Abstract
Muscle strength, usually measured as the peak torque during maximal contraction, is impaired in persons with stroke. Time-dependent properties of muscle contraction may also be altered but have not been quantified. We quantified both magnitude (peak torque) and time-dependent parameters (times to develop and reduce torque) in eight different isometric joint actions. Parameters were compared among the more and less affected arms of 20 persons with chronic stroke and the non-dominant arms of 10 similarly aged healthy persons. Torque-generation parameters were independent from one another (i.e., low correlations) and highly reliable between trials and days. All parameters were impaired in the more affected arm, whereas peak torque and time to develop torque were impaired in the less affected arm. Following stroke, torque generation impairments include both magnitude and time-dependent properties and exist not only in the more but also in the less affected arm. Clinicians attempting to improve upper extremity function should employ therapeutic exercises that challenge patients to improve both their strength and speed of muscle contraction.
Keywords: arm, hemiparesis, muscle, rehabilitation, stroke
Introduction
Muscle strength, the ability to generate muscular force, is generally measured by the magnitude of force or torque (e.g., peak torque) that can be generated, whereas weakness is defined as a decrease in the maximum voluntary torque or force when compared to normative values5. The inability to generate torque is recognized as a primary obstacle to recovery following a stroke2 and is related to decreased function of the upper8 or lower26 extremities.
The magnitude of force generated is the most common measure of muscle contraction. However, time-dependent properties of muscle contraction may also be altered by neurological disease and are potentially important for function. For example, the rate of force generation is impaired and related to functional performance in individuals with Parkinson’s disease12. The speed with which the torque profile rises and falls may also be an important descriptor of muscle contraction after a stroke. In healthy individuals, peak torque is achieved within 1 second of the initiation of a maximum voluntary contraction and is consequently available for use in everyday tasks10. During the acute stage of stroke, however, a reduced peak torque is accompanied by a prolonged time to generate peak torque for knee extensors7 and elbow flexors/extensors10.
Given the structural muscle changes associated with chronic stroke, including a relative decrease in fast-twitch fibers36, we hypothesized that persons with chronic stroke would also exhibit an increase in the time required to generate torque. In persons with stroke, the magnitude of and joints affected by deficits in peak force production varies across individuals3,11, depending on lesion site and volume. However, the nature and anatomical distribution of deficits in time required to generate torque are unknown for muscles of the upper extremity. It is also unknown whether impairment in the time required to generate torque is accompanied by impairments in the time to reduce torque. Lastly, the relationship between the magnitude of torque production and time-dependent muscle properties (i.e., time to develop and reduce torque) has not been defined. Joint torque parameters represent the collective behaviour of active muscles and characterize the net output of the neuromuscular system during a task.
We have evaluated torque-generation by measuring both the magnitude (peak torque) and time-dependent parameters (time to develop and time to reduce torque) in eight different isometric joint actions across four conditions: (1) the more and (2) less affected side of individuals with chronic stroke, and (3) the dominant and (4) non-dominant sides of healthy control participants. The objectives of the study were to compare these torque parameters across the four conditions and across upper extremity muscle groups within each condition, and to determine the relationship between the magnitude of peak torque and the time-dependent variables.
Methods
PARTICIPANTS
Twenty older adults (mean 60.9, SD 6.1, range 49–72 years, 13 men and 7 women) were recruited from the community with the following inclusion criteria: (1) a minimum of 1-year post-stroke, (2) present with hemiparesis secondary to first stroke, (3) able to provide informed consent, (4) able to follow one- and two-step commands, and (5) able to voluntarily flex/abduct the shoulder 45 degrees and extend the elbow 30 degrees. For this group of 20 adults with stroke, 12 had ischemic strokes and the other had hemorrhagic strokes, 17 were right hand dominant prior to their stroke, 13 had hemiparesis on the right side of the body, and the time since stroke was a mean 4.3, SD 2.6 years. Ten healthy adults of similar age (mean 61.0, SD 9.0, range 51–77 years) and gender (6 men and 4 women) were recruited from the community. Musculoskeletal or neurological conditions (in addition to the stroke for the test participants) that would affect upper-extremity function were exclusion criteria for all participants. The study protocol was approved by local university and hospital ethics committees, and all participants gave informed consent.
