Abstract
Intracerebral hemorrhage (ICH) is a devastating disease lacking an effective treatment. While the initial injury occurs within minutes, an inflammatory response contributes to ongoing tissue damage over hours to days. Relatively little is known about leukocyte trafficking into the brain in the hours after ICH onset. Understanding these events may lead to identification of new therapeutic targets. Using the blood injection mouse model of ICH, the numbers of leukocytes in the ipsilateral and contralateral brain were quantified by flow cytometry 12 hours after surgery. Perihematomal inflammation was confirmed by histology and chemokines and cytokines in the brain quantified by multiplex ELISA. Few neutrophils were detected in the brain 12 hours after ICH. The majority of leukocytes consisted of inflammatory macrophages (CD45.1hiCD3−Ly6G−CD11c−CD11b+Gr1+ cells) and inflammatory dendritic cells (CD45.1hiCD3−Ly6G−CD11cintCD11b+Gr1+ cells). Microglia numbers did not differ between the hemispheres. These results indicate that blood-derived monocyte populations traffic into brain early after ICH and outnumber neutrophils at 12 hours.
Keywords: Intracerebral Hemorrhage, Inflammation, Stroke, Macrophages, Dendritic cells, Neutrophils, Neuroinflammation
Introduction
Intracerebral hemorrhage (ICH) is a devastating stroke subtype affecting nearly 2 million patients worldwide each year[1]. While the initial neurological deficit is caused by mass effect and shear forces on brain tissue, there is increasing recognition that an inflammatory process contributes to further injury over ensuing days[2]. Therapies that target inflammation have appeal, as a prolonged treatment window may allow many patients to benefit.
Peripheral leukocytes migrate from blood to the perihematomal region and may lead to progressive tissue damage and/or clearance of the hematoma and repair. In a rat model of ICH, leukocyte and platelet depletion by whole body irradiation significantly reduced perihematomal edema[3]. In addition, splenectomy three days prior to ICH reduced cerebral edema and infiltrating macrophages and neutrophils, further implicating the peripheral immune response in ICH injury[4]. These studies suggest that inhibition of leukocyte trafficking into the brain may have therapeutic benefit. However, further work is needed to clarify which cell populations are migrating into brain, at what time points, and how each population contributes to injury and recovery.
Most previous studies have focused on quantifying the inflammatory response beginning at 24 hours. Cellular trafficking events occurring earlier after ICH onset have not been well studied. Many investigations of the early events after ICH have relied on histological methods, which are limited in the ability to differentiate blood-derived macrophages and other myeloid cells from the resident microglia. Based on these studies, neutrophils have been considered first-responders, followed by monocyte/macrophage infiltration days into the disease. The present study aimed to quantify the perihematomal leukocyte and microglia populations at 12 hours using flow cytometry in a mouse model of ICH. We determined that neutrophils comprise fewer than 8% of leukocytes that traffic into the damaged hemisphere by 12 hours. The majority of leukocytes consisted of inflammatory macrophages and inflammatory dendritic cells. The appropriate chemokines for recruitment of these leukocyte populations were elevated in perihematomal brain tissue.
Methods
Animals
C57BL/6J, which express the pan-leukocyte marker CD45.2, and B6.SJL-Ptprca Pep3b/BoyJ, the congenic strain expressing CD45.1, male mice were purchased from Jackson Labs. All mice were 8–16 weeks old when used for experiments. Animal procedures were performed in accordance with NIH guidelines for the care and use of laboratory animals and approved by the Animal Care Committee of the University of Connecticut Health Center.
Intracerebral hemorrhage surgery
Eleven male B6.SJL-PtprcaPep3b/BoyJ mice were anesthetized with isoflurane and subjected to striatal blood injection to model ICH as previously described[5]. 15 μL of blood from a C57BL/6J donor mouse was injected at 0.5 μL/min by microinfusion pump (WPI) without anticoagulation at 2.5 mm right of bregma and 3 mm deep while body temperature was maintained at 37 ± 0.5° C. Eight sham surgeries were performed including all procedures except blood injection.
