Abstract
The aim of the present study was to investigate the expression of hypoxia-inducible factor-1α (HIF-1α) in tongue squamous cell carcinoma (TSCC) and to assess its possible impact on prognosis. A total of 49 tumor samples and 15 adjacent non-tumor samples from 49 patients treated between January 2000 and December 2005 at the Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Tongji University (Shanghai, China) were obtained for investigation with immunohistochemistry and reverse transcription-polymerase chain reaction (RT-PCR). The expression of HIF-1α was detected in 87.76% (43/49) of the TSCC samples and in 33.33% (5/15) of the adjacent non-tumor tissues. The expression of vascular endothelial growth factor (VEGF) was also observed in 83.67% (41/49) of the TSCC samples and in only 20% (3/15) of the adjacent non-tumor samples at a low level. RT-PCR revealed that the mRNA expression of HIF-1α and VEGF was present in the tumor tissues; however, it was barely detected in the corresponding adjacent normal tissues. The overexpression of HIF-1α was significantly associated with T classification (P=0.01), lymphatic metastasis (P=0.05) and histological differentiation (P<0.001). Furthermore, HIF-1α overexpression was significantly associated with poor overall (P=0.001) and disease-free survival rates (P=0.01), independent of T stage and lymphatic metastasis. The Cox proportional hazards regression model demonstrated that the level of HIF-1α expression may be an independent prognostic factor for TSCC. HIF-1α overexpression was observed in TSCC and its overexpression suggests a poor prognosis. HIF-1α may be a molecular marker for predicting the prognosis of TSCC.
Keywords: hypoxia, hypoxia-inducible factor-1α, vascular endothelial growth factor, tongue squamous cell carcinoma, prognosis
Introduction
Hypoxia is a general characteristic of malignant tumors (1,2) which occurs in numerous solid tumors (3–5). It regulates a variety of transcription factors, including hypoxia-inducible factor-1 (HIF-1) which is a heterodimeric transcription factor consisting of 2 subunits; a constitutively stable β subunit and an oxygen sensitive α subunit (6). The α subunit is rapidly degraded through the ubiquitin-proteasome pathway in the physiological microenvironment (7). However, the α subunit is stabilized and accumulates under hypoxic conditions (8) due to the inactivation or absence of the von Hippel-Lindau (VHL) tumor suppressor gene (9,10). HIF-1α products regulate cell adaptation to hypoxic microenvironments by modulating a number of downstream genes involved in vascular growth and cellular metabolism. Among these genes, vascular endothelial growth factor (VEGF) is vital as a regulatory gene of angiogenesis in the adaptation to hypoxic microenvironments (11). Studies have demonstrated the correlation between HIF-1α and VEGF in tumor cells (12) and solid tumors (13) and high levels of HIF-1α expression appear to predict a poor prognosis for various types of cancer (4,14,15).
However, relevant studies on tongue squamous cell carcinoma (TSCC) are rare. As a type of common malignant tumor in the oral cavity, TSCC has a high mortality rate due to early metastasis and recurrence. Although the level of healthcare has greatly improved, the cure rate of TSCC remains unsatisfactory and the 5-year survival rate is ∼50% (16).
The aim of the present study was to investigate the association of HIF-1α expression with various clinical parameters using reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemistry and western blot analysis to evaluate the impact of HIF-1α expression on the prognosis of TSCC.
Patients and methods
Clinical cases
A cohort of 49 patients (28 males and 21 females; mean age, 69.2 years; range, 45–84) was eligible for the present study. All the patients were treated between January 2000 and December 2005 at the Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Tongji University (Shanghai, China). A total of 49 paraffin-embedded tumor specimens of TSCC and 15 adjacent non-tumor tissue specimens were obtained for immunohistochemistry. Additionally, 15 fresh frozen tumor tissue specimens and their corresponding adjacent tissue specimens were obtained for RT-PCR. All the patients included in the study had a primary tumor in the oral cavity which was diagnosed as a squamous cell carcinoma (SCC) by clinical, radiological and pathological examination. Surgical treatment involved radical resection of the whole tumor with a free histopathological margin of at least 15 mm from the tumor borders. Bilateral selective neck dissection was performed in cases of suspect results from pre-operative tumor staging by computerized tomography and sonographic examination or in cases of a tumor size of >2 cm. The tissues were confirmed post-surgically as TSCC or adjacent non-tumor tissue using hematoxylin and eosin (H&E) staining. All tumors were classified according to the UICC TNM staging system which was revised in 2002 (17).