The level of motor impairment for the more affected arm in participants with stroke was assessed by the upper extremity portion of the Fugl-Meyer scale19 (maximum function=66) and by the modified Ashworth Scale (MAS) for spasticity6 (0=no increase in muscle tone and 4 = affected part rigid in flexion or extension). The Fugl-Meyer score was 38.2 (SD 19) and MAS was 1.3 (SD 1.1). Twelve participants with stroke were evaluated a second time, 2 to 3 days following the first assessment, to establish intersession reliability.
TORQUE GENERATION ASSESSMENT
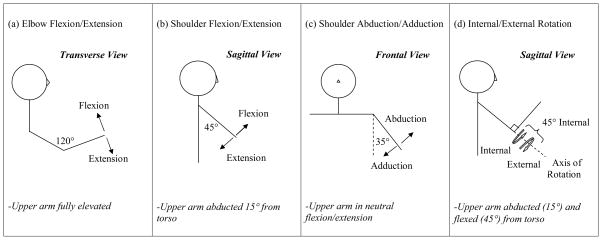
Torque generation was measured using the isometric mode (static contractions) of a seated dynamometer system (KinCom, Chattanooga, TN). In this mode, both the joint and the distal point of force application (lever) are stabilized and supported by straps or an arm trough, depending on the movement direction. The trunk was restrained by a set of crossing seat and lap belts; further stabilization at the level of the clavicle was applied by a clinician. Maximal voluntary isometric contraction of eight different upper-extremity joint actions was tested in midrange in the shoulder (flexion, extension, abduction, adduction, internal and external rotation), and elbow (flexion and extension) (see Fig. 1). We measured torque generation in four conditions: (1) the more and (2) less affected arms of participants with stroke, and in (3) the non-dominant and (4) dominant arms of healthy participants.
Figure 1.
Posturing for isometric testing. From left to right, the illustrations show postures for (a) elbow flexion/extension, (b) shoulder flexion/extension, (c) shoulder abduction/adduction, and (d) internal/external rotation. The mid-range isometric testing angle is labeled on each illustration. Additional posturing is described below each illustration.
For each trial, participants started in a relaxed state and were instructed: “At the sound of the tone (an auditory cue), quickly push as hard as you can (in the appropriate direction). Immediately stop pushing at the second tone.” Participants understood that in each trial they were required to (1) develop torque as fast as possible, (2) sustain a maximal level of torque (i.e., peak), and (3) reduce torque as fast as possible. Within each trial, participants received continuous visual feedback about the rate and level of force production via a bar graph displayed on a computer monitor. This information was removed from the computer screen at the end of the trial. Verbal encouragement was used to ensure the best performance. Three contractions (each of 3 seconds) were performed for each joint action, and rest breaks of 1 minute between trials were given to minimize fatigue. A 3-minute rest was given between joint actions. Blood pressure and heart rate were monitored with a digital blood pressure cuff (Lifesource, Milpitas, CA) throughout testing to ensure that it remained within the exercise testing guidelines of the American College of Sports Medicine1.
ISOMETRIC JOINT TORQUE ANALYSIS
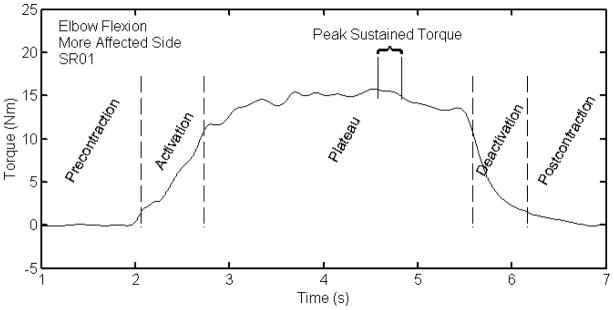
Resultant torque profiles for each contraction were corrected for gravity. Peak torque was defined as the maximum torque value that could be sustained for a period of 250 ms15. Peak torques were normalized by body mass. Gender and age, two other factors that affect strength, were not statistically different in their means or distribution between the healthy and stroke participants. The locations of 10% and 70% peak torque values were identified on the ascending and descending portions of the torque profile. These values were used to divide the contraction into five segments: pre-contraction, activation, plateau, deactivation, and post-contraction (Fig. 2). The times to develop and reduce torque were calculated as the durations between the 10 and 70% thresholds; these parameters are useful for characterizing the shape of the torque profile and are independent of peak torque. Trials that had segmented or noisy profiles were eliminated from further analysis. The percentage of trials that were eliminated was < 1%.
