-GADD45 gene (D-GADD45/D-GADD45 and D-GADD45/+ flies, respectively) (B and C) under different irradiation conditions. Significant differences in survival are marked by asterisks (for the variants “without irradiation” and “40 cGy”) or by triangles (for the variants “30 Gy” and “40 cGy + 30 Gy” variants), * - p < 0.001, ** - p < 0.05 (Kolmogorov-Smirnov test).
Table 1. The influence of different irradiation conditions on the longevity parameters of Drosophila individuals of the wild-type Canton-S strain and with mutation in the D-GADD45 gene.
| Genotype | Sex | Treatment | М | ± Δm | 90% | Min | Max | MRDT | α | R0 | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Canton-S |
♂♂ |
W/o irradiation |
47.0 |
47.9 ± 0.6 |
58 |
6 |
72 |
7.4 |
0.09 |
0.0006 |
379 |
| |
|
40 cGy * |
54.0 * |
52.2 ± 0.6 |
65 ** |
5 |
71 |
6.7 |
0.10 |
0.0003 |
478 |
| |
|
30 Gy |
20.0 |
22.0 ± 0.3 |
26 |
7 |
60 |
6.7 |
0.10 |
0.0088 |
399 |
| |
|
40 cGy + 30 Gy * |
45.0 * |
40.2 ± 0.7 |
56 * |
7 |
60 |
8.0 |
0.09 |
0.0016 |
358 |
| |
♀♀ |
W/o irradiation |
58.0 |
55.0 ± 0.8 |
68 |
6 |
72 |
7.1 |
0.10 |
0.0002 |
328 |
| |
|
40 cGy * |
65.0 |
63.0 ± 0.6 |
71 |
5 |
77 |
4.4 |
0.16 |
0.0000 |
378 |
| |
|
30 Gy |
28.0 |
29.8 ± 0.5 |
45 |
7 |
53 |
7.5 |
0.09 |
0.0039 |
420 |
| |
|
40 cGy + 30 Gy * |
46.0 * |
44.4 ± 0.7 |
60 * |
7 |
66 |
7.8 |
0.09 |
0.0010 |
397 |
|
D-GADD45/ D-GADD45 |
♂♂ |
W/o irradiation |
48.0 |
50.2 ± 1.5 |
82 |
6 |
94 |
15.3 |
0.05 |
0.0033 |
195 |
| |
|
40 cGy * |
45.0 * |
43.0 ± 1.2 |
58 * |
4 |
72 |
9.2 |
0.08 |
0.0018 |
144 |
| |
|
30 Gy |
43.0 |
42.7 ± 1.3 |
69 |
2 |
85 |
14.9 |
0.05 |
0.0050 |
197 |
| |
|
40 cGy + 30 Gy ** |
37.5 ** |
40.5 ± 1.2 |
67 |
5 |
85 |
15.7 |
0.04 |
0.0060 |
228 |
| |
♀♀ |
W/o irradiation |
56.0 |
52.6 ± 1.4 |
80 |
2 |
95 |
14.5 |
0.05 |
0.0026 |
242 |
| |
|
40 cGy * |
44.0 * |
45.1 ± 1.2 |
63 * |
4 |
85 |
11.6 |
0.06 |
0.0027 |
193 |
| |
|
30 Gy |
38.0 |
43.8 ± 1.3 |
74 |
4 |
100 |
18.4 |
0.04 |
0.0063 |
203 |
| |
|
40 cGy + 30 Gy ** |
39.0 |
41.5 ± 1.2 |
65 * |
4 |
82 |
12.9 |
0.05 |
0.0043 |
175 |
|
D-GADD45/+ |
♂♂ |
W/o irradiation |
22.0 |
24.9 ± 0.8 |
35 |
4 |
75 |
12.2 |
0.06 |
0.0137 |
173 |
| |
|
40 cGy |
22.0 |
25.8 ± 0.9 |
43 |
2 |
63 |
10.9 |
0.06 |
0.0108 |
180 |
| |
|
30 Gy |
24.0 |
26.6 ± 0.7 |
38 |
4 |
65 |
9.0 |
0.08 |
0.0079 |
190 |
| |
|
40 cGy + 30 Gy * |
19.0 * |
23.1 ± 0.8 |
32 |
4 |
66 |
15.0 |
0.05 |
0.0188 |
167 |
| |
♀♀ |
W/o irradiation |
33.5 |
33.8 ± 1.2 |
56 |
4 |
85 |
15.2 |
0.05 |
0.0088 |
154 |
| |
|
40 cGy * |
30.0 * |
28.8 ± 0.8 |
41* |
4 |
61 |
7.6 |
0.09 |
0.0046 |
151 |
| |
|
30 Gy |
32.0 |
31.1 ± 1.0 |
45 |
4 |
82 |
12.3 |
0.06 |
0.0087 |
140 |
| 40 cGy + 30 Gy | 32.0 | 31.4 ± 0.9 | 45 | 4 | 72 | 9.0 | 0.08 | 0.0051 | 152 |
M, median lifespan; ± Δm, mean lifespan and standard error of the mean; 90%, age of 90% mortality; Min and Max, minimum and maximum lifespan; α and R0, Gompertz equation parameters; MRDT, mortality rate doubling time (ln2/α); N, number of individuals in the population; * - p < 0.001, ** - p < 0.05 (between “w/o irradiation” and “40 cGy” variants, and between “30 Gy” and “40 cGy + 30 Gy” variants; treatment, Kolmogorov-Smirnov test; median lifespan, Gehan-Breslow-Wilcoxon and Mantel-Cox tests; age of 90% mortality, Wang-Allison test).
In males and females with a heterozygous mutation in the D-GADD45 gene, the median lifespan was 42–53% lower when compared with Canton-S wild-type flies (p < 0.001) (Table 1). In homozygous males and in females with heterozygous D-GADD45 mutation, differences in the median lifespan were insignificant when compared with the wild-type strain Canton-S; however, maximum lifespan was expanded. Thus, the age of 90% mortality was increased by 17–40% in male and female D-GADD45/D-GADD45 flies (p < 0.001) (Table 1). It is possible that this effect is the result of the genetic background of the strains. Nevertheless, in contrast to the wild-type strain Canton-S, both homo- and heterozygous flies with the mutation in D-GADD45 had higher rates of mortality (MRDT increased by 1.6–2.1 times) and a reduced resistance to ionizing irradiation. Homo- and heterozygous males and females with a mutation in D-GADD45 had a 4–32% (p < 0.05) decrease in median lifespan after chronic (40 cGy) or acute (30 Gy) gamma-irradiation exposure, or successive irradiation of both dose rates. It is important to point out that in flies with the D-GADD45 mutation, both the effect of hormesis and a radioadaptive response were absent. Instead, preliminary chronic irradiation at 40 сGy increased the negative effect of subsequent acute exposure in males by 13–21%. The most negative effect due to gamma-irradiation was observed after successive exposure at dosages of 40 cGy and 30 Gy. In female flies, the median survival rate after acute exposure to 30 Gy and sequential exposure at 40 cGy and 30 Gy did not differ significantly. However, in homozygous D-GADD45/D-GADD45 females, a 12% reduction of 90% mortality rate was observed, which indicates accelerated aging in Drosophila with this given genotype after exposure to low doses of pre-irradiation. These results confirm the analysis of the survival curve and other lifespan indicators (Fig. 4 and Table 1).
Therefore, a 2–3 times decrease in D-GADD45 gene expression is enough to eliminate the effects of hormesis and radioadaptive response. Therefore, D-GADD45 plays a key role in the formation of response reactions to the influence of ionizing radiation.
Radiation resistance during D-GADD45 overexpression in the nervous system
On the basis of our results (see above), we suggested that the overexpression of D-GADD45 would lead to increased resistance to ionizing radiation and would cause an additional radioprotective effect. For this reason, we investigated lifespan changes in flies with constitutive D-GADD45 overexpression in the nervous system following chronic exposure to ionizing radiation at a chronic (40 cGy) or acute (30 Gy) dose rate.