Follow-up
All patients were followed up by telephone or postoperative questionnaires monthly and underwent ultra-sound or computed tomography examinations at least once every 3 months at the outpatient clinic, particularly in the first 2 years. The average overall survival time was 36.73 months (ranging from 3 to 72 months).
RT-PCR
Total RNA was isolated from the frozen tissues using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and cDNA was synthesized using a PrimeScript RT reagent kit (Takara, Beijing, China). mRNA expression was evaluated quantitatively using real-time RT-PCR with SYBR Premix Ex Taq™ (Takara) and an ABI PRISM® 7900HT real-time PCR system. The thermocycler conditions were pre-denaturing at 95°C for 30 sec, followed by 35 cycles of denaturing at 95°C for 30 sec, annealing at 60°C for 30 sec and extension at 68°C for 1 min. The relative amount of the PCR product was defined as the threshold cycle (CT value) of the sample divided by that of β-actin. All experiments were performed in triplicate. The primers were synthesized (Sangon Biotech, Shanghai, China) and the sequences were as follows: for HIF-1α, the forward primer was 5′-GAA CCT GAT GCT TTA AAC T-3′ and the reverse primer was 5′-CAA CTG ATC GAA GGA ACG-3′; for VEGF, the forward primer was 5′-TTT CTG CTG TCT TGG GTG CAT TGG-3′ and the reverse primer was 5′-TCT GCA TGG TGA TGT TGG ACT CCT-3′; for β-actin, the forward primer was 5′- TGG, CAC, CCA, GCA, CAA, TGA, A- 3′ and the reverse primer was 5′-CTA AGT CAT AGT CCG CCT AGA AGC A-3′.
Immunohistochemisty
Sections (thickness, 4 μm) of paraffin-embedded TSCC and adjacent tissues were dewaxed in xylene and rehydrated. Antigen retrieval was performed by heating the sections with 10 mM citrate buffer (pH 6.0) for 15 min in a microwave. The sections were washed in PBS buffer and blocked for 30 min in 10% goat serum containing 1% BSA and 0.02% Triton X-100. Serial sections were incubated with rabbit anti-human HIF-1α 67 monoclonal antibody (1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and rabbit anti-human VEGF polyclonal antibody (1:100; Santa Cruz Biotechnology Inc.) separately for 20 min (with PBS instead of primary antibody as the negative control) and washed in PBS 3 times for 15 min. Subsequently, a catalyzed signal amplification system (Santa Cruz Biotechnology Inc.) was used for HIF-1α staining according to the manufacturer’s instructions. The antibodies were detected using a standard avidin-biotin complex method [biotinylated rabbit anti-mouse antibody (Santa Cruz Biotechnology Inc.) and an avidin-biotin complex (Santa Cruz Biotechnology Inc.)] and developed with diaminobenzidine. All sections were then counterstained for 45 sec with hematoxylin and dehydrated in alcohol and xylene prior to mounting. Non-tumor tissue samples adjacent to the paraffin-embedded tumor specimens were examined in a similar way for H&E HIF-1α staining.
Classification of HIF-1α expression
The HIF-1α protein was mainly present inside the nucleus, shown as brown or brown-yellow granules located inside the tumor cell. The VEGF protein was present inside the tumor cell or the cell membrane. Each section was examined independently by 2 pathologists. Five fields of view were randomly selected under an optical microscope at a x200 magnification. The positive cells were examined for staining intensity and counted 3 times to calculate the average number in each section. Based on the relative number of positive cells and staining intensity, 4 levels were defined to identify the staining activity of tumor cells: level I, no positive cells; level II, <10% positive cells, weak staining; level III, 10–50% positive cells, moderate staining; level IV, >50% positive cells, strong staining.
Statistical analysis
Correlations between clinicopathological features and the expression of HIF-1α were evaluated using the Chi-square test. Disease-free survival (DFS) and overall survival (OS) were used as the 2 end-points of the survival analysis. OS was defined as the number of months from the day the patient left the hospital to patient mortality. Patients who succumbed to other causes or remained alive at the final follow-up were considered to be review events. DFS was defined as the tumor-free time between the initial treatment and the first local recurrence or distant metastasis. Survival curves of DFS and OS were analyzed using the Kaplan-Meier method. The log-rank test was used to assess the differences between the groups and multivariate survival analysis was performed using Cox’s regression model. P<0.05 was considered to indicate statistically significant differences.
