Abstract
Muscle weakness is commonly cited as a cause of crouch gait in individuals with cerebral palsy; however, outcomes after strength training are variable and mechanisms by which muscle weakness may contribute to crouch gait are unclear. Understanding how much muscle strength is required to walk in a crouch gait compared to an unimpaired gait may provide insight into how muscle weakness contributes to crouch gait and assist in the design of strength training programs. The goal of this study was to examine how much muscle groups could be weakened before crouch gait becomes impossible. To investigate this question, we first created muscle-driven simulations of gait for three typically-developing children and six children with cerebral palsy who walked with varying degrees of crouch severity. We then simulated muscle weakness by systematically reducing the maximum isometric force of each muscle group until the simulation could no longer reproduce each subject’s gait. This analysis indicated that moderate crouch gait required significantly more knee extensor strength than unimpaired gait. In contrast, moderate crouch gait required significantly less hip abductor strength than unimpaired gait, and mild crouch gait required significantly less ankle plantarflexor strength than unimpaired gait. The reduced strength required from the hip abductors and ankle plantarflexors during crouch gait suggests that weakness of these muscle groups may contribute to crouch gait and that these muscle groups are potential targets for strength training.
Keywords: cerebral palsy, crouch gait, strength, muscle, simulation
Introduction
Cerebral palsy is caused by a lesion to the brain that occurs at or near the time of birth and results in impaired movement. It is the most common childhood movement disorder and affects approximately 3.6 of every 1000 children born in the United States (Yeargin-Allsopp et al., 2008). Many individuals with cerebral palsy walk in a crouch gait pattern characterized by excessive flexion of the hip, knee, and ankle during the stance phase of gait. Walking in a crouch is inefficient and can lead to joint pain (Jahnsen et al., 2004) and bone deformities (Graham and Selber, 2003).
There are many suggested causes of crouch gait, including contracted hamstrings, contracted hip flexors, impaired balance, foot deformities, impaired proprioception, and muscle weakness (Gage, 1990). Muscle weakness can be caused by various mechanisms such as impaired motor control and reduced physiological cross-sectional area of muscles. Muscle weakness is common in individuals with cerebral palsy (Damiano et al., 1995b; Wiley and Damiano, 1998), and strength training is often incorporated into therapy (Dodd et al., 2002; Pippenger and Scalzitti, 2004; Mockford and Caulton, 2008). Although individuals with cerebral palsy can increase their muscle strength after completing a strength training program (Damiano et al., 1995b; Dodd et al., 2002; Mockford and Caulton, 2008), the improvements in gait after strength training are variable. Previous studies have reported improvements in knee extension (Damiano et al., 1995a) and functional walking tests after strength training (Blundell et al., 2003; Dodd et al., 2003); however, other studies have found no significant change in walking tests (Kerr et al., 2006; Liao et al., 2007) or deterioration in gait for some subjects (Damiano et al., 2010).
A rationale for strength training is that individuals with crouch gait may not have adequate strength to walk in an upright posture. However, previous studies have shown that walking in a crouch gait requires a greater knee extensor moment (Hsu et al., 1993; Lin et al., 2000) and greater total muscle force (Steele et al., 2010) than walking in an upright gait. Although crouch gait requires larger forces in some muscles, there may be other muscles that are too weak to achieve an upright gait pattern. Unimpaired gait has been shown to be most sensitive to weakness of the ankle plantarflexors, hip abductors, and hip flexors (van der Krogt et al, 2012). Comparing the amount of muscle strength required during crouch gait to unimpaired gait can provide insight into how muscle weakness contributes to crouch gait and how strength training could improve gait.
The aim of this study was to examine how much muscle groups could be weakened before walking in a crouch gait becomes impossible. We used a musculoskeletal model in which it was possible to reduce the force-generating capacity of particular muscle groups to simulate weakness. We created muscle-driven simulations of unimpaired subjects and subjects with cerebral palsy who walked in varying degrees of crouch gait. For each subject, we examined how much particular muscle groups could be weakened before the simulation could no longer reproduce the subject’s gait pattern. Identifying muscle groups that require less strength to walk in a crouch gait than unimpaired gait may suggest which muscles may be too weak to walk in an upright posture and may be targets for strength training programs.
Methods
Three typically-developing children and six children with spastic diplegic cerebral palsy (Table 1) were selected from a database of subjects who had visited Gillette Children’s Specialty Healthcare for motion analysis. Three-dimensional motion analysis data was collected using a 12-camera system (Vicon Motion Systems, Lake Forest, CA) and a standard marker measurement protocol (Davis et al., 1991). Ground reaction forces were measured with four force plates (AMTI, Watertown, MA), sampled at 1080 Hz, and low-pass filtered at 20 Hz. All subjects walked barefoot at their self-selected speed without walking aids.