Tissue harvesting
At 12 ± 1 hour after surgery mice were euthanized, transcardially perfused with 40 mL cold PBS, and brains harvested. The brainstem and cerebellum were removed and the brain was divided along the interhemispheric fissure into ipsilateral and contralateral hemispheres.
Flow cytometry
Each hemisphere to be analyzed by flow cytometry was placed in complete RPMI 1640 (Life Technologies) medium and mechanically and enzymatically digested in collagenase/dispase (1 mg/mL) and DNAse (10 mg/mL)(both Roche Diagnostics). Leukocytes were harvested from the interphase of a 70%/30% Percoll gradient. Cells were washed and blocked with mouse Fc Block (BD Biosciences) prior to staining with CD45.2–FITC (BD Pharmingen), CD45.1–PE, CD11b–PerCp-Cy5.5, CD11c–PE-Cy7, Gr1–APC-efluor780 (all eBioscience), CD3–v500 and Ly6G–v450 (both BD Horizon). Data were acquired on a LSRII using FACsDIVA 6.0 (BD Biosciences) and analyzed using FlowJo (Treestar Inc.). Donor leukocytes were identified as CD45.2hi, host leukocytes CD45.1hi, T lymphocytes as CD45.1hiCD3+, dendritic cells as CD45.1hiCD3−Ly6G−CD11chi, neutrophils as CD45.1hiCD3−CD11b+Ly6G+, macrophages as CD45.1hiCD3−CD11c−Ly6G−CD11b+Gr1−, and inflammatory macrophages as CD45.1hiCD3−Ly6G−CD11c−CD11b+Gr1+. A separate CD45.1hiCD3−Ly6G−CD11cintCD11b+Gr1+ population was identified as inflammatory dendritic cells. A population of cells expressing low to intermediate levels of CD45 and which were positive for CD11b expression (CD45.1intCD11b+) were identified as microglia.
ELISA
The perihematomal region of each ipsilateral hemisphere used for cytokine/chemokine quantification was homogenized and sonicated in RIPA buffer (Cell Signaling) with protease inhibitors, then centrifuged at 14,000×g. The protein concentration of the supernatant was determined using the BCA Protein Assay Kit (Thermo Fisher Scientific Inc.). 100 μg total protein were used for cytokine/chemokine quantification by multiplex ELISA (mouse inflammation panel I, Millipore) according to manufacturer’s instructions.
Immunofluorescence
Brains were immediately frozen in Tissue-tek O.C.T. (Andwin Scientific) and stored at −80°C. Six-micron thick sections were blocked in normal goat serum and incubated with rat anti-mouse Ly6G (clone 1A8, 5 μg/mL, BD Biosciences), rabbit anti-mouse GFAP (1:100, Dako) or rat anti-mouse CD11b (5 μg/mL, eBioscience) followed by Cy3 Affinipure goat anti-rat IgG or DL488 goat anti-rabbit IgG (Jackson Immunoresearch) at 1:500. DAPI was used at 0.25 μg/mL (Roche Diagnostics).
Statistical analyses
Excess numbers of ipsilateral leukocytes were calculated to account for any residual leukocytes in the circulation of each mouse after perfusion and reported as mean±standard deviation. After testing for normality, excess ipsilateral cell counts were compared between ICH and sham groups by t-test or Mann-Whitney test as appropriate. Microglia numbers were compared between the ipsilateral and contralateral hemispheres by t-test. Cytokine and chemokine levels were compared between ICH and sham groups by t-test with the Holm-Bonferroni correction for multiple hypotheses.