Figure 2.
Torque profile regions
STATISTICAL ANALYSIS
Statistical analyses were performed on the three torque generation parameters (peak normalized torque, time to develop torque, and time to reduce torque). Relative reliability using intraclass correlations, ICC(1,1)35 and absolute reliability using the standard error of measurement (SEM)17 of the parameters was determined for each muscle group across the three trials (intrasession) and across the two days (intersession) for the stroke participants; SEMs were expressed as a percentage of mean scores. Subsequent analyses used the mean values from tests on the first day. Preliminary analyses found no difference between the non-dominant and dominant arms of the healthy participants for any of the three parameters, so further analyses included only the non-dominant arm.
The effect of arm condition (more and less affected side of individuals with chronic stroke, and non-dominant side of healthy control participants) on peak normalized torque, time to develop torque, and time to reduce torque was evaluated using an ANOVA followed by post-hoc Duncan’s multiple comparison tests. Within each condition, the effect of the eight different muscle groups on the muscle contraction parameters was tested by an ANOVA followed by post-hoc Duncan’s multiple comparison tests. Finally, the relationship of parameters (i.e., times to develop and reduce torque and peak torque) was evaluated by performing three Pearson product correlations for each arm condition. For the correlational analyses, data for all eight muscle groups were included within each arm condition. All statistical calculations were performed on SPSS 11.0 (Statistical Package for the Social Sciences, Chicago, Illinois) using p=0.05.
Results
RELIABILITY
For brevity, only the averaged values of intersession reliabilities across joint actions are reported. For the more affected arm, the mean ICCs were 0.97, 0.91, and 0.94 for peak torque, time to develop torque, and time to reduce torque respectively; the associated SEM percentages were 12.3%, 12.4%, and 18.4%. For the less affected arm, the mean ICCs were 0.98, 0.88, and 0.94 and the SEM percentages were 7.9%, 14.2%, and 15.6%.
PEAK TORQUE
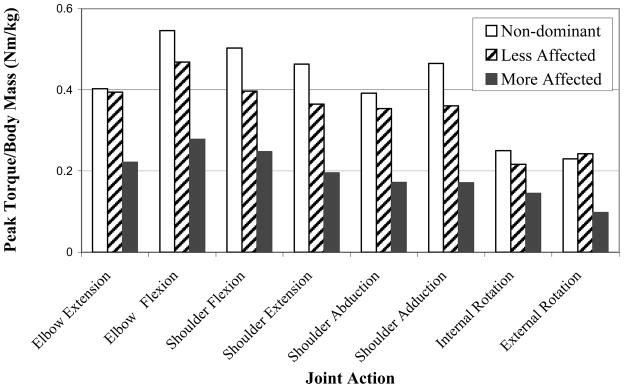
There was a significant effect of condition on the peak normalized torque [F(2,397)=72.72,p<.001] (Figure 3). The post-hoc multiple comparison Duncan test separated the condition means such that the healthy non-dominant arm was the greatest (0.406 Nm/kg), followed by the less affected (0.350 Nm/kg), and then the more affected (0.191 Nm/kg) condition.
Figure 3.
Normalized peak torque versus joint action for each arm condition.
There was a significant effect of joint action on the peak normalized torque on the non-dominant arm [F(7, 72)=10.45, p<.001], less affected condition [F(7,152)=6.11, p<.001], and the more affected condition [F(7,152)=4.80, p<.001]. For all three conditions, the post-hoc analyses resulted in similar ranking of joint actions; the peak normalized torque values were largest for shoulder flexion and elbow flexion joint actions and smallest for internal and external rotation joint actions. The peak normalized torque values associated with shoulder abduction and adduction, shoulder extension, and elbow extension were intermediate to these values.