Results
Immunohistochemical analysis of HIF-1α and VEGF expression in TSCC
Fig. 1 shows the results of the immunohistochemisty analysis. In the 49 cases of TSCC, there were 6 (12.24%) negative cases, 21 (42.86%) weak positive cases (Fig. 1A), 12 (24.49%) medium positive cases (Fig. 1B) and 10 (20.41%) strong positive cases (Fig. 1C); the total positive rate was 87.76%. By contrast, there were only 5 (33.33%) cases of weak expression of HIF-1α in the 15 adjacent non-tumor tissue specimens (Fig. 1D). For VEGF, there were 8 (16.33%) negative cases, 17 (34.69%) weak positive cases (Fig. 1E), 16 (32.65%) medium positive cases (Fig. 1F) and 8 (16.33%) strong positive cases in the present study (Fig. 1G) and the overall positive rate was 83.67%. There were only 3 (20%) cases of weak expression of VEGF in the 15 adjacent non-tumor tissue specimens (Fig. 1H). The differences were observed to be significant (P<0.001).
Figure 1.
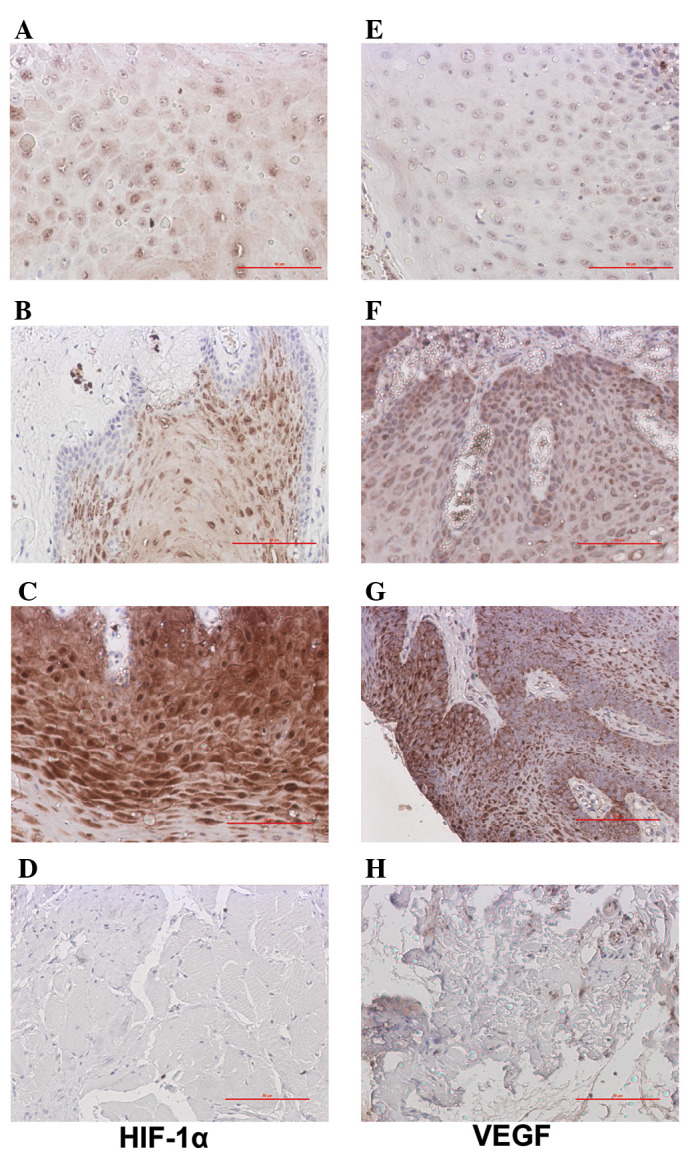
Expression of HIF-1α and VEGF (immunohistochemisty, magnification, x200). (A–C) Expression of HIF-1α at various levels in TSCC: (A) weak positive, (B) medium positive and (C) strong expression. (D) No expression of HIF-1α in the adjacent tissue. (E–G) Expression of VEGF in TSCC. (H) Weak expression of VEGF in adjacent normal tissue. Red lines, 50 μm; HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; TSCC, tongue squamous cell carcinoma.