Table 1.
Subject characteristics
| N | Age (years) | Height (cm) | Weight (kg) | |
|---|---|---|---|---|
| Unimpaired | 3 | 10 ± 3 | 144 ± 16 | 36 ± 9 |
| Mild Crouch | 3 | 9 ± 1 | 124 ± 10 | 24 ± 4 |
| Moderate Crouch | 3 | 11 ± 2 | 136 ± 6 | 43 ± 31 |
The subjects with cerebral palsy all walked in a crouch gait and had a minimum knee flexion angle during stance greater than 15° and a maximum ankle dorsiflexion angle during stance greater than 5°. The crouch subjects were divided evenly into two groups based upon crouch severity: mild crouch gait (N=3, minimum knee flexion angle of 15-30°) and moderate crouch gait (N=3, minimum knee flexion angle of 30-50°).
Muscle-driven simulations were created that represent the gait dynamics of each subject using OpenSim, a freely available biomechanical simulation package (Delp et al., 2007), http://opensim.stanford.edu). Using simulation allowed us to selectively weaken particular muscle groups, account for some of the complexities of the musculoskeletal system, such as the force-length-velocity relationship of muscle, and examine the compensatory actions from other muscles in response to simulated muscle weakness. A generic musculoskeletal model (lower extremities from Delp et al., 1990, and torso from Anderson and Pandy, 1999) with 19 degrees of freedom and 92 musculotendon actuators was scaled to each subject using anthropometric measurements (Figure 1). The degrees of freedom in this model included three rotations and translations of the pelvis, a ball-and-socket joint between the pelvis and torso at the third lumbar vertebra, a ball-and-socket joint at each hip, a planar joint with coupled translations at each knee, and a revolute joint at each ankle. The maximum isometric forces of the muscles in the model were geometrically scaled by height-squared of each subject. We scaled muscle strength by height-squared because muscle strength is proportional to a muscle’s physiological cross-sectional area, which is correlated with height-squared in adults and children (Jaric, 2002; Parker et al., 1990; Schantz et al., 1983).
Figure 1.
Musculoskeletal models used to create dynamic simulations of gait for individuals that walked in an unimpaired gait pattern (left) and individuals with cerebral palsy who walked in mild (center) and moderate (right) crouch gait.
Joint angles were calculated for one walking trial for each subject by computing the joint angles that minimize the difference between experimental marker positions and virtual markers placed on the model (Figure 2A). Joint moments were calculated using inverse dynamics (Figure 2B). To determine the motion of the torso, experimental marker data was available for all of the unimpaired subjects and three of the six subjects with crouch gait. For the individuals without torso markers, torso position was set to 20° lumbar flexion, 0° lumbar bending, and 0° lumbar rotation. To test the effects of torso position, we completed the analysis for the unimpaired subjects with and without torso markers and found no significant differences in the results (Supplementary Figure 1).
Figure 2.
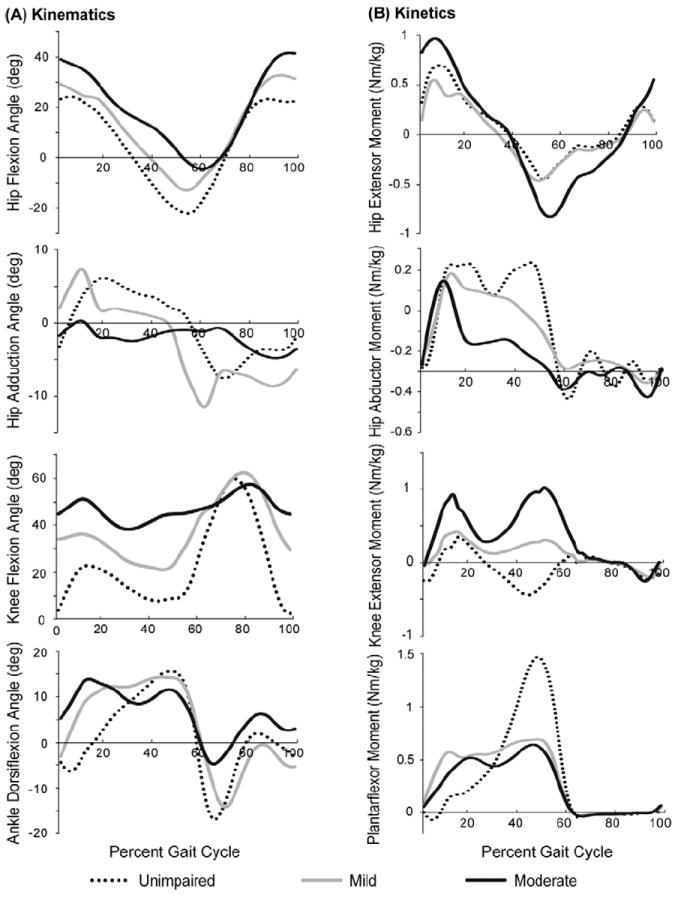
The average hip, knee, and ankle kinematics (A) and kinetics (B) during unimpaired gait (dotted) and mild (light gray) and moderate (dark gray) crouch gait. Joint moments are normalized by body mass (kg).