TIME TO DEVELOP TORQUE
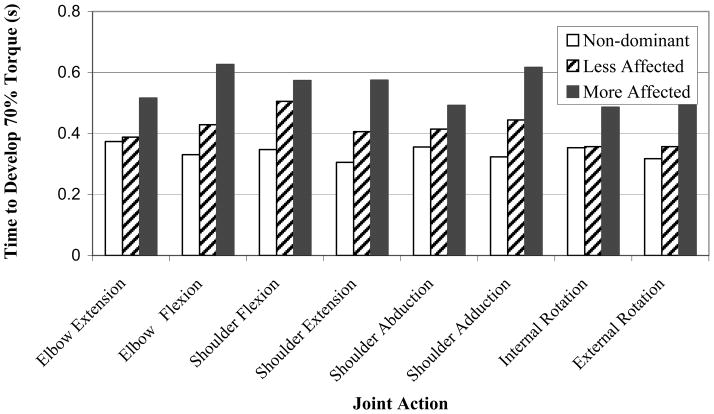
There was a significant effect of arm condition on the time to develop torque [F(2,397)=21.88, p<.001; Fig. 4]. The post-hoc test separated the condition means such that the more affected (0.548 s) arm was slower than the less affected arm (0.413 s), whereas the healthy non-dominant arm (0.339 s) was the fastest. There was no significant effect of joint action on the time to develop torque (p > 0.05) for any of the arm conditions.
Figure 4.
Time to develop torque versus joint action for each arm condition
TIME TO REDUCE TORQUE
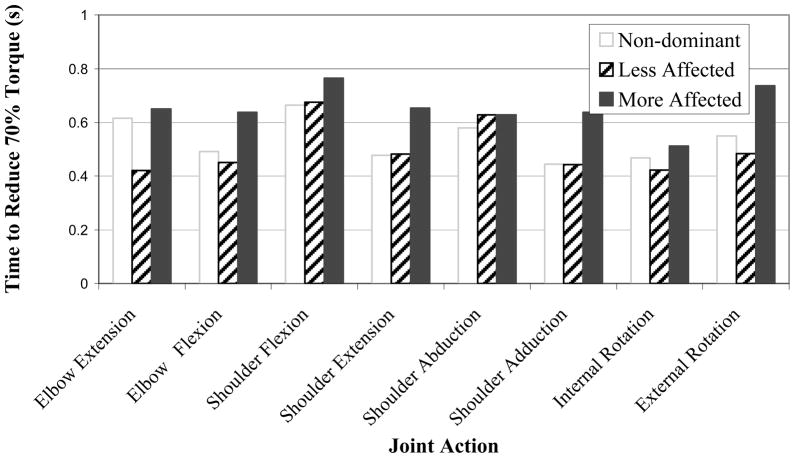
There was a significant effect of arm condition on the time to reduce torque, [F(2,397)=8.71, p<.001; Fig. 5]. Similar to the time to develop torque, the post-hoc test separated the means such that the more affected arm was the slowest (0.653 s) of the three arm conditions; there was, however, no statistical difference between the times of the less affected arm (0.501 s), and the healthy non-dominant arm (0.536 s) conditions. There was no significant effect of joint action on the time to reduce torque for the non-dominant or more affected arms (p> 0.05), but there was a significant effect of joint action on the time to reduce torque for the less affected condition [F(7,152)=2.19, p<.05].
Figure 5.
Time to reduce torque versus joint action for each arm condition
RELATIONSHIP BETWEEN MUSCLE PARAMETERS
No significant correlations were found amongst any of the parameters (i.e., times to develop and reduce torque and peak torque) in the non-dominant arm. In the less affected arm, significant (p<.01), but low correlations were found between peak torque and time to reduce torque (−0.322) and between time to develop torque and time to reduce torque (0.275). All correlations among the torque parameters were significant (p<.01) but low within the more affected arm: peak torque and time to develop torque (−0.301), peak torque and time to reduce torque (−0.329), and time to develop torque and time to reduce torque (0.275).
Discussion
PEAK TORQUE
In comparison to the non-dominant arm of healthy participants, the magnitude of joint torque (peak normalized torque) was impaired by 53% in the more affected arm and by 15% in the less affected arm in persons with stroke. This finding confirms and extends recent evidence3,11,24 that strength is also impaired in the less-affected upper extremity following stroke. The apparent weakness of the less affected side may be due to the small percentage of descending cortical tracts that originate from the lesion site and remain ipsilateral15 or from the generally sedentary lifestyle of a person with stroke who may fail to maintain the strength of a more regularly exercised non-dominant arm of a healthy person.