HIF-1α was observed to be markedly expressed in the TSCC tissue, while no expression was observed in the adjacent tissue of the representative section shown in Fig. 2. In the same regions, HIF-1α and VEGF were expressed at relatively low levels. This was supported by the results of the RT-PCR.
Figure 2.
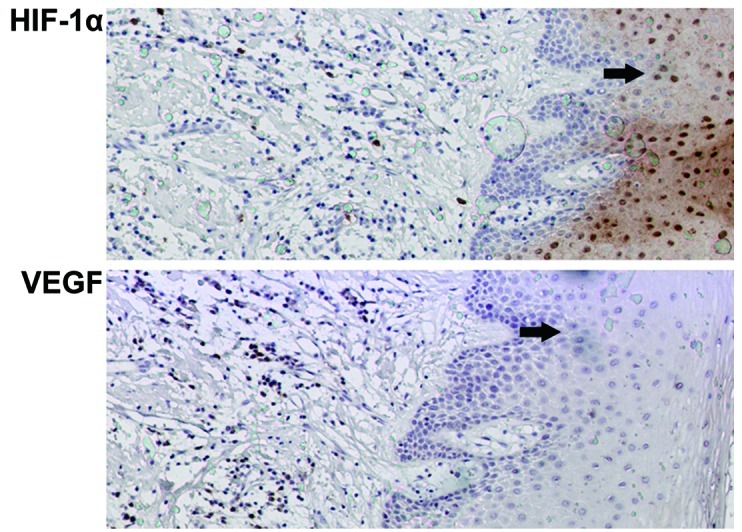
Expression of HIF-1α and VEGF (immunohistochemistry, magnification, x200) in a single section of adjacent non-tumor tissue. HIF-1α and VEGF were expressed at relatively low levels compared with the same regions of the TSCC sections. Arrows indicate cell nuclei at the same region of the serial TSCC sections. HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; TSCC, tongue squamous cell carcinoma.
RT-PCR analysis of HIF-1α and VEGF expression in TSCC
As shown in Fig. 3A, the mRNA expression levels in fresh tissues obtained from 2 cases were observed, demonstrating that HIF-1α mRNA was highly expressed in the tumor tissue but expressed at a low level in the adjacent tissue. However, VEGF mRNA expression was observed in the tumor tissue and the adjacent tissue, although the expression in tumor was higher. These data indicated that HIF-1α expression was markedly higher in tumor tissue when compared with that of the corresponding adjacent tissue specimens and that for VEGF, the difference in mRNA expression levels was less significant (Fig. 3B).
Figure 3.
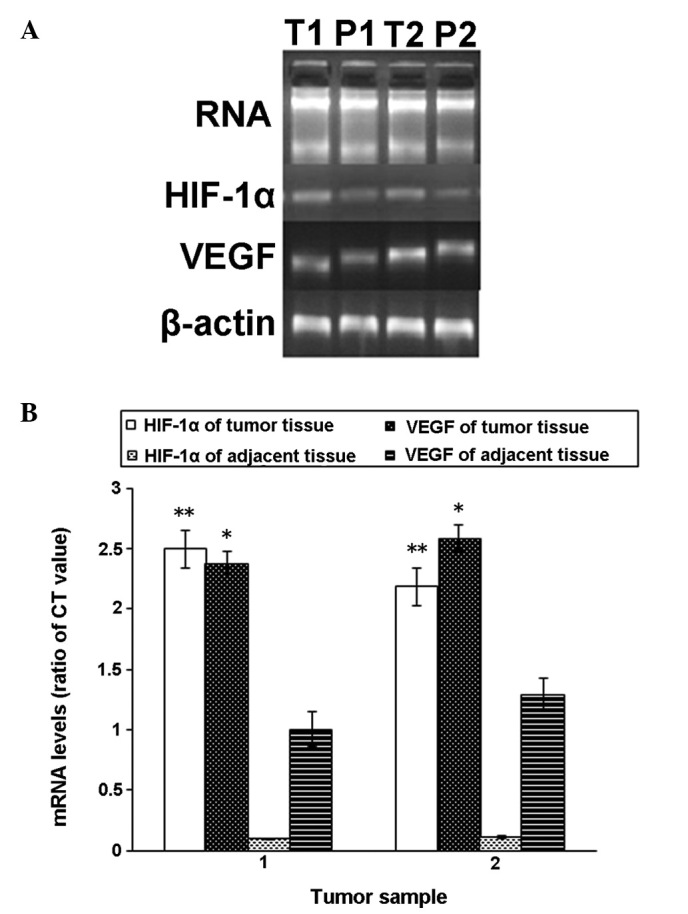
Expression levels of HIF-1α and VEGF in fresh tissue samples. (A) mRNA expression levels of HIF-1α and VEGF by RT-PCR between (T1, T2) tumor and (A1, A2) adjacent tumor samples (2 cases). (B) Quantitative analysis of HIF-1α and VEGF mRNA expression levels (*P<0.05, **P<0.001, compared with adjacent tissue). HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; RT-PCR, reverse transcription-polymerase chain reaction.