The Residual Reduction Algorithm was used to improve the dynamic consistency of the simulation (Delp et al., 2007). This algorithm makes small changes to kinematics (< 2°) and the torso mass center location (< 2cm) to reduce the residual forces applied to the pelvis. After running the Residual Reduction Algorithm, the residual forces were less than ten percent of body-weight and the residual moments were less than ten percent of body-weight times height for all subjects. Muscle excitations were estimated using the Computed Muscle Control (CMC) algorithm, which determines a set of muscle excitations that recreates the subject’s kinematics (Thelen et al., 2003; Thelen and Anderson, 2006). At each time step, CMC determines the set of muscle excitations that produce the forces that generate measured accelerations for all degrees of freedom taking into account muscle activation dynamics and force-length-velocity properties. The distribution of muscle excitations among redundant actuators was determined by minimizing the sum of activations-squared at each time step. Idealized torque actuators, known as reserve actuators, were included for each degree of freedom in the model to provide extra torque if the muscles could not generate the measured accelerations. Reserve actuator torques were used to determine when the muscles could no longer produce the subject’s motion.
To assess the accuracy of the simulation we compared the simulated kinematics and kinetics to experimental data. The simulated joint angles reproduced the joint angles calculated by inverse kinematics with a maximum difference of less than two degrees. Additionally, the sum of the moments generated by the muscle forces at each degree of freedom reproduced the joint moments computed using the Residual Reduction Algorithm with an average root mean square error of 1.7 ± 0.9 Nm across all subjects.
To determine how much particular muscle groups could be weakened, each of seven muscle groups were sequentially weakened by systematically reducing the maximum isometric force of each muscle in the group. Table 2 lists the muscles in each group. Each muscle group was weakened in isolation. We reduced the maximum isometric force until the CMC algorithm could no longer determine a set of muscle excitations that would recreate the subject’s dynamics with an error less than 2° for all degrees of freedom and torques from the reserve actuators that were less than ten percent of the maximum joint moment at each degree of freedom. We used the bisection method (Burden and Faires, 1985) to determine how much the maximum isometric force of each muscle group could be reduced. This method searches through the space of all possible values for the maximum isometric force by iteratively taking the middle of an interval representing the maximum and minimum possible values from previous iterations until it converges upon the minimum value that could recreate the subject’s dynamics. This point was termed the required muscle strength for each muscle group and was expressed as a percent of the muscle group’s maximum isometric force.
Table 2.
Description of muscle groups
| Name | Description |
|---|---|
| Gluteus Maximus | Three musculotendon actuators representing the medial, intermediate, and lateral components of the muscle |
| Hip Abductors | Gluteus medius and gluteus minimus each modeled as three musculotendon actuators representing the anterior, intermediate, and posterior components of the muscle |
| Iliopsoas | Iliacus and psoas each modeled by a single musculotendon actuator |
| Hamstrings | Three musculotendon actuators representing the bi-articular hamstrings including the biceps femoris long head, semimembranosus, and semitendinousus |
| Quadriceps | Four musculotendon actuators representing the rectus femoris, vastus medials, vastus intermedius, and vastus lateralis |
| Ankle Plantarflexors | Three musculotendon actuators representing the soleus and the medial and lateral heads of the gastrocnemius |
| Ankle Dorsiflexors | Musculotendon actuators representing the anterior tibialis, extensor digitorum, extensor hallus longus, and peroneus tertius |
Because of the limited number of subjects and three groups for comparison, we used a protected Fisher’s Least Significant Difference test to determine if the required muscle strength was different between unimpaired gait and mild and moderate crouch gait (Meier, 2006; Liu and Tu, 2008). In this analysis a global test, one-way ANOVA, was performed for each muscle group, and only if the p-value of this test was less than 0.1 did we perform a Fisher’s Least Significant Difference test for differences between the three gait patterns with a significance level of 0.05. Using the protected Fisher’s Least Significance Difference test reduced the chance of Type I errors (i.e. false positives), although the limited sample size increased the chance of Type II errors (i.e. false negatives). We also performed regression analyses to determine if required muscle strength changed with crouch severity.