We found differences in the peak torque across the eight joint actions within the healthy participants that are likely dependent on the sizes and moment arms of muscles contributing to the movement. The relative ranking of the magnitudes of the peak torques for the eight joint actions was the same for all three conditions, i.e., the strongest joint actions in the more affected condition were also the strongest joint actions in the less affected and non-dominant arm conditions; moreover, the weakest joint actions were the same in all three arm conditions. This agrees with other studies3,11 that have not found evidence supporting the clinical belief that strength deficits increase in the proximal-to-distal and the flexor-to-extensor directions in the upper limb following stroke. It could be argued that the distribution of strength deficits in chronic stroke is individual and related to the size and the location of the brain lesion, but this individualized weakness cannot be detected when data are averaged over a large sample size uncontrolled for lesion size or location.
TIME-DEPENDENT CHANGES
We found that the times to develop and reduce torque, indicators of the ability to modulate muscle force in a timely fashion, are impaired in chronic stroke. Time to develop torque was impaired by 61% in the more affected arm and by 22% in the less affected arm of persons with chronic stroke, compared to the non-dominant arm of healthy persons. We also found that the time to reduce torque was impaired by 22% in the more affected arm condition compared to the less affected arm and non-dominant arm conditions.
There is a natural slowing of force generation and muscle activation that occurs with age resulting from factors that include a reduction in motor drive and a decrease in the ability of aged skeletal muscle to generate tension rapidly29. Reduction in the number of fast-fatigable muscle fibers (type II), and their denervation with age18 may also contribute to natural declines in the speed of muscle contraction, as the loss of type II muscle fibers would specifically produce difficulty in the initiation and achievement of rapid and high-force movements. Accelerated type II fiber atrophy caused by a sedentary lifestyle and reduced muscle activity32 may contribute to the moderate slowing of torque development times observed in the less affected arm. The further slowing of torque development in the more affected arm is likely due to motor unit loss of up to 50%13,31 that is specific to motor units associated with type II fibers13,16,31. In addition to muscle inactivity, Dattola et al.13 suggested that mechanisms contributing to this type II atrophy may include transsynaptic degeneration of type II motoneurons, collateral reinnervation, and motor unit transformation. Other factors contributing to reduced torque control following stroke include motor unit recruitment deficits and decreased firing frequency33. The inability to respond quickly to changes in force requirements may also relate to abnormal motor unit discharge patterns. Persons with stroke also demonstrate atypical electromyographic interspike intervals that could manifest clinically as a difficulty in maintaining a steady force or in adapting to rapid changes in force requirements2, 20, 34, 37.
In contrast to our results, Canning et al.10 did not find deficits in the time to develop torque during isometric elbow flexion and extension movements in older adults who were 25 weeks post-stroke. There are two major methodological differences that we believe improved our ability to detect differences in time to develop and reduce torque. First, they used time to 90% of peak torque, which we found in pilot work to be less reliable than time to 70% of peak torque. The torque profile tends to both fluctuate and flatten out near the peak torque, inherently making unrealiable time-dependent measurements near the peak. Second, we used a larger sample size (20 vs.10) and compressed values over multiple muscle groups, thus enabling us to increase our ability to detect differences among arm conditions.
Impairments in the time to reduce torque, unique to the more affected arm, may be due to changes in motoneuron membrane firing behaviour. Changes in neuromodulators as a consequence of stroke can shift the synaptic current – frequency relations so that a cell can maintain a prolonged tonic firing rate following a brief period of excitation25. Both animal4, 22, 28 and human21 studies suggest that this behaviour, known as a ‘plateau potential’22, plays a facilitating role in the regulation of motor unit firing rates. It follows that slower torque relaxation times of the more affected side during isometric contractions are associated with motoneurons that self-sustain tonic firing when a descending neural stimulus is removed.