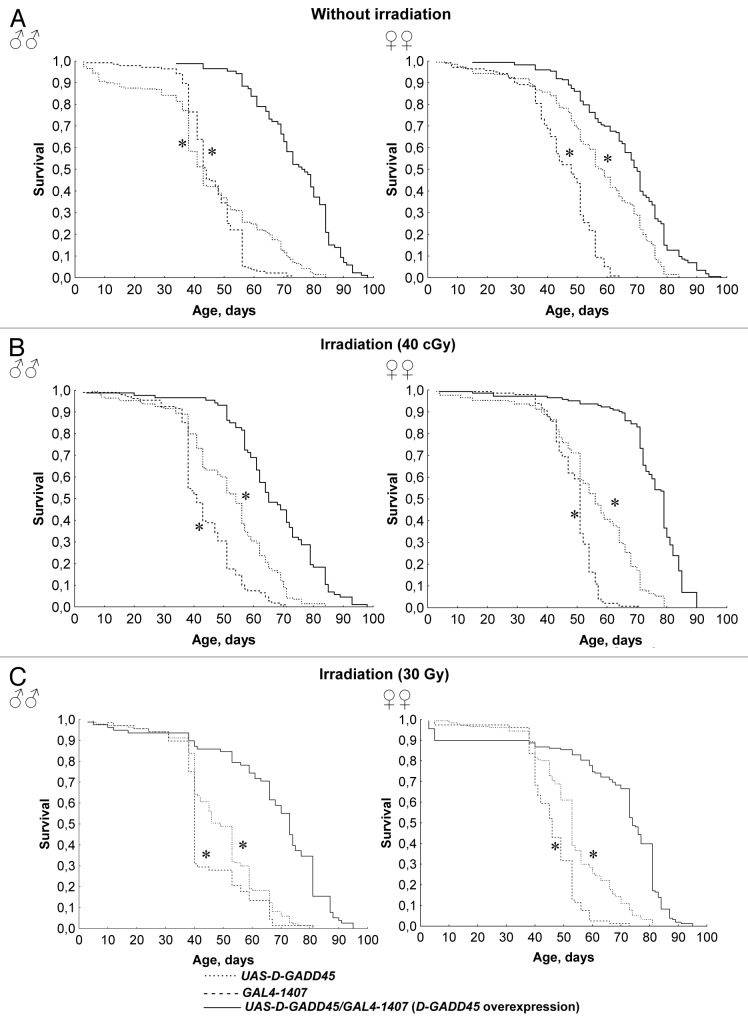
Comparison of the lifespan effects for different variants in our irradiation experiments demonstrated that flies with constitutive D-GADD45 overexpression in the nervous system and parental strain flies UAS-D-GADD45 and GAL4–1407 either do not experience any significant changes in mortality rate, or show the effects of radiation hormesis after gamma-radiation exposure (Table 2). Drosophila females with D-GADD45 overexpression showed a 6–13% increase in median lifespan after chronic and acute gamma-irradiation at 40 cGy and 30 Gy dose rates (p < 0.05). However, in males with overexpressed D-GADD45 in their nervous system, gamma-irradiation had the opposite effect. The median lifespan of these flies was reduced by 17% after chronic irradiation at 40 cGy (p < 0.001), and did not observably change after acute exposure (Table 2). Therefore, D-GADD45 gene overexpression in the nervous system did not cause any additional radiation resistance.
Table 2. The influence of different irradiation conditions on the longevity parameters of Drosophila individuals with the D-GADD45 overexpression in the nervous and without overexpression.
| Genotype | Sex | Treatment | М | ± Δm | 90% | Min | Max | α | R0 | MRDT | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
UAS-D-GADD45 |
♂♂ |
W/o irradiation |
43.0 |
45.0 ± 1.2 |
71 |
3 |
84 |
0.05 |
0.0041 |
3.7 |
258 |
| |
|
40 cGy * |
54.0 * |
51.3 ± 1.1 |
70 |
6 |
84 |
0.07 |
0.0011 |
3.6 |
190 |
| |
|
30 Gy ** |
49.0 ** |
50.0 ± 1.1 |
67 ** |
10 |
81 |
0.08 |
0.0011 |
3.6 |
134 |
| |
♀♀ |
W/o irradiation |
57.5 |
56.6 ± 1.2 |
76 |
3 |
84 |
0.07 |
0.0007 |
3.6 |
211 |
| |
|
40 cGy |
56.0 |
55.0 ± 1.2 |
71 * |
3 |
79 |
0.08 |
0.0006 |
3.7 |
170 |
| |
|
30 Gy * |
53.0 ** |
53.0 ± 1.0 |
73 ** |
5 |
81 |
0.08 |
0.0009 |
3.5 |
180 |
|
GAL4–1407 |
♂♂ |
W/o irradiation |
44.0 |
45.6 ± 0.8 |
56 |
4 |
73 |
0.10 |
0.0006 |
3.2 |
136 |
| |
|
40 cGy * |
41.0 ** |
43.5 ± 1.1 |
57 |
3 |
71 |
0.09 |
0.0012 |
3.3 |
108 |
| |
|
30 Gy * |
40.0 ** |
44.0 ± 1.6 |
66 * |
10 |
81 |
0.07 |
0.0024 |
3.4 |
168 |
| |
♀♀ |
W/o irradiation |
48.0 |
45.0 ± 1.0 |
56 |
6 |
64 |
0.11 |
0.0004 |
3.2 |
138 |
| |
|
40 cGy * |
51.0 ** |
48.8 ± 0.6 |
57 |
9 |
71 |
0.15 |
0.0001 |
3.0 |
147 |
| |
|
30 Gy ** |
46.0 |
45.8 ± 1.1 |
56 |
5 |
73 |
0.11 |
0.0005 |
3.6 |
179 |
|
UAS-D-GADD45/ GAL4–1407 |
♂♂ |
W/o irradiation |
76.0 |
74.2 ± 1.4 |
89 |
34 |
98 |
0.09 |
0.0001 |
3.5 |
186 |
| |
|
40 cGy |
65.0 ** |
66.7 ± 1.7 |
85 ** |
4 |
98 |
0.07 |
0.0003 |
3.6 |
187 |
| |
|
30 Gy ** |
73.0 |
67.1 ± 2.3 |
87 |
3 |
95 |
0.07 |
0.0003 |
3.9 |
178 |
| |
♀♀ |
W/o irradiation |
70.0 |
67.0 ± 1.1 |
84 |
15 |
98 |
0.08 |
0.0003 |
3.5 |
173 |
| |
|
40 cGy * |
79.0 * |
74.8 ± 1.1 |
85 |
4 |
90 |
0.12 |
0.0000 |
3.7 |
142 |
| 30 Gy * | 74.0 ** | 66.8 ± 1.9 | 84 | 3 | 95 | 0.07 | 0.0003 | 4.1 | 158 |
M, median lifespan; ± Δm, mean lifespan and standard error of the mean; 90%, age of 90% mortality; Min and Max, minimum and maximum lifespan; α and R0, Gompertz equation parameters; MRDT, mortality rate doubling time (ln2/α); N, number of individuals in the population; * - p < 0.001, ** - p < 0.05 (treatment, Kolmogorov-Smirnov test; median lifespan, Gehan-Breslow-Wilcoxon and Mantel-Cox tests; age of 90% mortality, Wang-Allison test).
Recent experiments demonstrated a lifespan extension of 22–77% in males and females with constitutive overexpression of D-GADD45 in the nervous system compared with flies of the parental strains UAS-D-GADD45 and GAL4–1407. It is important to mention that in conditions of chronic 40 cGy and acute 30 Gy gamma-irradiation doses, the lifespan of males and females with D-GADD45 overexpression remained 20–40% higher compared with the lifespan of the parental strain flies (p < 0.001) (Fig. 5 and Table 2). Consequently, the overexpression of the D-GADD45 in the nervous system increases the lifespan of Drosophila flies under standard conditions as well as under gamma-irradiation conditions.
Figure 5. Survival of flies with D-GADD45 overexpression and with parental genotypes under different irradiation conditions. (A–C) Survival curves of Drosophila males and females with constitutive D-GADD45 overexpression in the nervous system, and with the UAS-D-GADD45 and GAL4–1407 parental genotypes under standard conditions (A), and chronic 40 cGy (B) and acute 30 Gy gamma-irradiation (C). Significant differences in survival are marked by * - p < 0.001, ** - p < 0.05 (Kolmogorov-Smirnov test).
Аge-dependent dynamics of spontaneous activity of the D-GADD45 gene
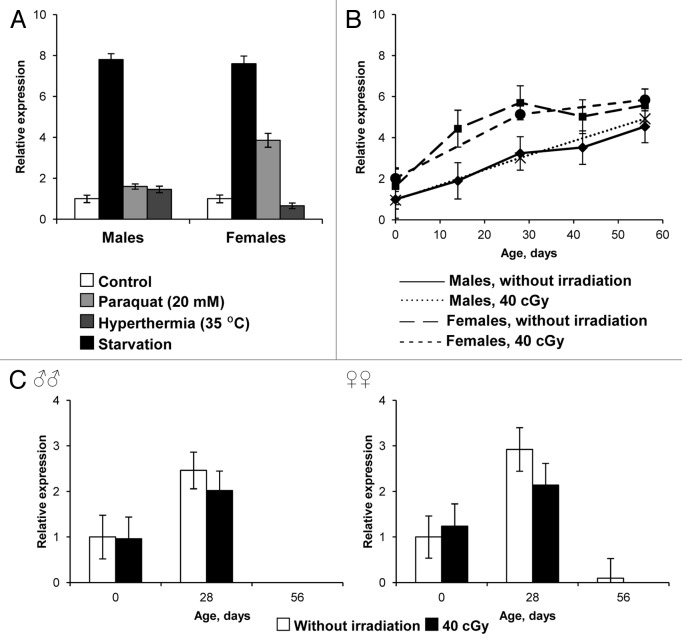
We also observed the age-dependent dynamics of spontaneous activity of D-GADD45 within the whole body and nervous system of adult flies. During aging, flies of the wild-type Canton-S strain experienced a widespread increase in D-GADD45 gene expression of 4.9–5.6 times homeostatic levels (Fig. 6B). At the same time, during evaluation of D-GADD45 gene expression, an expression level increase of 2.4–3.3 times homeostatic levels up to the second week of life was noted, which was then followed by a reduction in expression to levels lower than those seen in the first days following adult emergence (Fig. 6C).