Results
Perihematomal Leukocytes Infiltrate from the Periphery
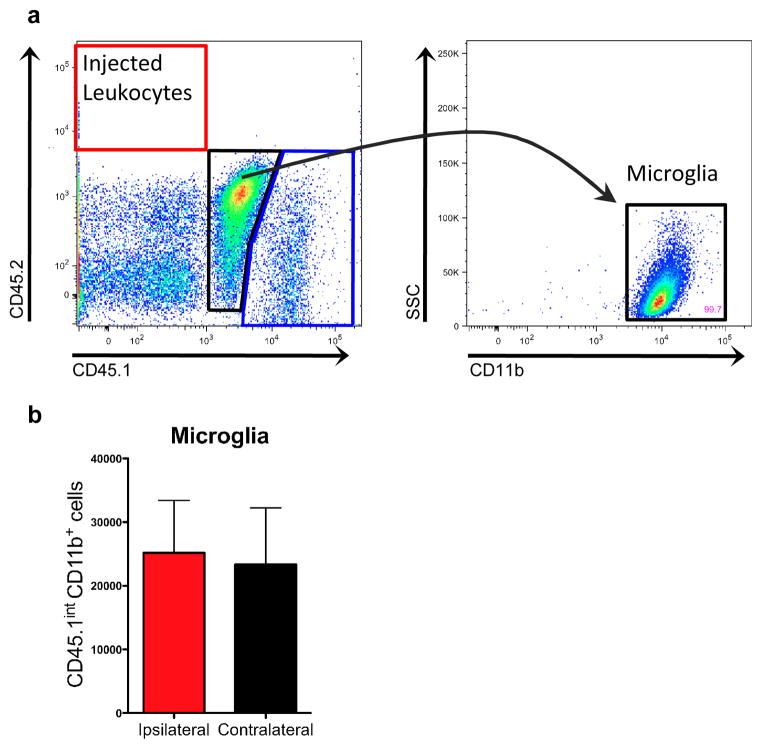
Blood from a congenic donor mouse expressing the CD45.2 pan-leukocyte marker was used to create the intracerebral hemorrhage in mice expressing the CD45.1 allele. This approach allowed leukocytes originating from the circulation of the host mouse (CD45.1hi cells) to be distinguished from any surviving leukocytes within the injected blood (CD45.2hi cells; Fig. 1a). At 12 hours, approximately 8.2% of live leukocytes identified by flow cytometry originated from within the hemorrhage itself. There were 1167.2 ±579.2 excess blood-derived leukocytes in the ipsilateral hemisphere 12 hours after ICH, compared to 435.7 ±124.1 in shams. Microglia numbers in ICH brains did not differ between hemispheres at this early time point (Fig. 1b).
Fig. 1.
Microglia and blood-derived leukocytes can be distinguished from injected leukocytes. a Representative plots from ipsilateral brain samples showing few donor CD45.2+ cells at 12 hours. The CD45.1int population (black gate, center) is 99.7% CD11b+ and are classified as microglia. The blue gate (lower right corner) represents the host-derived CD45.1hi leukocyte population. b Total microglia numbers are not different between hemispheres. n=5
Inflammatory Macrophages and Dendritic Cells Dominate the Inflammatory Infiltrate
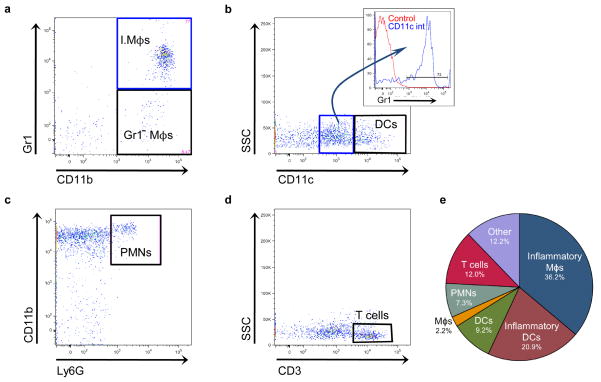
Once we confirmed the majority of leukocytes identified by flow cytometry had trafficked into the brain from the host during the inflammatory response, we calculated the excess ipsilateral numbers of individual leukocyte populations in ICH and sham brains (Table 1). Inflammatory Gr1+ macrophages (I.Mϕs) accounted for the largest leukocyte population, while there were few Gr1− macrophages (Gr1− Mϕs; Fig. 2a). The inflammatory dendritic cell population was the second most numerous, while there were many fewer CD11chi dendritic cells (Fig. 2b). Neutrophils (PMNs) and T cells accounted for 7.3% and 12.0% of excess ipsilateral leukocytes, respectively (Fig. 2c, d). The composition of the inflammatory infiltrate is shown in Fig. 2e.