Results
The strength, or force-generating capacity, required from each muscle group could be reduced substantially while the model could still produce the dynamics of unimpaired gait and crouch gait (Figure 3). This occurs in the simulations because neither unimpaired gait nor crouch gait requires maximum strength, and other muscles can compensate for the diminished strength of a particular muscle group. When we reduced the maximum isometric force of one muscle group, the activation level of that muscle group increased and other compensatory actions arose from muscles with similar function (Supplementary Figure 2). For example, the strength required from the gluteus maximus could be reduced to near zero in the simulations and the dynamics of unimpaired gait and crouch gait could be maintained. The gluteus maximus represents about 35% of the hip extension moment generating capacity; thus, when its strength is reduced, other muscles, such as the gluteus medius and bi-articular hamstrings increase their activity level.
Figure 3.
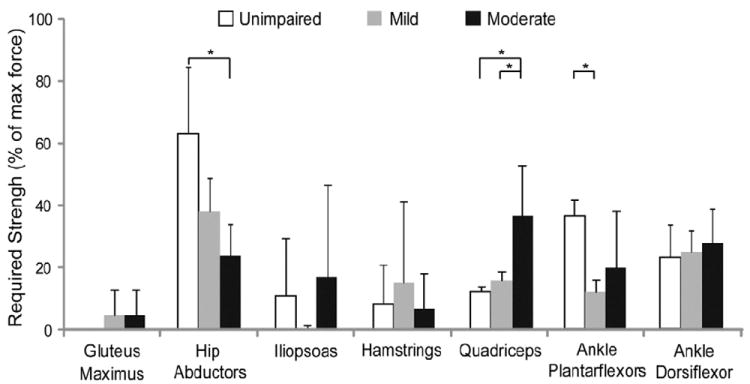
Required strength for each muscle group that was necessary to recreate the subjects’ gait patterns expressed as percent of the maximum isometric force (average ± 1 SE, * p < 0.05 from Fisher’s Least Significant Difference test). The Fisher’s Least Significant Difference test was only performed if the p-value from the one-way ANOVA was less than 0.1. The ANOVA p-values for the hip abductors, quadriceps, and ankle plantarflexors were 0.05, 0.04, and 0.09, respectively.
Moderate crouch gait required significantly more quadriceps strength than unimpaired gait and mild crouch gait (Figure 3). Furthermore, the quadriceps strength required increased quadratically with crouch severity (r2=0.75). This trend is largely due to the greater knee extensor moment required from inverse dynamics during stance in crouch gait compared to unimpaired gait (see knee extensor moment in Figure 2B).
The hip abductor strength required was significantly less during moderate crouch gait than unimpaired gait (Figure 3) and decreased linearly with crouch severity (r2= 0.58). The hip abductor moment required from inverse dynamics was also reduced in crouch gait compared to unimpaired gait, especially during terminal stance (see hip abductor moment in Figure 2B). The muscles that were weakened in the simulation included the gluteus medius and the gluteus minumus, which represent about 70% of the hip abductor moment generating capacity, and few other muscles can compensate for weakness of these muscles.
The ankle plantarflexor strength required was significantly less during mild crouch gait than during unimpaired gait (Figure 3). The ankle plantarflexor moment required from inverse dynamics during early and mid stance was larger during crouch gait; however, the peak plantarflexor moment required in terminal stance was reduced during crouch gait (see ankle plantarflexor moment in Figure 2B).
The ankle dorsiflexor strength required was similar across all gait patterns (Figure 3). No consistent differences in the amount of hamstring or iliopsoas strength required were identified between unimpaired gait and crouch gait.
Discussion
Our results indicate that the required knee extensor strength increased with crouch severity, required hip abductor strength was reduced in moderate crouch gait, and required ankle plantarflexor strength was reduced in mild crouch. Identifying which muscle groups require more or less strength during crouch gait than unimpaired gait can provide insight into how muscle weakness, or a reduced force-generating capacity, could contribute to crouch gait and provide guidance for strength training programs. Weakness of muscle groups that require more strength during crouch gait than unimpaired gait, such as the quadriceps, are not likely contributors to crouch gait; however, weakness of muscle groups that require less strength during crouch gait than unimpaired gait could contribute to crouch gait. Weakness of the hip abductors and ankle plantarflexors may preclude individuals with crouch gait from achieving an upright gait pattern, and these muscle groups may be important targets for strength training programs.