Figure 6. The influence of stress factors on the expression levels of the D-GADD45 gene. (A) Expression levels in wild-type Canton-S flies after oxidative stress (20 mM paraquat), hyperthermia (35°C) and starvation. (B) The age-dependent changes in ubiquitous D-GADD45 gene expression of wild-type Canton-S flies. (C) The age-dependent changes in D-GADD45 gene expression in the nervous system of the wild-type Canton-S males and females.
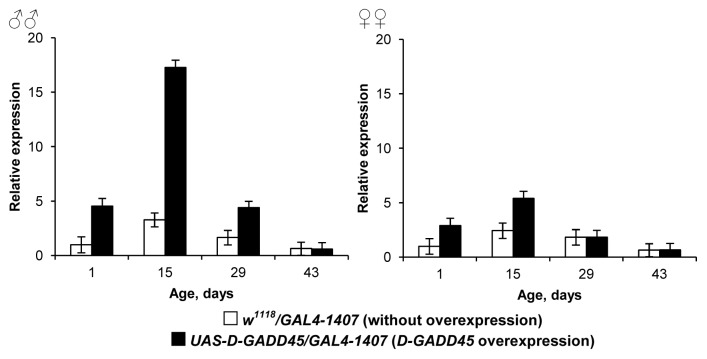
Moreover, flies with constitutive D-GADD45 overexpression in the nervous system showed a reduction in the level of increased transcriptional activity of the transgene. Between 0–5 d of age, Drosophila with the parental genotypes UAS-D-GADD45/GAL4–1407 showed increased levels of D-GADD45: expression in males was higher by 4.5–5.3 times, and in females by 2.2–2.9 times, levels in flies without the transgene. However, by age 29 d, levels had decreased in transgenic males to only 2.7 times higher than that of flies without the transgene, while in females there was no statistically significant difference between transgenic and non-transgenic flies (Fig. 7).
Figure 7. The age-dependent changes in D-GADD45 expression in flies with constitutive D-GADD45 gene overexpression in the nervous system.
The influence of oxidative stress, genotoxic stress, hyperthermia and starvation on the D-GADD45 gene expression
To understand the mechanisms involved in D-GADD45-mediated stress resistance in Drosophila, we studied the way in which the expression of the gene changes in response to various stress factors. The 5-d-old wild-type Canton-S flies were exposed to paraquat, hyperthermia and starvation for 24 h followed by quantitative real-time RT-PCR and the calculation of the relative levels of D-GADD45 expression.
Treatment with 20 mM paraquat enhanced the D-GADD45 expression by 7.6–7.8-fold both in Drosophila males and in females. The lower 1.6–3.9-fold increase was detected after 35°С hyperthermia. However, starvation increased the expression level of the D-GADD45 gene 1.5-fold in males only. In females, the transcriptional activity of investigated gene was 1.5 times lower in starved flies compared with flies that lived in standard conditions (Fig. 6A).
Age-dependent dynamics of D-GADD45 gene expression were observed in Drosophila adults of the wild-type Canton-S strain, within a standard environment and after chronic ionizing irradiation (genotoxic stress) in the pre-imago developmental stages at levels of 40 cGy. We described that ionizing irradiation at this dosage does not affect the expression of D-GADD45, neither in the nervous system nor in the whole body, throughout the life of adults (Fig. 6B and C).
Stress-induced D-GADD45 gene expression and the induction of D-GADD45-dGFP
The expression of D-GADD45 in vivo was evaluated using the reporter construct D-GADD45-dGFP. Inflammation arose as a response to damage to the chitinous exoskeleton after 1 h (data not shown). This induced the expression of D-GADD45-dGFP in both males and females. However, acute gamma-irradiation exposure (at a dosage of 30 Gy) and chronic (40 cGy) gamma-irradiation exposure, as well as oxidative stress (20 mM paraquat), thermal stress (35°С), dehydration and starvation, did not lead to any changes in the activity of GADD45 protein, as measured by the reporter construct D-GADD45-dGFP (data not shown).
Discussion
GADD45 is a key regulator of stress resistance in mammals and acts in the pathogenesis of many age-dependent diseases, including tumors, atherosclerosis and neurodegeneration.1 Previously, we demonstrated that the overexpression of the Drosophila ortholog to GADD45, D-GADD45, in both male and female flies, caused an increase in their median and maximum lifespan.6 At the same time, no deterioration in fertility or neuro-muscular activity was detected.6 The spontaneous level of damaged DNA measured within larval neuroblasts of the third generation that had D-GADD45 overexpression was reduced.6 Thus, the overexpression of D-GADD45 is associated with an increase in efficiency DNA damage detection and repair.
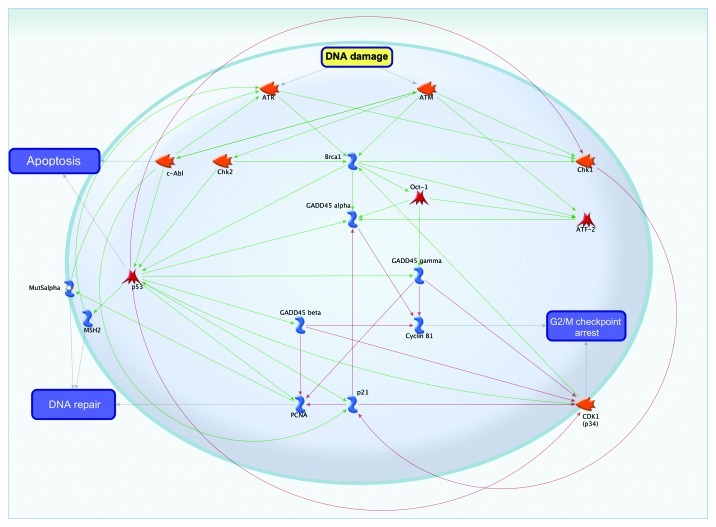
The role of GADD45 in signal transduction pathways of DNA damage and repair is generalized in Figure 8. Gadd45 proteins function in DNA damage, and repair is mediated by a complex interplay of physical interactions with other cellular proteins (Fig. 8). These include PCNA, ATM, ATR and BRCA1.3,23,24 In response to DNA damage, GADD45 may be activated by ATF-2,25 Oct-126 and p5327 transcription factors. Additionally, the DNA repair process can be activated through intermediaries such as MutSα28 or through the transcription factor p53 and MSH-family proteins.29 The binding of GADDβ with the kinase CDK1 (p34)30 or with cyclin B1 results in G2/M checkpoint arrest.
Figure 8. The role of GADD45 in DNA repair.
Besides, it is found that under certain conditions, p53 suppress senescence.31,32 While arresting cell cycle, p53 may simultaneously suppress the senescence program, thus causing quiescence. p53 suppresses senescence presumably by inhibition of mTOR (mammalian target of rapamycin).33,34 Senescence occurs when p53 fails to inhibit mTOR. Low concentrations of DNA-damaging drugs induce p53 at levels that do not inhibit mTOR, thus causing senescence.33,34 In this context, the p53-dependent activation of GADD45 may complete the anti-aging and stress-resistance effects of p53.
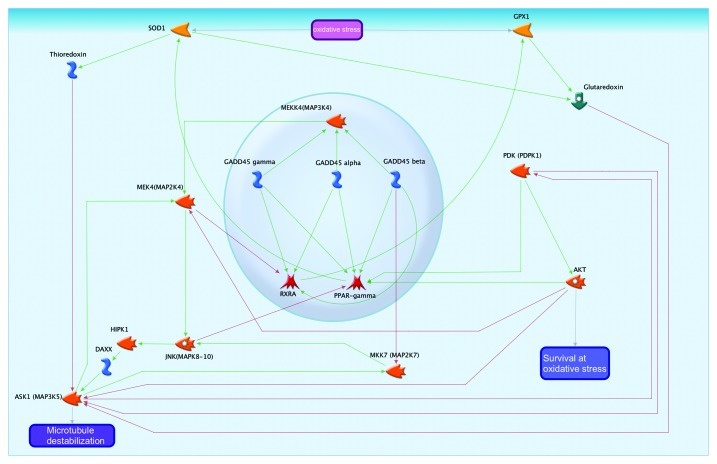
At an organismal level, one possible mechanism for the increase in longevity associated with D-GADD45 overexpression is an increased resistance to oxidative stress. The involvement of GADD45 in oxidative stress response is summarized in Figure 9. The expression levels of antioxidant enzymes SOD1 and GPX1 are regulated by the transcription factors PPARγ35 and RXRA,36 respectively. All three forms of the protein GADD45 have a positive effect on these transcription factors by increasing their transcription levels. Downstream of the oxidative pathway, SOD1 activates thioredoxin and glutaredoxin, redox enzymes that inhibit ASK1,37 a protein that leads to the dissociation of microtubules. Thus, the activity of GADD45 family proteins prevents cellular damage.