Association between HIF-1α and clinicopathological parameters
Table I shows the correlation between the parameters, including gender, age, histological differentiation, surgical approach, lymphatic metastasis, T stage and HIF-1α expression. In the present study, the 4 classes of expression of HIF-1α were combined into 2 classes: class I/II and class III/IV. No significant correlation was observed between gender, age, surgical approach and expression of HIF-1α (class I/II and class III/IV) using the Chi-square test. However, the T stage (Tis/T1 vs. T2/T3) and histological differentiation (G1 vs. G2 + G3) were observed to correlate with HIF-1α overexpression and the results were statistically significant (P<0.05).
Table I.
Correlation between HIF-1α expression and clinical parameters in TSCC.
| HIF-1α
|
||||
|---|---|---|---|---|
| Parameter | n (%) | I/II | III/IV | P-value |
| Total | 49 (100) | 27 | 22 | - |
| Gender | 0.158 | |||
| Male | 28 (57.14) | 18 | 10 | |
| Female | 21 (42.86) | 9 | 12 | |
| Age (years) | 0.246 | |||
| <70 | 21 (42.86) | 14 | 7 | |
| ≥70 | 28 (57.14) | 13 | 15 | |
| Neck dissection | 0.395 | |||
| Yes | 30 (61.22) | 15 | 15 | |
| No | 19 (38.78) | 12 | 7 | |
| T stage | 0.010a | |||
| T1 + Tis | 24 (48.98) | 18 | 6 | |
| T2 + T3 | 25 (51.02) | 9 | 16 | |
| Lymphatic metastasis | 0.005a | |||
| Negative | 43 (87.76) | 27 | 16 | |
| Positive | 6 (12.24) | 0 | 6 | |
| Histological differentiation | 0.000a | |||
| G1 | 23 (46.94) | 21 | 2 | |
| G2 + G3 | 26 (53.06) | 6 | 20 | |
Data were analyzed using the Chi-square test.
P<0.05 was considered to indicate statistically significant differences. HIF-1α, hypoxia-inducible factor-1α; TSCC, tongue squamous cell carcinoma.
Association between HIF-1α and prognosis
Univariate analysis of clinical parameters with DFS and OS
Table II shows the results of the Kaplan-Meier analysis. The results revealed significantly poorer DFS (P<0.05) and OS (P<0.05) with histological differentiation (P= 0.020, P=0.008), lymphatic metastasis (P<0.001, P<0.001) and HIF-1α expression (P= 0.001, P<0.001). No significant associations between survival rates and gender, age or neck dissection were observed.
Table II.
Correlation of clinical parameters with DFS and OS in TSCC.
| Parameter | DFS (months) | P-value | OS (months) | P-value |
|---|---|---|---|---|
| Gender | 0.107 | 0.115 | ||
| Male | 33.00±21.96 | 39.93±25.04 | ||
| Female | 26.00±18.99 | 32.48±21.14 | ||
| Age (years) | 0.292 | 0.218 | ||
| <70 | 31.81±20.53 | 39.38±23.60 | ||
| ≥70 | 28.64±21.32 | 34.75±23.675 | ||
| Neck dissection | 0.094 | 0.088 | ||
| Yes | 25.53±20.06 | 31.33±22.561 | ||
| No | 37.05±20.57 | 45.26±22.99 | ||
| T stage | 0.132 | 0.108 | ||
| T1 + Tis | 31.33±20.31 | 39.42±22.58 | ||
| T2 + T3 | 28.72±21.66 | 34.16±24.55 | ||
| Lymphatic metastasis | 0.000b | 0.000b | ||
| Negative | 32.98±20.46 | 40.33±22.78 | ||
| Positive | 8.67±5.20 | 11.00±6.29 | ||
| Histological differentiation | 0.020a | 0.008a | ||
| G1 | 36.35±20.00 | 46.83±21.96 | ||
| G2 + G3 | 24.38±20.28 | 27.81±21.45 | ||
| HIF-1α expression | 0.001a | 0.000b | ||
| Negative/weak | 37.63±20.61 | 46.96±21.85 | ||
| Moderate/strong | 20.64±17.30 | 24.18±19.30 |
Data were analyzed using the log-rank test.