Table 1.
Brain leukocyte counts 12 hours after ICH or sham surgery as detected by flow cytometry. The number of excess ipsilateral leukocytes for each population quantified is shown.
| Leukocyte population | Gating | Sham (Mean ± SD) | ICH (Mean ± SD) |
|---|---|---|---|
| Inflammatory macrophages | CD45.1hiCD3−Ly6G−CD11c−CD11b+Gr1+ | 71 ± 22 | 422 ± 333* |
| Inflammatory dendritic cells | CD45.1hiCD3−Ly6G−CD11cintCD11b+Gr1+ | 71 ± 39 | 243 ± 205 |
| Dendritic cells | CD45.1hiCD3−Ly6G−CD11chi | 52 ± 22 | 108 ± 50 |
| T cells | CD45.1hiCD3+ | 75 ± 67 | 141 ± 53 |
| Neutrophils | CD45.1hiCD3−CD11b+Ly6G+ | 4.3 ± 1.2 | 86 ± 101 |
| Macrophages | CD45.1hiCD3−Ly6G−CD11c−CD11b+Gr1− | 17 ± 2.1 | 26 ± 26 |
Inflammatory macrophages were significantly higher in ICH brain versus sham (*p<0.05 by Mann-Whitney test). n=3–5/group
Fig. 2.
Representative plots showing leukocyte populations present after ICH. a Inflammatory macrophages (I.Mϕs) and Gr1− macrophages (Gr1− Mϕs) are both present in brain, but the inflammatory macrophages are much more numerous. This plot is gated on CD45.1hiCD3−Ly6G−CD11c− cells. b Plot showing CD11cint (blue gate, center) and CD11chi dendritic cell populations (DCs, black gate, right) is gated on CD45.1hiCD3−Ly6G− cells. The majority of CD11cint cells express Gr1 (inset) and are termed inflammatory dendritic cells. c Few neutrophils (PMNs) are found in brain at 12 hours. The plot is gated on CD45.1hiCD3−. d T cells are found in the ipsilateral hemisphere by 12 hours. The plot is gated on CD45.1hi cells. e Pie chart showing the distribution of leukocyte populations expressed as the mean percentage of infiltrating cells from 5 mice. “Other” indicates CD45.1hi cells that do not fall into any of the aforementioned gating strategies
In order to confirm the flow cytometry results, four mice were euthanized 12 hours after ICH or sham surgery for analysis by immunohistochemistry. Visualization of the perihematomal region confirmed the presence of larger, amoeboid CD11b+ cells resembling macrophages, which was not seen after sham surgery (Fig. 3a, b). Few Ly6G+ cells with multi-lobed nuclei could be found after ICH and none after sham (Fig. 3c, d).
Fig. 3.