Figure 9. GADD45 in the oxidative stress pathway.
GADD45β acts through the protein kinases MEKK4 and JNK.38,39 Activation of these cascades increases levels of the protein ASK1,40 which acts in opposition to the protein SOD1. Moreover, JAK and MEK kinase cascades inhibit the activity of the transcription factors PPAR and RXRA,41,42 thus reducing the level of SOD1 and GPX1 expression. The overexpression of SOD143 and GPX144 increases the resistance of cells to oxidative stress in transgenic mouse models. However, the equilibrium of SOD1 and GPX1 activity is also critical for the cell fate. On the one hand, too little SOD relative to GPX could lead to an accumulation of O2–, which is toxic to macromolecules; on the other hand, too much SOD relative to GPX could lead to increased production of the H2O2 intermediate.45 Thus, in the process of oxidative stress response, GADD45 family proteins are involved in the control of the activity and maintenance of the balance between antioxidant enzymes and can both positively and negatively affect the fate of cells. It can also be noted that the ASK1 protein has a mutually inhibitory relationship with the kinase PDK,46 which inhibits AKT47 and thereby reduces the chances of cell survival in response to oxidative stress.
Paraquat (N,N′-dimethyl-4,4′-bipyridinium dichloride, C12H14N2) is a molecular compound that causes the formation of free radicals within the cell. By receiving an electron from cellular redox enzymes, paraquat interacts with molecular oxygen, forming the superoxide free radical О2-.48 It has been previously established that in adult Drosophila, treatment with paraquat leads to a decrease in longevity. At the same time, selection for longevity increases resistance to paraquat.11 In our experiment, adult mortality under the effects of paraquat was significantly lower during constitutive and conditional overexpression of D-GADD45 in the nervous system in males as well as females. Moreover, we observed the 7.6–7.8-fold increase in expression level of the D-GADD45 gene after treatment of wild-type flies with paraquat. These results indicate the involvement of GADD45 in oxidative stress response and resistance. GADD45 family proteins in mammals can increase resistance to oxidative stress through the activation of nucleotide and base excision repair mechanisms (for example, by binding to PCNA, XPC and XPG)3,49,50 (Fig. 8) or through JNK-dependent longevity that is activated in response to an increased level of pro-oxidants (Fig. 9).51
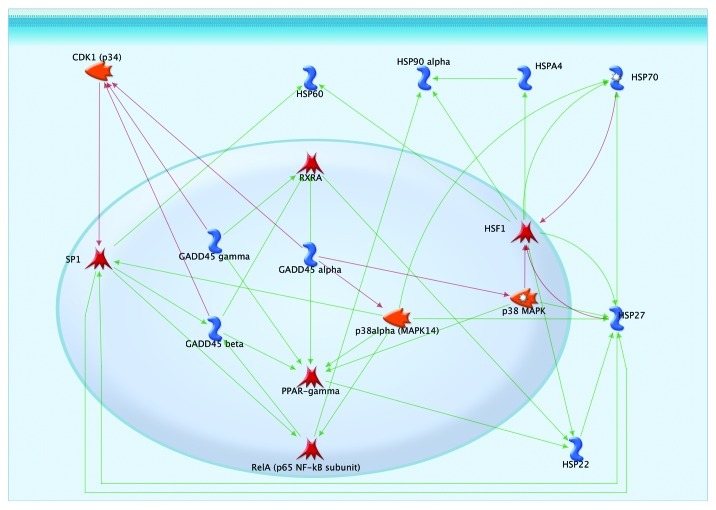
The negative effect of thermal stress factors was reduced when D-GADD45 was overexpressed in the nervous system. Heat shock causes damage within the tertiary structure of proteins, negatively impacts their aggregation and causes endoplasmic stress.17 GADD45 proteins affect the activity of heat shock proteins in several ways (Fig. 10). All three orthologs of GADD45 inhibit the protein kinase CDK1, which phosphorylates the transcription factor SP1, thereby inhibiting its activity.52 The tanscription factor SP1 activates the expression of heat shock protein HSP27;53 thus, GADD45 proteins increase the activity of HSP27 by preventing its inactivation. In turn, this increases the activity of another heat shock protein, HSP70.54 SP1 also increases the expression of another heat shock protein, HSP60.55
Figure 10. Involvement of GADD45 in heat shock response.
The GADD45 family of proteins also activates the transcription factor PPAR-γ, which increases the expression of the heat shock protein HSP22,35 which can also be activated by the upregulation of RXRA by GADD45 proteins. HSP22 is responsible for activating another heat shock protein, HSP27.56 Thus, when activated, members of the GADD45 family are able to trigger a cascade of effects on heat shock proteins.
GADD45, by inhibiting the kinase p38MAPK, activates the transcription factor HSF1,57 which is a cornerstone element for pathways activating heat shock proteins, such as HSP60, HSP90, HSPA4, HSP70, HSP27 and HSP22 (Fig. 10). D-GADD45 overexpression through MAPKs could cause an increase in HSF-1 and heat shock protein activity, contributing to an organism's resistance to stressors. In addition, due to the intensification of metabolism, heat shock results in the appearance of additional reactive oxygen.58 In our experiment, the D-GADD45 expression increased 1.6–3.9-fold at 35°C, confirming its role in thermal stress response.
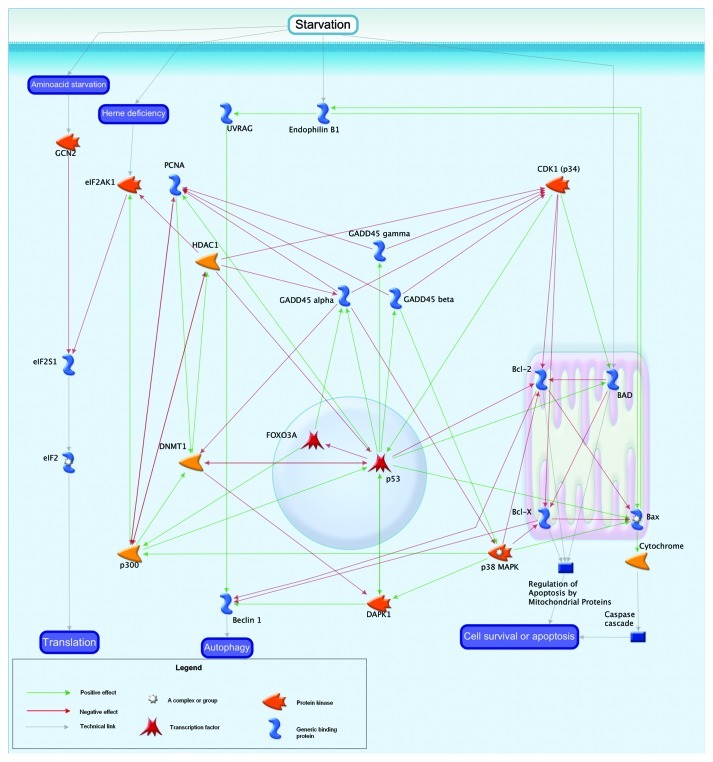
In our experiments, increased resistance to starvation was demonstrated in males with D-GADD45 overexpression in the nervous system, compared with flies without overexpression. Starved wild-type males but not females demonstrated a slight increase (1.5-fold) in GADD45 expression. Recent studies demonstrated that GADD45 is a critical protein in the starvation-induced pathway that reprograms muscle gene expression to cause skeletal muscle atrophy.59 Gadd45α represses genes involved in anabolic signaling and energy production, and it induces pro-atrophy genes. As a result, Gadd45α reduces multiple barriers to muscle atrophy (including PGC-1α, Akt activity, and protein synthesis) and stimulates pro-atrophy mechanisms (including autophagy and caspase-mediated proteolysis).59 GADD45’s implication in the response to starvation is shown in Figure 11. GADD45α activity is redundant with respect to β and γ forms. However, the α form of GADD45 inhibits p38MAPK, and the β form activates it. Starvation results in the oppression of amino acid composition and affects protein levels of endophilin B1 and BAD. The reduction in amino acids begins with the protein GCN2, which inhibits the elF family proteins that regulate translation in the cell.60 GADD45 affects this process through the intermediaries PCNA61 and HDAC1,62 which have negative effects on the entire family of elF proteins63 through the p300 protein. Thus, GADD45 protein can inhibit the activity of translation during starvation. Proteins such as DMNT1 and p38MAPK, are also involved in this process as additional mediators of regulation of translation by GADD45 protein.
Figure 11. Starvation and GADD45.