P<0.05 was considered to indicate statistically significant differences,
P<0.001. DFS, disease-free survival; OS, overall survival; HIF-1α, hypoxia-inducible factor-1α; TSCC, tongue squamous cell carcinoma.
Multivariate analysis of clinical parameters with DFS and OS
In multivariate Cox analysis the OS and DFS were compared according to clinical parameters (lymphatic metastasis, histological differentiation) with the HIF-1α expression. Lymphatic metastasis and HIF-1α expression were identified by Cox regression as independent predictors of DFS and OS. Lymphatic metastasis (P=0.010, P=0.011) and HIF-1α expression (P=0.050, P=0.030) were also predictors of tumor-free survival in multivariate regression (Table III).
Table III.
Multivariate analysis of DFS and OS in TSCC.
| DFS
|
OS
|
|||
|---|---|---|---|---|
| Parameter | P-value | RR | P-value | RR |
| Lymphatic metastasis | ||||
| Negative vs. Positive | 0.010a | 0.080–0.705 | 0.011a | 0.086–0.732 |
| Histological differentiation | ||||
| G1 vs. G2 + G3 | 0.749 | 0.329–2.224 | 0.619 | 0.296–2.066 |
| HIF-1α expression | ||||
| Negative/weak vs. moderate/strong | 0.050a | 0.146–1.001 | 0.030a | 0.122–0.898 |
The Cox partial nonparametric regression model was used to evaluate the predictive power of various combinations of prognosticators in a multivariate manner.
P<0.05 was considered to indicate statistically significant differences. DFS, disease-free survival; OS, overall survival; RR, relative risk; HIF-1α, hypoxia-inducible factor-1α; TSCC, tongue squamous cell carcinoma.
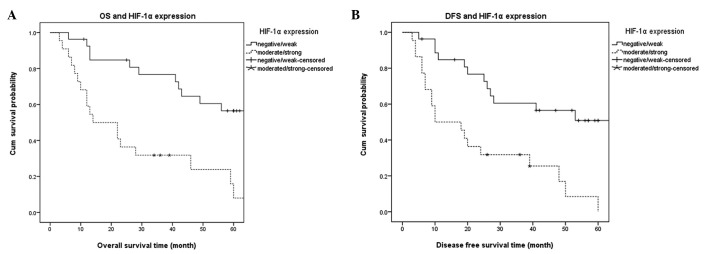
Fig. 4 shows the Kaplan-Meier survival curve analysis of DFS and OS. The expression of HIF-1α (class I/II and class III/IV) affected the OS (Fig. 4A) and DFS (Fig. 4B) of patients with TSCC. The patients with high HIF-1α expression levels had poorer DFS and OS, suggesting that the overexpression of HIF-1α was associated with a poor prognosis.
Figure 4.
Survival analysis of HIF-1α overexpression with overall survival (OS) and disease-free survival (DFS) (Kaplan-Meier curve). The survival timetable of all patients was divided into two groups for comparison based on the expression levels of HIF-1α: Negative/weak group and Moderate/strong group. (A) OS curve; (B) DFS curve. HIF-1α, hypoxia-inducible factor-1α.