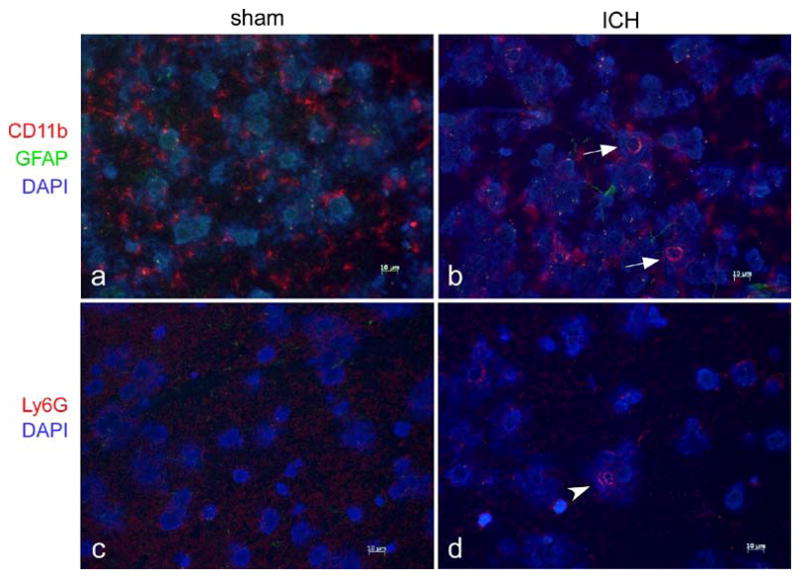
Immunofluorescence staining demonstrates local inflammation. Representative perihematomal sections from 12 hours after ICH or sham surgery are shown in a–d. a–b Staining for CD11b, a marker for myeloid cells, demonstrates most CD11b+ cells after sham surgery (a) have branched cytoplasmic processes consistent with resting microglia. b Arrows indicate larger, spherical CD11b+ cells consistent with macrophages at the border of the ICH that were not found after sham surgery. Astrocytic processes, identified by GFAP+ staining, were difficult to identify after sham surgery but became more evident after ICH. c–d Neutrophils, identified by staining with Ly6G, could not be found after sham surgery and were found in small numbers around the ICH at 12 hours (arrowhead). Scale bar indicates 10 μm
Chemokines for Recruitment of Myeloid Cells Increase by 12 Hours
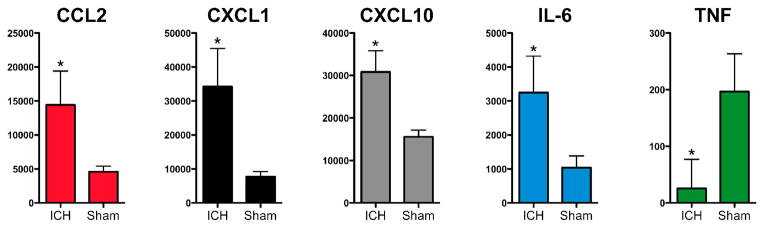
Once the composition of the inflammatory cellular infiltrate was determined, we sought to determine whether the appropriate chemokines were increased in the brain after ICH at this early time point. Chemokines for neutrophils (CXCL1/KC), inflammatory monocytes (CCL2/MCP-1), mononuclear and TH1 T cells (CXCL10/IP10), as well as the pro-inflammatory cytokine IL-6 were increased in the ipsilateral hemisphere after ICH compared to sham (Fig. 4). Unexpectedly, tumor necrosis factor (TNF) levels were reduced in ipsilateral ICH brain when compared to sham at 12 hours.
Fig. 4.
ICH induces elevations of several chemokines and cytokines within 12 hours. The chemokines CCL2, CXCL1, and CXCL10 are significantly higher in ipsilateral ICH (n=4) brain versus ipsilateral sham (n=3) brain. The pro-inflammatory cytokine IL-6 is also elevated, but TNF is lower in ipsilateral ICH brain. All values are shown in pg/g brain protein. * p<0.05 by t-test after Holm-Bonferroni correction for multiple hypotheses
Discussion
Blood-derived leukocytes begin to migrate to the perihematomal region within hours after ICH. Interestingly, inflammatory macrophages and inflammatory dendritic cells, rather than neutrophils, comprised the majority of leukocytes 12 hours after ICH. Other studies of the perihematomal leukocyte populations using immunohistochemistry describe neutrophils as rarely observed beginning at 4 hours after ICH and increasing in numbers until 48 hours[6,7]. This is consistent with our finding of few neutrophils at 12 hours. However, these and other studies have found macrophages did not appear until 48 and 72 hours[6,8], which contrasts with our finding of robust numbers of these populations at 12 hours when examining the brains by flow cytometry.