The starvation response is also associated with mitochondrial proteins; in particular, Bcl 2, BAD and Blx-x. Apoptosis is activated during starvation by these proteins. GADD45 family of proteins can affect the activity of mitochondrial apoptotic proteins through the intermediaries Cdk1 (p34)30 and p38MAPK,17 as well as the transcription factor p53. In this way, the pathway influences the susceptibility of cells to starvation and, consequently, the fate of cells.
Starvation can also lead to autophagy, mediated by the proteins UVRAG and Beclin 1.64 In turn, the Beclin 1 is affected by mitochondrial proteins65 as well as the protein DAPK1.66 DAPK1, through intermediaries such as p38MAPK and DMNT1,67 is directly regulated by the GADD45 family. In addition, excessive reduction in food intake causes oxidative stress and leads to a decrease in the efficacy of DNA repair mechanisms.68 It is possible that D-GADD45 overexpression contributes to a reduction in the negative effects of a given stress factor and contributes to survival of the organism by modulating cellular metabolism (Fig. 11) and DNA repair (Fig. 8).
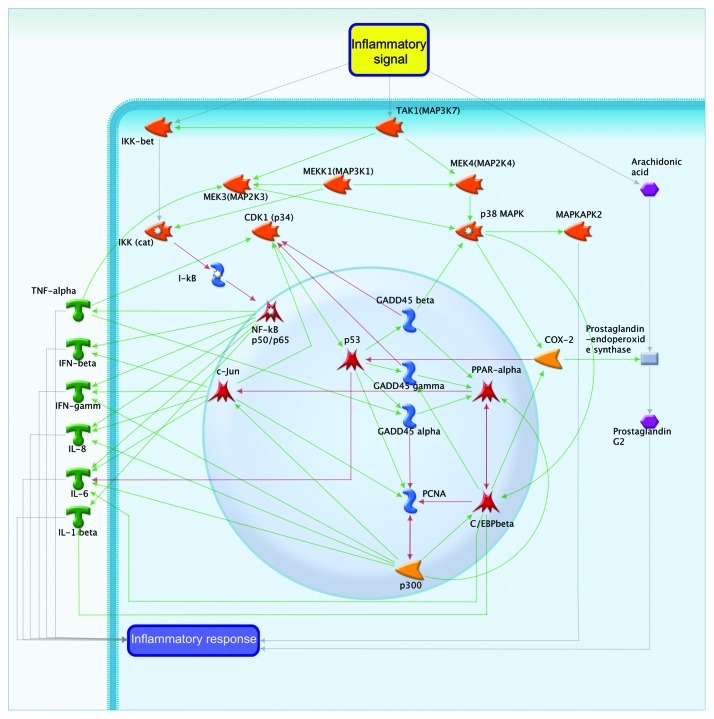
The inflammation is another stress factor that increases not only the level of D-GADD45 mRNA5 but also protein (our data). The implication of D-GADD45 in inflammation is shown in Figure 12. The inflammatory process is thought to be mediated through three major pahways: the IKK-NF pathway, the arachidonic acid and prostaglandin pathway and the MEK-MAPK pathway. The main function of GADD45 in the inflammatory processes is determined by its interactions with other proteins, such as p38MAPK,69 p34CDK30 and PCNA.3 The interaction of GADD45 with the transcription factors PPAR, C/EBPβ and c-Jun can also trigger activation of the inflammatory response.
Figure 12. Inflammation and GADD45.
The GADD45 family of proteins affects the transcription of IFN gamma by interacting with PCNA-p300 and some representatives of the IL (receptor)70 family. Mediation through this pathway adjusts the force of the inflammatory process that is distributed along the IKK-NF pathway. Regulation of this pathway can occur indirectly by transcription factors such as C/EBPβ,71 PPARα72 and c-Jun.73 The inflammatory pathway first involves the activation of PPARα,72 which activates the downstream transcription factors C/EBPβ and c-Jun.
GADD45 proteins can also act through a prostaglandin-endoperoxid synthase COX-2 (PRGS2). COX-2 converts arachidonic acid into prostaglandin endoperoxide. GADD45 family proteins affect COX-2 (PTGS2) through p38MAPK,74 or indirectly through the transcription factors PPARα and C/EBPβ.75
We demonstrated that flies with constitutive and conditional D-GADD45 gene overexpression in the nervous system were more resistant to the actions of stress factors (such as paraquat, hyperthermia and starvation) than flies without overexpression, and the wild-type strain showed increased expression of D-GADD45. This does not confirm the previous reports that demonstrated that D-GADD45 gene expression in Drosophila is only induced during inflammation in response to microbial exposure and wounds, but not to paraquat, arsenite, UV radiation and X-rays.5
Ionizing irradiation is genotoxic and leads to oxidative stress.76 In the wild-type strain Canton-S, hormesis was observed during chronic exposure of low doses of gamma-irradiation, as indicated by an increase in median lifespan of irradiated flies. In addition, there was a change in radioadaptive response: following acute exposure at 30 Gy after chronic pre-irradiation at 40 cGy, exposed flies showed a 1.6- to 2.3-fold reduction in the damaging effect of irradiation. In contrast, in the homo- and heterozygous D-GADD45 mutants, chronic irradiation caused a decrease in lifespan, while pre-irradiation did not cause radioadaptation to the subsequent acute irradiation. Therefore, D-GADD45 appears to play a role in the adaptive response to ionized irradiation. At the same time, chronic irradiation at a dose rate of 40 cGy did not induce D-GADD45 expression, which is congruent with reports in the literature.5
Despite the fact that acute exposure at a high dose rate of 30 Gy caused a reduction in lifespan of flies with constitutive D-GADD45 overexpression, the lifespan of irradiated flies remained higher overall compared with the lifespan of non-irradiated flies (without overexpressed D-GADD45). Consequently, D-GADD45 gene expression in the nervous system prolongs lifespan in Drosophila both in the control environment and after gamma-irradiation.
It is known that with aging there is a change in the level of the expression of stress-resistance genes. The expression of one type of gene reduces the response to the accumulation of damage (for example, DNA-damage response and DNA repair genes),19 while, at the same time, the expression of other genes compensatively increases this response (for example, heat shock response genes, oxidative stress inducible genes and DNA damage-inducible genes).19,77 Previous studies demonstrated that aging negatively affects the ability of cells to express GADD45 proteins in response to various stress factors. For example, exposure of cardiomyocytes of young mice to paraquat led to the increased expression of GADD45 proteins, but did not lead to the increased expression in the myocardium of old animals.78 Our data shows that, despite the fact that the overall spontaneous activity of the D-GADD45 gene increases with age (Fig. 6B), the level of expression of this gene in the nervous system is practically eliminated (Figs. 6C and 7). As the overexpression of D-GADD45 within the nervous system increases lifespan and increases the resistance to various forms of stress, the dramatic decrease of its activity within the nervous system with age might be one of the reasons for the decrease in the organism's stress resistance. The demonstrated reduction in mRNA levels of D-GADD45 with age and during artificial, constitutive overexpression in the nervous system may indicate an epigenetic cause of the low level of activity of this gene in old flies.
Thus, we have demonstrated an interconnection between the induction of D-GADD45 and resistance to oxidative (paraquat), genotoxic (gamma-irradiation) and heat stress (35°C), as well as to starvation in Drosophila. At the same time, we demonstrated that most stress factors induce the mRNA expression of D-GADD45, and that the effect of an increase in stress resistance in Drosophila is associated with the D-GADD45 gene activity.
There are some possible mechanisms by which GADD45 may play a role in stress resistance. Research performed on mammals demonstrates that GADD45 family proteins take part in protein-protein interactions, along with cell cycle and DNA replication regulators, mitogen-activated protein kinases and nuclear hormone receptors.1 GADD45a participates in demethylation of CpG in DNA, and is associated with the nucleosome.79 Finally, GADD45 binds RNA but not single- or double-stranded DNA or methylated DNA in vitro.80 GADD45a also displays RNase-sensitive co-localization in nuclear speckles with the RNA helicase p68 and the RNA-binding protein SC35.80
Materials and Methods
Drosophila melanogaster strains
The following laboratory strains of Drosophila melanogaster were used: Canton-S and w1118 as a wild-type strains; D-GADD45/D-GADD45 [homozygous flies with y* w*; P(GawB)Gadd45NP0351 genotype] and D-GADD45/+ [heterozygous flies with y* w*; P(GawB)Gadd45NP0351/CyO, P(UAS-lacZ.UW14)UW14 genotype] with P transposable element insertion site in the coding region of D-GADD45 (provided by Kyoto Stock Center); UAS-D-GADD45, containing an additional copy of the D-GADD45 gene controlled by the UAS promoter (a kind gift from Dr. Uri Abdu, Ben-Gurion University); GAL4–1407 [genotype: w*; P(GawB)1407], carrying the constitutively expressed GAL4 promoter in the nervous system (kindly provided by the Bloomington Stock Center); ELAV [genotype: y w; P(ELAV-GeneSwitch)] with the mifepristone-inducible GAL4 driver in the nervous system (kindly granted by Dr. Haig Keshishian, Yale University).