The focus of this study was to isolate the effects of reduced force-generating capacity on the dynamics of crouch gait; however, multiple mechanisms including reduced physiological cross-sectional area, impaired neural drive, altered selective motor control, and reduced voluntary force capacity can contribute to muscle weakness. Neural mechanisms such as reduced voluntary force capacity (Rose and McGill, 2005; Elder et al. 2007) and muscle properties such as reduced physiological cross-sectional area (Moreau et al. 2009) have been documented in individuals with cerebral palsy. Future investigations are required to differentiate between these mechanisms.
The small number of subjects available for simulation and analysis is a limitation of this study. Some of the muscle groups, such as the hamstrings and iliopsoas, had variable results which may require a larger number of subjects to understand the relationship between required strength and crouch gait.
Our analysis demonstrated that moderate crouch gait places a greater demand on the quadriceps than unimpaired gait. This suggests that if subjects are able to walk in a crouch gait that they have sufficient force-generating capacity to walk in an upright gait. Previous studies have reported an increase in quadriceps activity with crouch severity measured using electromyography taken during a static crouch posture (Hsu et al., 1993) or estimated from cadavers positioned in a crouch posture (Perry et al., 1975). Strength training programs designed for individuals with cerebral palsy and crouch gait have targeted the hip and knee extensors (Damiano et al., 1995a; Damiano et al., 2010) and reported inconsistent improvements in knee extension. The results of our study may help to explain the inconsistent outcomes after these strength training programs because crouch gait requires greater quadriceps force than unimpaired gait and may not contribute to crouch gait in some individuals. However, case studies have documented a lack of force-generating capacity when the knee is near full extension in some individuals with cerebral palsy (Gage and Novacheck, 2001). Thus, although crouch gait required more quadriceps strength than unimpaired gait, if individuals with crouch gait cannot generate sufficient force when the knee is in full extension, quadriceps weakness may still contribute to crouch gait. Examining the relationship between quadriceps strength and knee flexion angle is an important area for further investigation in individuals with cerebral palsy.
Moderate crouch gait required less hip abductor strength than unimpaired gait and mild crouch gait required less ankle plantarflexor strength than unimpaired gait. In the subjects included in this study, we also observed from inverse dynamics reduced hip abductor moments and ankle plantarflexor moments during stance. To investigate if reduced joint moments were present in a larger population, we examined the kinematic and kinetic data for all crouch gait subjects who visited Gillette Children’s Specialty Healthcare from February 1994 to January 2010. This analysis included 82 typically-developing children, 976 mild crouch gait subjects and 209 moderate crouch gait subjects using the same criteria described in the methods. A comparison of the hip abductor moment in this larger population indicated that the hip abductor moment during crouch gait was significantly reduced compared to unimpaired gait (Figure 4), as determined by a one-way ANOVA (p < 0.001). Additionally, the ankle plantarflexor moment during terminal stance was significantly reduced during crouch gait (p < 0.001) and the ankle dorsiflexion angle during stance increased with crouch severity (p < 0.001). Our simulations revealed a significant decrease in the ankle plantarflexor strength required during mild crouch gait compared to unimpaired gait, but not during moderate crouch gait. As the ankle dorsiflexion angle increases with crouch severity, the ankle plantarflexion moment arms of the gastrocnemius and soleus decrease, which would require greater ankle plantarflexor force to produce the required ankle plantarflexor moment.
Figure 4.