Research on monocyte biology focuses on two distinct populations of circulating monocytes: “Inflammatory” monocytes, characterized by high expression of Ly-6C(Gr1) and the CC chemokine receptor 2 (CCR2), and “resident” monocytes, which express low levels of Ly-6C(Gr1) and CCR2 but express high levels of CX3CR1 (reviewed in[9]). The Gr1+ inflammatory monocytes are thought to be responsible for the M1-type macrophage response, characterized by phagocytosis, proteolysis, and inflammation and are able to differentiate into TNF-α/iNOS-producing dendritic cells (tip-DCs) in inflamed tissue[10]. Inflammatory monocytes are involved in pathogenesis during experimental autoimmune encephalomyelitis[11] and ischemic stroke[12,13] and may be responsible for neurological deficits in the first three days after ICH[14]. The Gr1− monocytes patrol blood vessels and migrate quickly into tissues at the site of injury[15], differentiating into the M2-type “healing” macrophages in both the myocardium and Listeria-infected peritoneum[16,17]. We found the numbers of ipsilateral Gr1− macrophages were low 12 hours after ICH while Gr1+ inflammatory macrophages were increased 5-fold. The roles of these macrophage populations in the hours after ICH have not yet been investigated.
Interestingly, we also found inflammatory (Gr1+) dendritic cells in the brain. These cells are derived from Gr1+ monocytes that have migrated to peripheral tissues and stimulate TH1 responses after infection[18]. Whether these cells are functioning to stimulate the T cells in the brain after sterile injury is unclear. Some blood-derived dendritic cells have recently been shown to limit inflammation and aid in remodeling of the myocardium after experimental myocardial infarction[19]. In our model higher numbers of CD11chi dendritic cells, as well as other myeloid populations, are found at 72 hours[20].
The prototypical chemokine for inflammatory monocyte recruitment (CCL2/MCP-1) was increased in the brain, consistent with their high numbers in brain. Dendritic cells are also known to cross the blood-brain barrier in response to CCL2[21]. Not surprisingly, CXCL1 (KC), the chemokine for neutrophil recruitment was also increased, as was the pro-inflammatory chemokine IL-6. However, TNF levels were unexpectedly lower in ipsilateral ICH brain versus sham. The TNF response after ICH has been studied by several groups. In some studies, TNF levels peaked by 4 hours and returned to baseline within the first day after ICH[22,23], while others have shown increased TNF protein or mRNA levels persisting for days after ICH[24,25]. In a human study, no difference was found in TNF mRNA levels between ICH samples evacuated within 24 hours of onset and control brains[26]. It is possible that the TNF response is multiphasic or that the species, model, and method of quantification of TNF contribute to the varying results. Our data represent one snapshot of TNF levels measured by multiplex ELISA after 15 μL blood injection.
These studies used a blood transfer model in which blood from a CD45.2-expressing mouse was used for the intracerebral hemorrhage in a CD45.1-expressing host. CD45.2-expressing leukocytes from within the hemorrhage could therefore be distinguished from the CD45.1-expressing leukocytes recruited to the area from the host’s circulation during the inflammatory response. Surprisingly few leukocytes originating from within injected blood were alive and recoverable from the brain at 12 hours.
Microglia are notoriously difficult to distinguish from macrophages by immunohistochemistry as both myeloid cell populations express CD11b, Iba1, CD68, and others[27], but have traditionally been separated from peripheral leukocytes in flow cytometry experiments by differential CD45 expression[28]. Here, the population identified as microglia are cells expressing low to intermediate levels of CD45.1 and high levels of CD11b. While we are confident the majority of microglia are included by these criteria, it is possible that some activated microglia may upregulate CD45, thus falling into the CD45.1hi classification.
Our findings of large numbers of the inflammatory macrophage and inflammatory dendritic cells in the brain by 12 hours after ICH challenge existing concepts of the kinetics of leukocyte infiltration after ICH. In addition, these findings may be relevant to ischemic stroke and other acute sterile brain injuries, in which neutrophils are also thought to be the earliest infiltrating cell population[29]. If these monocyte populations significantly contribute to the inflammatory cascade and injury, the time window for interference with this process may be shorter than previously considered.
Acknowledgments
This study was supported by NIH 5T32 NS041224 (MDH).