Overexpression of the D-GADD45 gene
For constitutive overexpression of D-GADD45 in the Drosophila nervous system, UAS-D-GADD45 females were crossed with GAL4–1407 males. Parental strain individuals were used as controls.
For conditional overexpression of D-GADD45 in the Drosophila nervous system, UAS-D-GADD45 females were crossed with ELAV males. The offspring were kept on a medium with mifepristone (a conditional inducer of GAL4 expression). It is known that mifepristone on its own does not affect the lifespan of Drosophila.81 Therefore, we can infer that responses observed in the experimental animals were caused by D-GADD45 overexpression and/or genetic background. Flies obtained by crossing UAS-D-GADD45 females with ELAV males and kept on a medium without mifepristone were used as a control when examining conditional D-GADD45 gene overexpression.
Mifepristone treatment
For mifepristone treatment, a stock solution of RU486 (Mifepristone, Sigma) with a 25 mg/ml concentration in 100% ethanol was used. It was mixed with yeast paste (60.4% water, 39% active yeast, 0.5% acetic acid, 0.1% ethanol) in a proportion of 0.1 ml initial RU486 solution per 100 ml of yeast paste. 0.3 ml of prepared yeast paste was then added to the surface of the medium. For experiments without mifepristone, the medium surface was covered with yeast paste that was prepared in the same way, but with an equivalent amount of ethyl alcohol added in place of the RU486 solution.
Drosophila maintenance
Flies were kept under standard conditions at 25°C in a 12:12 h light-dark regime, on an agar/semolina/sugar/yeast medium (but see above for mifepristone treatment).82 Twenty-five pairs of parents, with synchronized 24 h egg laying, were used to obtain experimental flies. The flies were extracted from vials immediately after imago eclosion.
Irradiation conditions
Flies were subjected to one of four different irradiation conditions: (1) no irradiation; (2) chronic exposure to 40 сGy of ionizing radiation from a gamma-source (Ra226) at the preimaginal stages of development. Treatment was conducted for 10 consecutive days per generation; (3) acute exposure to a gamma-source (Со60) for a period of 30 min, at a dosage of 30 Gy immediately after adults emerged; (4) flies that were already exposed to ionizing radiation earlier, at both doses, were re-exposed to determine if earlier exposure had preadapted individuals for subsequent exposure.
Quantitative real-time RT-PCR
Gene expression levels were tested using quantitative real-time RT-PCR (qRT-PCR), and evaluated in males and females separately. Ten whole flies (for analysis of ubiquitous expression level) or 30 heads (for analysis of expression level in the Drosophila nervous system) removed from their bodies, were placed in TRIzol Reagent (Invitrogen) and homogenized using a SilentCrusher S homogenizer (Heidolph). RNA was then extracted according to the manufacturer’s instructions. RNA was separated using phenol-clorophorm technique, followed precipitation with isopropyl alcohol. cDNA synthesis was performed with an Oligo(dT) primer (Invitrogen) and SuperScript III First-Stand cDNA Synthesis reverse transcriptase (Invitrogen) according to the manufacturer’s protocol.
In order to test the background level of DNA contamination in RNA samples, a separate transcription reaction without enzyme was performed. PCR was performed using β-tubulin primers (forward primer: 5′-GCAACTCCACTGCCATCC-3′; reverse primer: 5′-CCTGCTCCTCCTCGAACT-3′).
Real-time PCR was performed in a 30-μl volume, in 200-μl PCR tubes, according to the manufacturer’s instructions, using a reaction mixture containing SYBR Green PCR Master Mix dye (Applied Biosystems) and the following primers (Syntol, Russia): D-GADD45 (forward primer: 5′-GCAAACGCACAACCAAAC-3′; reverse primer: 5′-GGCCATCAGGCAGAAGAG-3′), β-tubulin primers (forward primer: 5′-GCAACTCCACTGCCATCC-3′; reverse primer: 5′-CCTGCTCCTCCTCGAACT-3′). qRT-PCR was performed in a CFX96 amplifier (BioRad) using the following program: (1) denaturing at 95°C for 10 min, (2) denaturing at 95°C for 15 sec, (3) annealing at 60°C for 30 sec, (4) elongation at 60°C for 30 sec, (5) stages 2–4 were repeated 50 times. D-GADD45 expression was normalized against the β-tubulin housekeeping gene. Amplification of D-GADD45 and β-tubulin was performed in individual tubes. Four to eight measurements were performed for each experiment. The relative level of D-GADD45 gene expression was calculated using CFX96 software (BioRad). The relative level of D-GADD45 expression was calculated by the 2-∆∆Ct method83 using cycle threshold (Ct). The ∆∆Ct value was calculated as ∆Ct(test sample) - ∆Ct(control sample), and each value of ∆Ct = Ct(D-GADD45) - Ct(β-Tubulin).
Lifespan assay
To estimate longevity, 150–250 flies were collected (approximately 30 adult flies per 120 ml vial) for each experiment. Males and non-virgin females were kept separately. Flies were transferred to fresh medium twice weekly. Dead flies were counted and removed daily.
Mortality data was used to plot survival curves and to calculate the mean, median, minimum and maximum lifespan, as well as the age of 90% mortality. In order to estimate the significant statistical differences between experimental and control groups, nonparametric methods were used. The Kolmogorov-Smirnov test was used for the comparison of survival functions between sample groups,84 and the Gehan-Breslow-Wilcoxon85 and Mantel-Cox tests86 were used for the comparison of median lifespan values. The significance of differences in maximum lifespan between groups was evaluated using the Wang-Allison test.87 For this test, each animal in each experiment was categorized into one of two groups: either lifespan above the 90th percentile or lifespan below the 90th percentile. A two-by-two contingency table was used to record data. An ordinary χ2-test was used for independent testing of two groups. In addition, the α and R0 parameters of the Gompertz equation [l(x) = R0 eαx] and the mortality rate doubling time [MRDT = ln(2)/α] were calculated, and the trajectories of the logarithmic mortality rate were plotted. The significance of differences in the age-dependent mortality rate and initial mortality rate (parameters α and R0 in the Gompertz equation) were evaluated using the maximum likelihood method.88 Statistical analyses was performed using Statistica version 6.1 (StatSoft, Inc.) and WinModest version 1.0.288 software.
Analysis of stress resistance
To evaluate stress resistance, 200–300 flies were selected (30 flies per vial) for each experiment. Males and females were examined separately. After 2–3 d, individuals were exposed to conditions of intense stress. To evaluate oxidative stress resistance, flies were starved for 2 h and then moved into vials where a filter paper had been soaked in a 20 mM paraquat (Methyl Viologen, Sigma) solution in 5% sucrose (1 ml), instead of the animals’ normal nutrient environment. To evaluate hyperthermia resistance, flies were placed at a temperature of 35°С in vials containing the standard agrarian-yeast nutrient environment. To estimate starvation resistance, Drosophila individuals were kept in vials where a water-soaked filter paper was used instead of the nutrient environment. Dead flies were counted twice daily. Survival curves were constructed and the percentages of dead flies after 48 h of exposure to specific stressors was calculated. To evaluate the difference between samples, the Fisher φ-test (for the comparison of percentage of dead flies after 48 h)89 and the Kolmogorov-Smirnov test (to compare the distribution of mortality within the samples) were used.84
D-GADD45 in vivo reporter
We used a 2-kb fragment upstream of the start site of the D-GADD45 coding region to drive expression of the destabilized form of GFP (dGFP) in vivo. dGFP is only stable for 8 h and is therefore a suitable temporal marker of transcriptional activity.90 To obtain transgenic flies containing a dGFP reporter of D-GADD45, 2 kb of DNA 5′ of D-GADD45 was cloned. This region included the ATG initiation codon located upstream of the D-GADD45 gene. dGFP was followed with the SV40 transcriptional stop signal commonly used in most fly vectors. This reporter construct was cloned into an attB vector for site-specific integration into the second chromosome. Cloning, site-specific integration, balancing and embryo injections were performed by GenetiVision. The resulting Drosophila strain with the D-GADD45 in vivo reporter was called D-GADD45-dGFP.