No joint action had significantly faster times to develop or reduce torque within any of the arm conditions, suggesting that anatomical location or muscle size did not influence time-dependent contraction parameters in the tested proximal upper-extremity muscles. The significant correlations between time-dependent parameters and peak normalized torque production in persons with stroke may be in part related to a selected atrophy of type II fast-twitch muscle fibers and a concomitant behavioural transition of type I-associated motoneurons that results in plateau-potential firing patterns. These changes would affect not only the peak force but also the rate at which it is generated and reduced. However, these correlations were very low, only accounting for less than 10% of the variability, suggesting that time-dependent parameters are largely unrelated to peak torque and represent a different dimension of joint action.
LIMITATIONS AND FUTURE CONSIDERATIONS
We measured the ability to generate torque under isometric conditions. Functional activities naturally require muscle to be working under concentric or eccentric conditions and so dynamic strength tests (isokinetic or isotonic) may be more functionally relevant. Isometric strength tests of the upper extremity, however, correlate well with the results from isokinetic and isometric tests27. Furthermore, the identification of time-dependent torque profile characteristics during dynamic tasks is complicated by the muscle length-tension and velocity effects on torque generation30.
We evaluated was a comprehensive evaluation of joint torque for eight upper extremity joint actions involved in gross motor arm function. In future studies, it would be clinically useful to characterize the joint actions of the more distal wrist and hand as they are critical for the orientation of the hand and the manipulation of objects. In fact, because of the natural proximal-to-distal gradient of increasing fast-twitch fiber content23, it is likely that comparisons between hand and arm joint actions would yield significant differences in both magnitude and time-dependent parameters of muscle contraction. It would also be beneficial to evaluate the relationship between the muscle contraction parameters with participation and activity restrictions.
CLINICAL IMPLICATIONS.
Our findings have certain practical applications. In particular, clinicians attempting to improve upper extremity function should employ therapeutic exercises that challenge patients to improve both their strength and speed of muscle contraction.
Acknowledgments
The authors acknowledge the support of the Canadian Institute of Health Research (MOP-57862) and a Rick Hansen Neurotrauma Studentship.
References
- 1.American College of Sports Medicine (ACSM) Guidelines for exercise testing and prescription. 6. Baltimore: Williams & Wilkins; 2000. [Google Scholar]
- 2.Andreassen S, Rosenfalck A. Impaired regulation of force and firing pattern of single motor units in patients with spasticity. J Neurol Neurosurg Psychiatry. 1980;43:907–916. doi: 10.1136/jnnp.43.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews AW, Bohannon RW. Distribution of muscle strength impairments following stroke. Clin Rehabil. 2000;14:79–87. doi: 10.1191/026921500673950113. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- 5.Bohannon RW. Measurement, nature, and implications of skeletal muscle strength in persons with neurological disorders. Clin Biomech. 1995;10:283–292. doi: 10.1016/0268-0033(94)00002-o. [DOI] [PubMed] [Google Scholar]
- 6.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 7.Bohannon RW, Walsh S. Nature, reliability, and predictive value of muscle performance measures in patients with hemiparesis following stroke. Arch Phys Med Rehabil. 1992;73:721–725. [PubMed] [Google Scholar]
- 8.Bohannon R, Warren ME, Cogman KA. Motor variables correlated with the hand-to-mouth maneuver in stroke patients. Arch Phys Med Rehabil. 1991;72:682–684. [PubMed] [Google Scholar]
- 9.Burke D. Spasticity as an adaptation to pyramidal tract injury. Adv Neurol. 1988;47:401–423. [PubMed] [Google Scholar]
- 10.Canning CG, Ada L, O’Dwyer N. Slowness to develop force contributes to weakness after stroke. Arch Phys Med Rehabil. 1999;80:66–70. doi: 10.1016/s0003-9993(99)90309-x. [DOI] [PubMed] [Google Scholar]