References
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 3.Kane PJ, Modha P, Strachan RD, Cook S, Chambers IR, Clayton CB, Mendelow AD. The effect of immunosuppression on the development of cerebral oedema in an experimental model of intracerebral haemorrhage: Whole body and regional irradiation. J Neurol Neurosurg Psychiatry. 1992;55:781–786. doi: 10.1136/jnnp.55.9.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 5.Sansing LH, Kasner SE, McCullough L, Agarwal P, Welsh FA, KK Autologous blood injection to model spontaneous intracerebral hemorrhage in mice. Journal of Visualized Experiments. 2011 doi: 10.3791/2618. http://www.jove.com/index/Details.stp?ID=2618. [DOI] [PMC free article] [PubMed]
- 6.Del Bigio MR, Yan H-J, Buist R, Peeling J, del Zoppo GJ. Experimental intracerebral hemorrhage in rats: Magnetic resonance imaging and histopathological correlates. Stroke. 1996;27:2312–2320. doi: 10.1161/01.str.27.12.2312. [DOI] [PubMed] [Google Scholar]
- 7.Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: Time course of inflammation and cell death. Neuroscience Letters. 2000;283:230–232. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- 8.Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Research. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- 9.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 10.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. Tnf/inos-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 11.King IL, Dickendesher TL, Segal BM. Circulating ly-6c+ myeloid precursors migrate to the cns and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor ccr2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 13.Schilling M, Strecker JK, Ringelstein EB, Schabitz WR, Kiefer R. The role of cc chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res. 2009;1289:79–84. doi: 10.1016/j.brainres.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Tsirka SE. The ccl2-ccr2 system affects the progression and clearance of intracerebral hemorrhage. Glia. 2012 doi: 10.1002/glia.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 16.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 18.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute t helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anzai A, Anzai T, Nagai S, Maekawa Y, Naito K, Kaneko H, Sugano Y, Takahashi T, Abe H, Mochizuki S, Sano M, Yoshikawa T, Okada Y, Koyasu S, Ogawa S, Fukuda K. Regulatory role of dendritic cells in post-infarction healing and left ventricular remodeling. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.052126. [DOI] [PubMed] [Google Scholar]
- 20.Sansing LH, Harris TH, Welsh FA, Kasner SE, Hunter CA, Kariko K. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann Neurol. 2011;70:646–656. doi: 10.1002/ana.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagar D, Foss C, El Baz R, Pomper MG, Khan ZK, Jain P. Mechanisms of dendritic cell trafficking across the blood-brain barrier. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke. 2001;32:162–167. doi: 10.1161/01.str.32.1.162. [DOI] [PubMed] [Google Scholar]
- 23.Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58:542–550. doi: 10.1227/01.NEU.0000197333.55473.AD. discussion 542–550. [DOI] [PubMed] [Google Scholar]
- 24.Mayne M, Ni W, Yan HJ, Xue M, Johnston JB, Del Bigio MR, Peeling J, Power C, Feuerstein GZ, Ho S, Wang X. Antisense oligodeoxynucleotide inhibition of tumor necrosis factor-{{alpha}} expression is neuroprotective after intracerebral hemorrhage editorial comment. 2001;32:240–248. doi: 10.1161/01.str.32.1.240. [DOI] [PubMed] [Google Scholar]
- 25.Liesz A, Middelhoff M, Zhou W, Karcher S, Illanes S, Veltkamp R. Comparison of humoral neuroinflammation and adhesion molecule expression in two models of experimental intracerebral hemorrhage. Exp Transl Stroke Med. 2011;3:11. doi: 10.1186/2040-7378-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmichael ST, Vespa PM, Saver JL, Coppola G, Geschwind DH, Starkman S, Miller CM, Kidwell CS, Liebeskind DS, Martin NA. Genomic profiles of damage and protection in human intracerebral hemorrhage. J Cereb Blood Flow Metab. 2008;28:1860–1875. doi: 10.1038/jcbfm.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: A review of function and identification. J Leukoc Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 28.Renno T, Krakowski M, Piccirillo C, Lin JY, Owens T. Tnf-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by th1 cytokines. J Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- 29.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]