Acknowledgments
This work was supported by the Presidium of the Russian Academy of Science, grant number 12-C-4–1007, and by the Russian Foundation for Basic Research, grant N 12-04-32261.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/22545
References
- 1.Moskalev AA, Smit-McBride Z, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, et al. Gadd45 proteins: relevance to aging, longevity and age-related pathologies. Ageing Res Rev. 2012;11:51–66. doi: 10.1016/j.arr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan J, Duan J, Zhang Z, Tong T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol. 2005;37:1407–20. doi: 10.1016/j.biocel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, et al. Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266:1376–80. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 4.Lee B, Morano A, Porcellini A, Muller MT. GADD45α inhibition of DNMT1 dependent DNA methylation during homology directed DNA repair. Nucleic Acids Res. 2012;40:2481–93. doi: 10.1093/nar/gkr1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peretz G, Bakhrat A, Abdu U. Expression of the Drosophila melanogaster GADD45 homolog (CG11086) affects egg asymmetric development that is mediated by the c-Jun N-terminal kinase pathway. Genetics. 2007;177:1691–702. doi: 10.1534/genetics.107.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plyusnina EN, Shaposhnikov MV, Moskalev AA. Increase of Drosophila melanogaster lifespan due to D-GADD45 overexpression in the nervous system. Biogerontology. 2011;12:211–26. doi: 10.1007/s10522-010-9311-6. [DOI] [PubMed] [Google Scholar]
- 7.Semenchenko GV, Khazaeli AA, Curtsinger JW, Yashin AI. Stress resistance declines with age: analysis of data from a survival experiment with Drosophila melanogaster. Biogerontology. 2004;5:17–30. doi: 10.1023/b:bgen.0000017681.46326.9e. [DOI] [PubMed] [Google Scholar]
- 8.Giannakou ME, Goss M, Jünger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- 9.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–30. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TE, de Castro E, Hegi de Castro S, Cypser J, Henderson S, Tedesco P. Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp Gerontol. 2001;36:1609–17. doi: 10.1016/s0531-5565(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen CJ, Van De Zande L, Bijlsma R. Resistance to oxidative stress induced by paraquat correlates well with both decreased and increased lifespan in Drosophila melanogaster. Biogerontology. 2005;6:387–95. doi: 10.1007/s10522-005-4903-2. [DOI] [PubMed] [Google Scholar]
- 12.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 13.Harshman LG, Moore KM, Sty MA, Magwire MM. Stress resistance and longevity in selected lines of Drosophila melanogaster. Neurobiol Aging. 1999;20:521–9. doi: 10.1016/s0197-4580(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 14.Salmon AB, Leonard S, Masamsetti V, Pierce A, Podlutsky AJ, Podlutskaya N, et al. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 2009;23:2317–26. doi: 10.1096/fj.08-122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis KN, Mele J, Hornsby PJ, Buffenstein R. Stress resistance in the naked mole-rat: the bare essentials - a mini-review. Gerontology. 2012;58:453–62. doi: 10.1159/000335966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajpathak SN, Liu Y, Ben-David O, Reddy S, Atzmon G, Crandall J, et al. Lifestyle factors of people with exceptional longevity. J Am Geriatr Soc. 2011;59:1509–12. doi: 10.1111/j.1532-5415.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rattan SI. Hormetic modulation of aging and longevity by mild heat stress. Dose Response. 2005;3:533–46. doi: 10.2203/dose-response.003.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arking R, Burde V, Graves K, Hari R, Feldman E, Zeevi A, et al. Forward and reverse selection for longevity in Drosophila is characterized by alteration of antioxidant gene expression and oxidative damage patterns. Exp Gerontol. 2000;35:167–85. doi: 10.1016/s0531-5565(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 19.Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, et al. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev. 2012 doi: 10.1016/j.arr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Sun H, Lu J, Li X, Chen X, Tao D, et al. Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J Exp Biol. 2005;208:697–705. doi: 10.1242/jeb.01439. [DOI] [PubMed] [Google Scholar]
- 21.Amrit FR, Boehnisch CM, May RC. Phenotypic covariance of longevity, immunity and stress resistance in the caenorhabditis nematodes. PLoS One. 2010;5:e9978. doi: 10.1371/journal.pone.0009978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskalev A. Radiation-induced life span alteration of Drosophila lines with genotype differences. Biogerontology. 2007;8:499–504. doi: 10.1007/s10522-007-9090-x. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes N, Sun Y, Chen S, Paul P, Shaw RJ, Cantley LC, et al. DNA damage-induced association of ATM with its target proteins requires a protein interaction domain in the N terminus of ATM. J Biol Chem. 2005;280:15158–64. doi: 10.1074/jbc.M412065200. [DOI] [PubMed] [Google Scholar]
- 24.Jang ER, Choi JD, Park MA, Jeong G, Cho H, Lee JS. ATM modulates transcription in response to histone deacetylase inhibition as part of its DNA damage response. Exp Mol Med. 2010;42:195–204. doi: 10.3858/emm.2010.42.3.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maekawa T, Sano Y, Shinagawa T, Rahman Z, Sakuma T, Nomura S, et al. ATF-2 controls transcription of Maspin and GADD45 α genes independently from p53 to suppress mammary tumors. Oncogene. 2008;27:1045–54. doi: 10.1038/sj.onc.1210727. [DOI] [PubMed] [Google Scholar]
- 26.Fan W, Jin S, Tong T, Zhao H, Fan F, Antinore MJ, et al. BRCA1 regulates GADD45 through its interactions with the OCT-1 and CAAT motifs. J Biol Chem. 2002;277:8061–7. doi: 10.1074/jbc.M110225200. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Liu G, Zhu J, Jiang J, Nozell S, Willis A. Isolation and characterization of fourteen novel putative and nine known target genes of the p53 family. Cancer Biol Ther. 2003;2:55–62. doi: 10.4161/cbt.180. [DOI] [PubMed] [Google Scholar]
- 28.Ohta S, Shiomi Y, Sugimoto K, Obuse C, Tsurimoto T. A proteomics approach to identify proliferating cell nuclear antigen (PCNA)-binding proteins in human cell lysates. Identification of the human CHL12/RFCs2-5 complex as a novel PCNA-binding protein. J Biol Chem. 2002;277:40362–7. doi: 10.1074/jbc.M206194200. [DOI] [PubMed] [Google Scholar]
- 29.Lyakhov IG, Krishnamachari A, Schneider TD. Discovery of novel tumor suppressor p53 response elements using information theory. Nucleic Acids Res. 2008;36:3828–33. doi: 10.1093/nar/gkn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Manicone A, Coursen JD, Linke SP, Nagashima M, Forgues M, et al. Identification of a functional domain in a GADD45-mediated G2/M checkpoint. J Biol Chem. 2000;275:36892–8. doi: 10.1074/jbc.M005319200. [DOI] [PubMed] [Google Scholar]
- 31.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci USA. 2010;107:9660–4. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poyurovsky MV, Prives C. P53 and aging: A fresh look at an old paradigm. Aging (Albany NY) 2010;2:380–2. doi: 10.18632/aging.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010;2:924–35. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2010;2:344–52. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamza MS, Pott S, Vega VB, Thomsen JS, Kandhadayar GS, Ng PW, et al. De-novo identification of PPARgamma/RXR binding sites and direct targets during adipogenesis. PLoS One. 2009;4:e4907. doi: 10.1371/journal.pone.0004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Zhang X, Bardag-Gorce F, Robel RC, Aguilo J, Chen L, et al. Retinoid X receptor alpha regulates glutathione homeostasis and xenobiotic detoxification processes in mouse liver. Mol Pharmacol. 2004;65:550–7. doi: 10.1124/mol.65.3.550. [DOI] [PubMed] [Google Scholar]
- 37.Lee YJ, Kim JH, Chen J, Song JJ. Enhancement of metabolic oxidative stress-induced cytotoxicity by the thioredoxin inhibitor 1-methylpropyl 2-imidazolyl disulfide is mediated through the ASK1-SEK1-JNK1 pathway. Mol Pharmacol. 2002;62:1409–17. doi: 10.1124/mol.62.6.1409. [DOI] [PubMed] [Google Scholar]
- 38.Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, et al. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004;6:146–53. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- 39.Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95:521–30. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- 40.Ko YG, Kang YS, Park H, Seol W, Kim J, Kim T, et al. Apoptosis signal-regulating kinase 1 controls the proapoptotic function of death-associated protein (Daxx) in the cytoplasm. J Biol Chem. 2001;276:39103–6. doi: 10.1074/jbc.M105928200. [DOI] [PubMed] [Google Scholar]
- 41.Gelman L, Michalik L, Desvergne B, Wahli W. Kinase signaling cascades that modulate peroxisome proliferator-activated receptors. Curr Opin Cell Biol. 2005;17:216–22. doi: 10.1016/j.ceb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Lee HY, Suh YA, Robinson MJ, Clifford JL, Hong WK, Woodgett JR, et al. Stress pathway activation induces phosphorylation of retinoid X receptor. J Biol Chem. 2000;275:32193–9. doi: 10.1074/jbc.M005490200. [DOI] [PubMed] [Google Scholar]