Discussion
HIF-1α is the most significant nuclear transcription factor identified as mediating the hypoxic response. As a global regulatory factor, it is able to activate a wide range of genes mediating physiological responses to hypoxia and consequently regulates oxygen concentration in cell metabolism (18). The transcriptional activity of HIF-1α is activated by hypoxia, thus triggering a series of adaptive responses leading to glucose metabolism, tumor angiogenesis and erythropoietin generation (19). Hypoxia is a general characteristic of malignant tumors which occurs in numerous solid tumors (20). Due to the continuous growth and expansion of the solid tumor, the intratumoral oxygen concentration is continuously reduced until hypoxia occurs. This causes HIF-1α accumulation inside the cell nucleus which activates various types of downstream genes for hypoxia adaptation. As a regulatory gene of angiogenesis in the adaptation to hypoxic microenvironments, VEGF is vital in tumor recurrence and metastasis (21). It is a highly specific vascular endothelial cell mitogen which directly stimulates endothelial cells, thus promoting endothelial cell proliferation, migration and increases in vascular permeability. The normal functions of VEGF include creating new blood vessels during embryonic development and following injury. However, under certain pathological conditions, VEGF may contribute to diseases such as tumors. Studies have demonstrated the function of VEGF in tumorigenesis and revealed it to be an independent indicator for predicting malignant tumors with poor prognoses (22–24). Sugiura et al examined 160 oral SCC specimens from the oral cavity using immunohistochemistry and revealed that VEGF-C may be used to predict the lymphatic metastasis of oral SCC (25).
In the present study, the expression of VEGF was observed to be significantly associated with that of HIF-1α. The results of RT-PCR suggested that the HIF-1α was present, overexpressed in TSCC and closely associated with VEGF. These results were consistent with those reported by Yasuda et al(13). The authors used in situ hybridization and immunohistochemistry to observe the expression of the HIF-1α gene and its association with the VEGF protein and microvessel density (MVD) and revealed that the expression of HIF-1α mRNA positively correlated with the VEGF protein expression and MVD in colorectal adenoma. Additionally, in the present study, HIF-1α and VEGF were mainly expressed in the TSCC tissue and were barely detected in the adjacent non-tumor tissue suggesting that HIF-1α was present and overexpressed in TSCC.
Since a number of studies have revealed HIF-1α overexpression to be significantly associated with poor prognoses in certain solid tumors, including pancreatic cancer, breast cancer, rectal adenocarcinoma and cervical cancer (26–29), it was investigated whether HIF-1α may function as a prognostic factor of TSCC. Using the the immunohistochemical staining performed on the 49 TSCC specimens of HIF-1α, the association between HIF-1α expression and the prognoses of patients with TSCC was studied. The data demonstrated that patients with no or weak expression of HIF-1α had higher survival rates (approximately 60%) than those with moderate or high expression of HIF-1α (approximately 30%). HIF-1α overexpression was closely associated with clinicopathological parameters, including histological differentiation, T stage and lymph node metastasis. The data also revealed unfavorable effects on survival rate. In multivariate Cox regression analysis, lymphatic metastasis and HIF-1α expression were significantly associated with DFS and OS, suggesting that the subgroup of patients with HIF-1α overexpression may have a high risk of TSCC and a poor prognosis. This hypothesis was supported by a number of studies in various research fields as mentioned previously. Bos et al(30) investigated the expression levels of HIF-1α, HER-2/neu, estrogen receptor and progesterone receptor in 150 patients with early-stage breast carcinoma using immunohistochemistry and HER-2/neu gene amplification with automated fluorescent in situ hybridization. The authors observed that increased levels of HIF-1α were associated independently with lower survival rates in patients with lymph node negative breast carcinoma.
However, the results of certain studies are inconsistent with those of the present study. Fillies et al(31) investigated 85 patients with histologically demonstrated surgically treated T1/2 SCC of the oral floor and the results suggested that HIF-1α overexpression was an indicator of favorable prognosis in T1 and T2 SCC of the oral floor. This contradiction may be due to a uniform cut-off point of HIF-1α expression or various types of tendentious treatment for the tumor. Considering this, HIF-1α may function as an independent prognostic marker of TSCC.
In conclusion, the present study is the first to clinically study patients with TSCC in the East China region. The findings suggested that the overexpression of HIF-1α predicts a poor prognosis in TSCC. Further studies should be performed to explore the potential functional role of HIF-1α in malignant tumors and to determine whether HIF-1α may be regarded as an indicator or target in the diagnosis and treatment of TSCC.
Acknowledgments
This study was supported by a grant from the Shanghai Science and Technology Committee (09QA1406400).