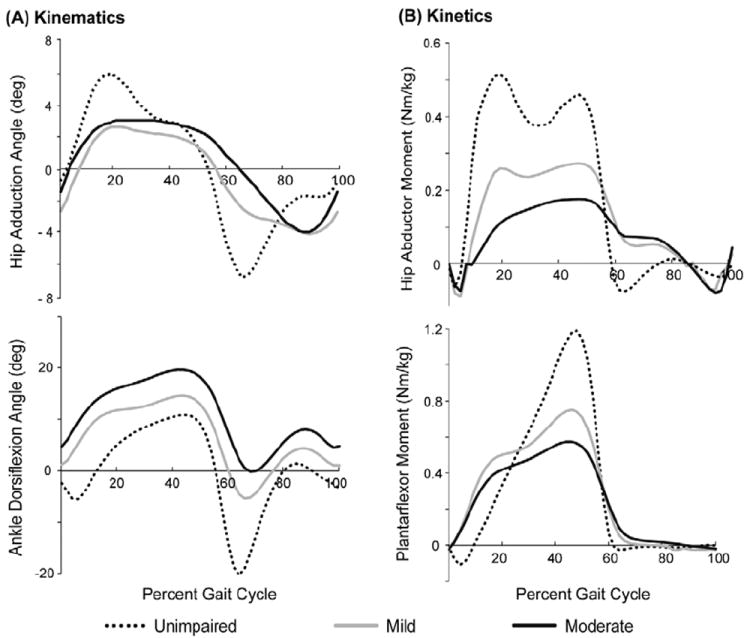
Kinematics (A) and kinetics (B) for hip abduction and ankle plantarflexion for unimpaired children (N=82, dotted black) and individuals with cerebral palsy who walked in mild (N = 976, light gray) and moderate (N=209, dark gray) crouch gait who visited Gillette Children’s Specialty Healthcare. The peak hip abductor moment and ankle plantarflexor moment are smaller during mild and moderate crouch gait compared to unimpaired gait (ANOVA, p < 0.001). Joint moments are normalized by body mass (kg).
Ankle plantarflexor weakness has previously been noted as a potential cause of crouch gait (Gage, 1990). Previous studies have reported that ankle plantarflexor strength in individuals with diplegic cerebral palsy is reduced to 40-60% of age-matched typically-developing peers (Wiley and Damiano, 1998; Elder et al., 2003). Potential causes of ankle plantarflexor weakness include impaired control, over-lengthening of the Achilles tendon, or effects from injections of neural toxins. Outcome studies after surgical lengthening of the Achilles tendon have noted an increased risk of crouch gait (Damron et al., 1994; Dietz et al., 2006). These results support the theory that some individuals may adopt crouch gait as a result of weak ankle plantarflexors. Engsberg et al. (2006) found that strengthening the ankle plantarflexors improved knee extension during stance in individuals with cerebral palsy.
Limited information is available regarding hip abductor strength in individuals with cerebral palsy. Wiley et al. (1998) reported that average hip abductor strength in individuals with diplegic cerebral palsy was 55% of age-matched typically-developing children. Hip abductor weakness has previously been associated with a Trendelenburg gait pattern, characterized by exaggerated torso bending in the coronal plane (Perry, 1992). To examine if torso motion contributed to the reduced hip abductor moment during crouch gait, we compared the torso motion of the subjects with crouch gait who had torso markers and crouch gait subjects from Gillette Children’s Specialty Healthcare who had torso markers (mild crouch gait N=31, moderate crouch gait N=10) to unimpaired gait (N=82). We found no significant difference in torso bending during mild and moderate crouch gait compared to unimpaired gait, suggesting that crouch posture rather than torso motion contributed to the reduced hip abductor strength required during crouch gait. Further study is required to determine the extent of hip abductor weakness in individuals with crouch gait and if strengthening the hip abductors improves crouch gait.
This study used muscle-driven simulations to examine how much the maximum isometric force of particular muscle groups could be reduced and maintain the dynamics of crouch gait compared to unimpaired gait. The simulations allowed us to examine the effects of individual muscle group weakness that could not be elucidated with other methods. The results of this study suggest that crouch gait requires greater quadriceps strength than unimpaired gait; however, moderate crouch gait requires less hip abductor strength and mild crouch gait requires less ankle plantarflexor strength than unimpaired gait. Future studies that investigate the effects of strengthening the hip abductors or ankle plantarflexors on crouch gait can further improve our understanding of how weakness of these muscles contributes to crouch gait and assist in the design of strength training programs that improve walking ability.
Supplementary Material
Supplementary Figure 1. Comparison of the required strength for each muscle group of the unimpaired subjects (N=3) when using torso markers for inverse kinematics and without torso markers (average ± 1 SE). There was no significant difference in the results with or without torso markers.
Supplementary Figure 2. Compensatory muscle action when a particular muscle group is weakened, expressed as the average change in muscle force over the gait cycle (times body-weight, BW). For each muscle group, the change in force of the weakened muscle group and the change in force of the three muscles with the greatest change in force are shown (average ± 1 SE).
Acknowledgments
The authors thank the staff of the James R. Gage Center for Gait and Motion Analysis at Gillette Children’s Specialty Healthcare. This work was funded by NIH grants R01 HD033929, R24 HD065690, and U54 GM072970, and an NSF Graduate Research Fellowship.