- 11.Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112:749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- 12.Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson’s disease: relationship to rate of force generation and clinical status. Ann Neurol. 1996;39:79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- 13.Dattola R, Girlanda P, Vita G, Santoro M, Roberto ML, Toscano A, Venuto C, Baradello A, Messina C. Muscle rearrangement in patients with hemiparesis after stroke: an electrophysiological and morphological study. Eur Neurol. 1993;3:109–114. doi: 10.1159/000116915. [DOI] [PubMed] [Google Scholar]
- 14.Davidoff RA. The pyramidal tract. Neurology. 1990;40:332–339. doi: 10.1212/wnl.40.2.332. [DOI] [PubMed] [Google Scholar]
- 15.Dewald JPA, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Dietz V, Ketelsen UP, Berger W, Quintern J. Motor unit involvement in spastic hemiparesis: relationship between leg muscle activation and histochemistry. J Neurol Sci. 1986;75:89–103. doi: 10.1016/0022-510x(86)90052-3. [DOI] [PubMed] [Google Scholar]
- 17.Eliasziw M, Young SL, Woodbury MG, Fryday-Field K. Statistical methodology for the assessment of interrater and intrarater reliability. Phys Ther. 1994;74:89–100. [Google Scholar]
- 18.Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci. 1995;50:124–129. doi: 10.1093/gerona/50a.special_issue.124. [DOI] [PubMed] [Google Scholar]
- 19.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. Poststroke hemiplegic patient: evaluation of physical performance. Scand J Reh Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 20.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 21.Gorassini MA, Bennet DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett. 1998;247:13–16. doi: 10.1016/s0304-3940(98)00277-8. [DOI] [PubMed] [Google Scholar]
- 22.Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurons in the decerebrate cat an in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jozsa L, Demel Z, Vandor E, Reffy A, Szilagyi I. Specific fibre composition of human hand and arm musceles (Article in German) Handchirurgie. 1978;10:153–157. [PubMed] [Google Scholar]
- 24.Jung HY, Yoon JS, Park BS. Recovery of proximal and distal arm weakness in the ipsilateral upper limb after stroke. Neurorehabilitation. 2002;17:153–159. [PubMed] [Google Scholar]
- 25.Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr Opin Neurobiol. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- 26.Kim CM, Eng JJ. The relationship of lower extremity muscle torque with locomotor performance in persons with stroke. Phys Ther. 2003;83:49–57. [PubMed] [Google Scholar]
- 27.Knapik JJ, Wright JE, Mawdsley RH, Braun BS. Isokinetic, isometric, and isotonic strength relationships. Arch Phys Med Rehabil. 1983;64:77–80. [PubMed] [Google Scholar]
- 28.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J of Neurophys. 1998;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- 29.Lewis RD, Brown JM. Influence of muscle activation dynamics on reaction time in the elderly. Eur J Appl Physiol Occup Physiol. 1994;69:344–349. doi: 10.1007/BF00392041. [DOI] [PubMed] [Google Scholar]
- 30.Mayer F, Horstmann T, Rocker K, Hetikamp HC, Dickhuth HH. Normal values of isokinetic maximum strength, the strength/velocity curve, and the angle at peak torque of all degrees of freedom in the shoulder. Int J Sports Med. 1994;15:S19–S25. doi: 10.1055/s-2007-1021105. [DOI] [PubMed] [Google Scholar]
- 31.McComas AJ, Sica RE, Upton AR, Aguilera N. Functional changes in motorneurones of hemiparetic subjects. J Neurol Neurosurg Psychiatry. 1973;36:183–193. doi: 10.1136/jnnp.36.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller RG. The effects of aging upon nerve and muscle function and their importance for neurorehabilitation. J Neurol Rehabil. 1995;9:175–181. [Google Scholar]
- 33.Rosenfalck A, Andreassen S. Impaired regulation of force and firing pattern of single motor units in patients with spasticity. J Neurol Neurosurg Psychiatry. 1980;43:897–906. doi: 10.1136/jnnp.43.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shahani BT, Wierzbicka MM, Parker SW. Abnormal single motor unit behavior in upper motor neuron syndrome. Muscle Nerve. 1991;14:64–69. doi: 10.1002/mus.880140111. [DOI] [PubMed] [Google Scholar]
- 34.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;2:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 35.Toffola E, Sparpaglione D, Pistorio A, Buonocore M. Myoelectric manifestations of muscle changes in stroke patients. Arch Phys Med Rehabil. 2001;82:661–665. doi: 10.1053/apmr.2001.22338. [DOI] [PubMed] [Google Scholar]
- 36.Yan K, Fang J, Shahani BT. Motor unit discharge behaviors in stroke patients. Muscle Nerve. 1998;21:1502–1506. doi: 10.1002/(sici)1097-4598(199811)21:11<1502::aid-mus20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]