References
- 1.Hochachka PW. Mechanism and evolution of hypoxia-tolerance in humans. J Exp Biol. 1998;201:1243–1254. doi: 10.1242/jeb.201.8.1243. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol. 2001;18:243–259. doi: 10.1385/MO:18:4:243. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama K, Kanzaki A, Hata K, et al. Hypoxia-inducible factor 1 alpha (HIF-1 alpha) gene expression in human ovarian carcinoma. Cancer Lett. 2002;176:215–223. doi: 10.1016/s0304-3835(01)00762-5. [DOI] [PubMed] [Google Scholar]
- 4.Kuwai T, Kitadai Y, Tanaka S, et al. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105:176–181. doi: 10.1002/ijc.11068. [DOI] [PubMed] [Google Scholar]
- 5.Ioannou M, Mylonis I, Kouvaras E, et al. Validated analysis of HIF-1α expression in cancer cells using a controlled and comparative immunoassay. Oncol Rep. 2010;24:161–169. doi: 10.3892/or_00000841. [DOI] [PubMed] [Google Scholar]
- 6.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 8.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19:5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- 10.Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Jr, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci USA. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianco F, Basini G, Santini S, Grasselli F. Angiogenic activity of swine granulosa cells: effects of hypoxia and the role of VEGF. Vet Res Commun. 2005;29(Suppl 2):157–159. doi: 10.1007/s11259-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 12.Choi SB, Park JB, Song TJ, Choi SY. Molecular mechanism of HIF-1-independent VEGF expression in a hepatocellular carcinoma cell line. Int J Mol Med. 2011;28:449–454. doi: 10.3892/ijmm.2011.719. [DOI] [PubMed] [Google Scholar]
- 13.Yasuda S, Arii S, Mori A, et al. Hexokinase II and VEGF expression in liver tumors: correlation with hypoxia-inducible factor 1 alpha and its significance. J Hepatol. 2004;40:117–123. doi: 10.1016/s0168-8278(03)00503-8. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi R, Tanaka S, Hiyama T, et al. Hypoxia-inducible factor-1α expression and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncol Rep. 2003;10:797–802. [PubMed] [Google Scholar]
- 15.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casiglia J, Woo SB. A comprehensive review of oral cancer. Gen Dent. 2001;49:72–82. [PubMed] [Google Scholar]
- 17.Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002;94:2511–2516. doi: 10.1002/cncr.10492. [DOI] [PubMed] [Google Scholar]
- 18.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 20.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 21.Richard DE, Berra E, Pouysségur J. Angiogenesis: how a tumor adapts to hypoxia. Biochem Biophys Res Commun. 1999;266:718–722. doi: 10.1006/bbrc.1999.1889. [DOI] [PubMed] [Google Scholar]
- 22.Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:624–630. doi: 10.1007/s00432-005-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal O, Soriano-Izquierdo A, Pera M, et al. Positive VEGF immunostaining independently predicts poor prognosis in curatively resected gastric cancer patients: results of a study assessing a panel of angiogenic markers. J Gastrointest Surg. 2008;12:1005–1014. doi: 10.1007/s11605-007-0336-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhao ZQ, Yang S, Lu HS. Expression of midkine and vascular endothelial growth factor in gastric cancer and the association of high levels with poor prognosis and survival. Mol Med Report. 2012;5:415–419. doi: 10.3892/mmr.2011.649. [DOI] [PubMed] [Google Scholar]
- 25.Sugiura T, Inoue Y, Matsuki R, et al. VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: Implications for use as a prognostic marker. Int J Oncol. 2009;34:673–680. doi: 10.3892/ijo_00000193. [DOI] [PubMed] [Google Scholar]
- 26.Shibaji T, Nagao M, Ikeda N, et al. Prognostic significance of HIF-1 alpha overexpression in human pancreatic cancer. Anticancer Res. 2003;23:4721–4727. [PubMed] [Google Scholar]
- 27.Gruber G, Greiner RH, Hlushchuk R, et al. Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res. 2004;6:R191–R198. doi: 10.1186/bcr775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu XG, Xing CG, Feng YZ, Chen J, Deng C. Clinical significance of immunohistochemical expression of hypoxia-inducible factor-1alpha as a prognostic marker in rectal adenocarcinoma. Clin Colorectal Cancer. 2006;5:350–353. doi: 10.3816/ccc.2006.n.005. [DOI] [PubMed] [Google Scholar]
- 29.Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–4696. [PubMed] [Google Scholar]
- 30.Bos R, van der Groep P, Greijer AE, et al. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–1581. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- 31.Fillies T, Werkmeister R, van Diest PJ, Brandt B, Joos U, Buerger H. HIF1-alpha overexpression indicates a good prognosis in early stage squamous cell carcinomas of the oral floor. BMC Cancer. 2005;5:84. doi: 10.1186/1471-2407-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]