Footnotes
Conflict of Interest Statement
None of the authors had financial or personal conflict of interest with regard to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson FC, Pandy MG. A Dynamic Optimization Solution for Vertical Jumping in Three Dimensions. Comput Methods Biomech Biomed Engin. 1999;2:201–231. doi: 10.1080/10255849908907988. [DOI] [PubMed] [Google Scholar]
- Blundell SW, Shepherd RB, Dean CM, Adams RD, Cahill BM. Functional strength training in cerebral palsy: a pilot study of a group circuit training class for children aged 4-8 years. Clin Rehabil. 2003;17:48–57. doi: 10.1191/0269215503cr584oa. [DOI] [PubMed] [Google Scholar]
- Burden RL, Faires JD, editors. Numerical analysis. Prindle, Weber & Schmidt; Boston, Mass: 1985. 2.1 The Bisection Algorithm; pp. x–676. [Google Scholar]
- Damiano DL, Arnold AS, Steele KM, Delp SL. Can strength training predictably improve gait kinematics? A pilot study on the effects of hip and knee extensor strengthening on lower-extremity alignment in cerebral palsy. Phys Ther. 2010;90:269–79. doi: 10.2522/ptj.20090062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano DL, Kelly LE, Vaughn CL. Effects of quadriceps femoris muscle strengthening on crouch gait in children with spastic diplegia. Phys Ther. 1995a;75:658–67. doi: 10.1093/ptj/75.8.658. discussion 668-71. [DOI] [PubMed] [Google Scholar]
- Damiano DL, Vaughan CL, Abel MF. Muscle response to heavy resistance exercise in children with spastic cerebral palsy. Dev Med Child Neurol. 1995b;37:731–9. doi: 10.1111/j.1469-8749.1995.tb15019.x. [DOI] [PubMed] [Google Scholar]
- Damron TA, Greenwald TA, Breed AL. Chronologic outcome of surgical tendoachilles lengthening and natural history of gastroc-soleus contracture in cerebral palsy. A two-part study. Clin Orthop Relat Res. 1994;301:249–255. [PubMed] [Google Scholar]
- Davis R, Ounpuu S, Tyburski D, Gage J. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575–587. [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54:1940–50. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37:757–67. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- Dietz FR, Albright JC, Dolan L. Medium-term follow-up of Achilles tendon lengthening in the treatment of ankle equinus in cerebral palsy. Iowa Orthop J. 2006;26:27–32. [PMC free article] [PubMed] [Google Scholar]
- Dodd KJ, Taylor NF, Damiano DL. A systematic review of the effectiveness of strength-training programs for people with cerebral palsy. Arch Phys Med Rehabil. 2002;83:1157–64. doi: 10.1053/apmr.2002.34286. [DOI] [PubMed] [Google Scholar]
- Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young people with cerebral palsy. Dev Med Child Neurol. 2003;45:652–7. doi: 10.1017/s0012162203001221. [DOI] [PubMed] [Google Scholar]
- Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A, Leahey L. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45:542–50. doi: 10.1017/s0012162203000999. [DOI] [PubMed] [Google Scholar]
- Engsberg JR, Ross SA, Collins DR. Increasing ankle strength to improve gait and function in children with cerebral palsy: a pilot study. Pediatr Phys Ther. 2006;18:266–75. doi: 10.1097/01.pep.0000233023.33383.2b. [DOI] [PubMed] [Google Scholar]
- Gage JR. Surgical treatment of knee dysfunction in cerebral palsy. Clin Orthop Relat Res. 1990:45–54. [PubMed] [Google Scholar]
- Gage JR, Novacheck TF. An update on the treatment of gait problems in cerebral palsy. J Pediatr Orthop B. 2001;10:265–74. [PubMed] [Google Scholar]
- Graham H, Selber P. Musculoskeletal aspects of cerebral palsy. Journal of Bone & Joint Surgery, British Volume. 2003;85:157–166. doi: 10.1302/0301-620x.85b2.14066. [DOI] [PubMed] [Google Scholar]
- Hsu AT, Perry J, Gronley JK, Hislop HJ. Quadriceps force and myoelectric activity during flexed knee stance. Clin Orthop Relat Res. 1993:254–62. [PubMed] [Google Scholar]
- Jahnsen R, Villien L, Aamodt G, Stanghelle JK, Holm I. Musculoskeletal pain in adults with cerebral palsy compared with the general population. J Rehabil Med. 2004;36:78–84. doi: 10.1080/16501970310018305. [DOI] [PubMed] [Google Scholar]
- Jaric S. Muscle strength testing: use of normalisation for body size. Sports Med. 2002;32:615–631. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed] [Google Scholar]