- 43.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991;88:11158–62. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss N, Zhang YY, Heydrick S, Bierl C, Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc Natl Acad Sci USA. 2001;98:12503–8. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Haan JB, Cristiano F, Iannello R, Bladier C, Kelner MJ, Kola I. Elevation in the ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum Mol Genet. 1996;5:283–92. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]
- 46.Seong HA, Jung H, Ichijo H, Ha H. Reciprocal negative regulation of PDK1 and ASK1 signaling by direct interaction and phosphorylation. J Biol Chem. 2010;285:2397–414. doi: 10.1074/jbc.M109.064295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–23. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le May N, Egly JM, Coin F. True lies: the double life of the nucleotide excision repair factors in transcription and DNA repair. J Nucleic Acids. 2010;2010 doi: 10.4061/2010/616342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma DK, Guo JU, Ming GL, Song H. DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle. 2009;8:1526–31. doi: 10.4161/cc.8.10.8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu N, Shao Y, Xu L, Yu L, Sun L. Gadd45-α and Gadd45-γ utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol Biol Rep. 2009;36:2075–85. doi: 10.1007/s11033-008-9419-9. [DOI] [PubMed] [Google Scholar]
- 52.Chuang JY, Wang SA, Yang WB, Yang HC, Hung CY, Su TP, et al. Sp1 phosphorylation by cyclin-dependent kinase 1/cyclin B1 represses its DNA-binding activity during mitosis in cancer cells. Oncogene. 2012 doi: 10.1038/onc.2011.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedman MJ, Li S, Li XJ. Activation of gene transcription by heat shock protein 27 may contribute to its neuronal protection. J Biol Chem. 2009;284:27944–51. doi: 10.1074/jbc.M109.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitlock NA, Lindsey K, Agarwal N, Crosson CE, Ma JX. Heat shock protein 27 delays Ca2+-induced cell death in a caspase-dependent and -independent manner in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:1085–91. doi: 10.1167/iovs.04-0042. [DOI] [PubMed] [Google Scholar]
- 55.Reed BD, Charos AE, Szekely AM, Weissman SM, Snyder M. Genome-wide occupancy of SREBP1 and its partners NFY and SP1 reveals novel functional roles and combinatorial regulation of distinct classes of genes. PLoS Genet. 2008;4:e1000133. doi: 10.1371/journal.pgen.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun X, Fontaine JM, Rest JS, Shelden EA, Welsh MJ, Benndorf R. Interaction of human HSP22 (HSPB8) with other small heat shock proteins. J Biol Chem. 2004;279:2394–402. doi: 10.1074/jbc.M311324200. [DOI] [PubMed] [Google Scholar]
- 57.Kim J, Nueda A, Meng YH, Dynan WS, Mivechi NF. Analysis of the phosphorylation of human heat shock transcription factor-1 by MAP kinase family members. J Cell Biochem. 1997;67:43–54. doi: 10.1002/(sici)1097-4644(19971001)67:1<43::aid-jcb5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 58.Kim IS, Moon HY, Yun HS, Jin I. Heat shock causes oxidative stress and induces a variety of cell rescue proteins in Saccharomyces cerevisiae KNU5377. J Microbiol. 2006;44:492–501. [PubMed] [Google Scholar]
- 59.Ebert SM, Dyle MC, Kunkel SD, Bullard SA, Bongers KS, Fox DK, et al. Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J Biol Chem. 2012;287:27290–301. doi: 10.1074/jbc.M112.374777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Haro C, Méndez R, Santoyo J. The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 1996;10:1378–87. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- 61.Hong R, Chakravarti D. The human proliferating Cell nuclear antigen regulates transcriptional coactivator p300 activity and promotes transcriptional repression. J Biol Chem. 2003;278:44505–13. doi: 10.1074/jbc.M303138200. [DOI] [PubMed] [Google Scholar]
- 62.Hua B, Tamamori-Adachi M, Luo Y, Tamura K, Morioka M, Fukuda M, et al. A splice variant of stress response gene ATF3 counteracts NF-kappaB-dependent anti-apoptosis through inhibiting recruitment of CREB-binding protein/p300 coactivator. J Biol Chem. 2006;281:1620–9. doi: 10.1074/jbc.M508471200. [DOI] [PubMed] [Google Scholar]
- 63.Singh VB, Pavithra L, Chattopadhyay S, Pal JK. Stress-induced overexpression of the heme-regulated eIF-2alpha kinase is regulated by Elk-1 activated through ERK pathway. Biochem Biophys Res Commun. 2009;379:710–5. doi: 10.1016/j.bbrc.2008.12.141. [DOI] [PubMed] [Google Scholar]
- 64.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–39. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, Eisenstein M, et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10:285–92. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bajbouj K, Poehlmann A, Kuester D, Drewes T, Haase K, Hartig R, et al. Identification of phosphorylated p38 as a novel DAPK-interacting partner during TNFalpha-induced apoptosis in colorectal tumor cells. Am J Pathol. 2009;175:557–70. doi: 10.2353/ajpath.2009.080853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Heininger K. Aging is a deprivation syndrome driven by a germ-soma conflict. Ageing Res Rev. 2002;1:481–536. doi: 10.1016/s1568-1637(02)00015-6. [DOI] [PubMed] [Google Scholar]
- 69.Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM. IL-18-stimulated GADD45 β required in cytokine-induced, but not TCR-induced, IFN-γ production. Nat Immunol. 2001;2:157–64. doi: 10.1038/84264. [DOI] [PubMed] [Google Scholar]
- 70.Nakayama A, Kawasaki H, Jin C, Munekata E, Taira K, Yokoyama KK. Transcriptional regulation of interferon gamma gene by p300 co-activator. Nucleic Acids Res Suppl. 2001:89–90. doi: 10.1093/nass/1.1.89. [DOI] [PubMed] [Google Scholar]
- 71.Basak C, Pathak SK, Bhattacharyya A, Mandal D, Pathak S, Kundu MNF. NF-kappaB- and C/EBPbeta-driven interleukin-1β gene expression and PAK1-mediated caspase-1 activation play essential roles in interleukin-1β release from Helicobacter pylori lipopolysaccharide-stimulated macrophages. J Biol Chem. 2005;280:4279–88. doi: 10.1074/jbc.M412820200. [DOI] [PubMed] [Google Scholar]
- 72.Yi YW, Kim D, Jung N, Hong SS, Lee HS, Bae I. Gadd45 family proteins are coactivators of nuclear hormone receptors. Biochem Biophys Res Commun. 2000;272:193–8. doi: 10.1006/bbrc.2000.2760. [DOI] [PubMed] [Google Scholar]
- 73.Zhang F, Nakamura T, Aune TM. TCR and IL-12 receptor signals cooperate to activate an individual response element in the IFN-gamma promoter in effector Th cells. J Immunol. 1999;163:728–35. [PubMed] [Google Scholar]
- 74.Guan Z, Buckman SY, Pentland AP, Templeton DJ, Morrison AR. Induction of cyclooxygenase-2 by the activated MEKK1 --> SEK1/MKK4 --> p38 mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:12901–8. doi: 10.1074/jbc.273.21.12901. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Y, Saunders MA, Yeh H, Deng WG, Wu KK. Dynamic regulation of cyclooxygenase-2 promoter activity by isoforms of CCAAT/enhancer-binding proteins. J Biol Chem. 2002;277:6923–8. doi: 10.1074/jbc.M108075200. [DOI] [PubMed] [Google Scholar]
- 76.Pomplun E, Terrissol M. Low-energy electrons inside active DNA models: a tool to elucidate the radiation action mechanisms. Radiat Environ Biophys. 1994;33:279–92. doi: 10.1007/BF01210450. [DOI] [PubMed] [Google Scholar]
- 77.Park SK, Prolla TA. Gene expression profiling studies of aging in cardiac and skeletal muscles. Cardiovasc Res. 2005;66:205–12. doi: 10.1016/j.cardiores.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 78.Edwards MG, Sarkar D, Klopp R, Morrow JD, Weindruch R, Prolla TA. Impairment of the transcriptional responses to oxidative stress in the heart of aged C57BL/6 mice. Ann N Y Acad Sci. 2004;1019:85–95. doi: 10.1196/annals.1297.017. [DOI] [PubMed] [Google Scholar]
- 79.Niehrs C, Schäfer A. Active DNA demethylation by Gadd45 and DNA repair. Trends Cell Biol. 2012;22:220–7. doi: 10.1016/j.tcb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Sytnikova YA, Kubarenko AV, Schäfer A, Weber AN, Niehrs C. Gadd45a is an RNA binding protein and is localized in nuclear speckles. PLoS One. 2011;6:e14500. doi: 10.1371/journal.pone.0014500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ford D, Hoe N, Landis GN, Tozer K, Luu A, Bhole D, et al. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp Gerontol. 2007;42:483–97. doi: 10.1016/j.exger.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ashburner M. Drosophila: A laboratory manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory, 1989. [Google Scholar]
- 83.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 84.Fleming TR, O'Fallon JR, O'Brien PC, Harrington DP. Modified Kolmogorov-Smirnov test procedures with application to arbitrarily right-censored data. Biometrics. 1980;36:607–25. [Google Scholar]
- 85.Breslow N. A generalized Kruskal-Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika. 1970;57:579–94. [Google Scholar]
- 86.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 87.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–32. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Pletcher SD. Model fitting and hypothesis testing for age-specific mortality data. J Evol Biol. 1999;12:430–9. [Google Scholar]
- 89.Fisher RA. Statistical methods for research workers. Edinburgh: Oliver and Boyd, 1954. [Google Scholar]
- 90.Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, et al. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem. 1998;273:34970–5. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]