- Kerr C, McDowell B, Cosgrove A, Walsh D, Bradbury I, McDonough S. Electrical stimulation in cerebral palsy: a randomized controlled trial. Dev Med Child Neurol. 2006;48:870–6. doi: 10.1017/S0012162206001915. [DOI] [PubMed] [Google Scholar]
- Liao HF, Liu YC, Liu WY, Lin YT. Effectiveness of loaded sit-to-stand resistance exercise for children with mild spastic diplegia: a randomized clinical trial. Arch Phys Med Rehabil. 2007;88:25–31. doi: 10.1016/j.apmr.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Guo LY, Su FC, Chou YL, Cherng RJ. Common abnormal kinetic patterns of the knee in gait in spastic diplegia of cerebral palsy. Gait Posture. 2000;11:224–32. doi: 10.1016/s0966-6362(00)00049-7. [DOI] [PubMed] [Google Scholar]
- Liu S, Tu D. On the Applications of Fisher’s Least Significant Difference (LSD) Procedure in Three-Arm Clinical Trials With Survival Endpoints. Drug Information Journal. 2008;42:81–91. [Google Scholar]
- Meier U. A note on the power of Fisher’s least significant difference procedure. Pharmaceutical Statistics. 2006;5:253–263. doi: 10.1002/pst.210. [DOI] [PubMed] [Google Scholar]
- Mockford M, Caulton JM. Systematic review of progressive strength training in children and adolescents with cerebral palsy who are ambulatory. Pediatr Phys Ther. 2008;20:318–33. doi: 10.1097/PEP.0b013e31818b7ccd. [DOI] [PubMed] [Google Scholar]
- Moreau NG, Teefey SA, Damiano DL. In vivo muscle architecture and size of the rectus femoris and vastus lateralis in children and adolescents with cerebral palsy. Dev Med Child Neurol. 2009;51:800–806. doi: 10.1111/j.1469-8749.2009.03307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DF, Round JM, Sacco P, Jones DA. A cross-sectional survey of upper and lower limb strength in boys and girls during childhood and adolescence. Ann Hum Biol. 1990;17:199–211. doi: 10.1080/03014469000000962. [DOI] [PubMed] [Google Scholar]
- Perry J, Antonelli D, Ford W. Analysis of knee-joint forces during flexed-knee stance. J Bone Joint Surg Am. 1975;57:961–7. [PubMed] [Google Scholar]
- Perry J. Gait analysis – normal and pathological gait. Slack Inc.; Thorofare, NJ: 1992. pp. 268–270. [Google Scholar]
- Pippenger WS, Scalzitti DA. What are the effects, if any, of lower-extremity strength training on gait in children with cerebral palsy? Phys Ther. 2004;84:849–58. [PubMed] [Google Scholar]
- Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev Med Child Neurol. 2005;47:329–336. doi: 10.1017/s0012162205000629. [DOI] [PubMed] [Google Scholar]
- Schantz P, Randall-Fox E, Hutchison W, Tyden A, Astrand PO. Muscle fibre type distribution, muscle cross-sectional area and maximal voluntary strength in humans. Acta Physiol Scand. 1983;117:219–26. doi: 10.1111/j.1748-1716.1983.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Steele KM, Seth A, Hicks JL, Schwartz MS, Delp SL. Muscle contributions to support and progression during single-limb stance in crouch gait. J Biomech. 2010;43:2099–2105. doi: 10.1016/j.jbiomech.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen DG, Anderson FC. Using computed muscle control to generate forward dynamic simulations of human walking from experimental data. J Biomech. 2006;39:1107–15. doi: 10.1016/j.jbiomech.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Anderson FC, Delp SL. Generating dynamic simulations of movement using computed muscle control. J Biomech. 2003;36:321–8. doi: 10.1016/s0021-9290(02)00432-3. [DOI] [PubMed] [Google Scholar]
- van der Krogt MM, Delp SL, Schwartz MH. How robust is human gait to muscle weakness? Gait Posture. 2012 doi: 10.1016/j.gaitpost.2012.01.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol. 1998;40:100–7. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547–54. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Comparison of the required strength for each muscle group of the unimpaired subjects (N=3) when using torso markers for inverse kinematics and without torso markers (average ± 1 SE). There was no significant difference in the results with or without torso markers.
Supplementary Figure 2. Compensatory muscle action when a particular muscle group is weakened, expressed as the average change in muscle force over the gait cycle (times body-weight, BW). For each muscle group, the change in force of the weakened muscle group and the change in force of the three muscles with the greatest change in force are shown (average ± 1 SE).


