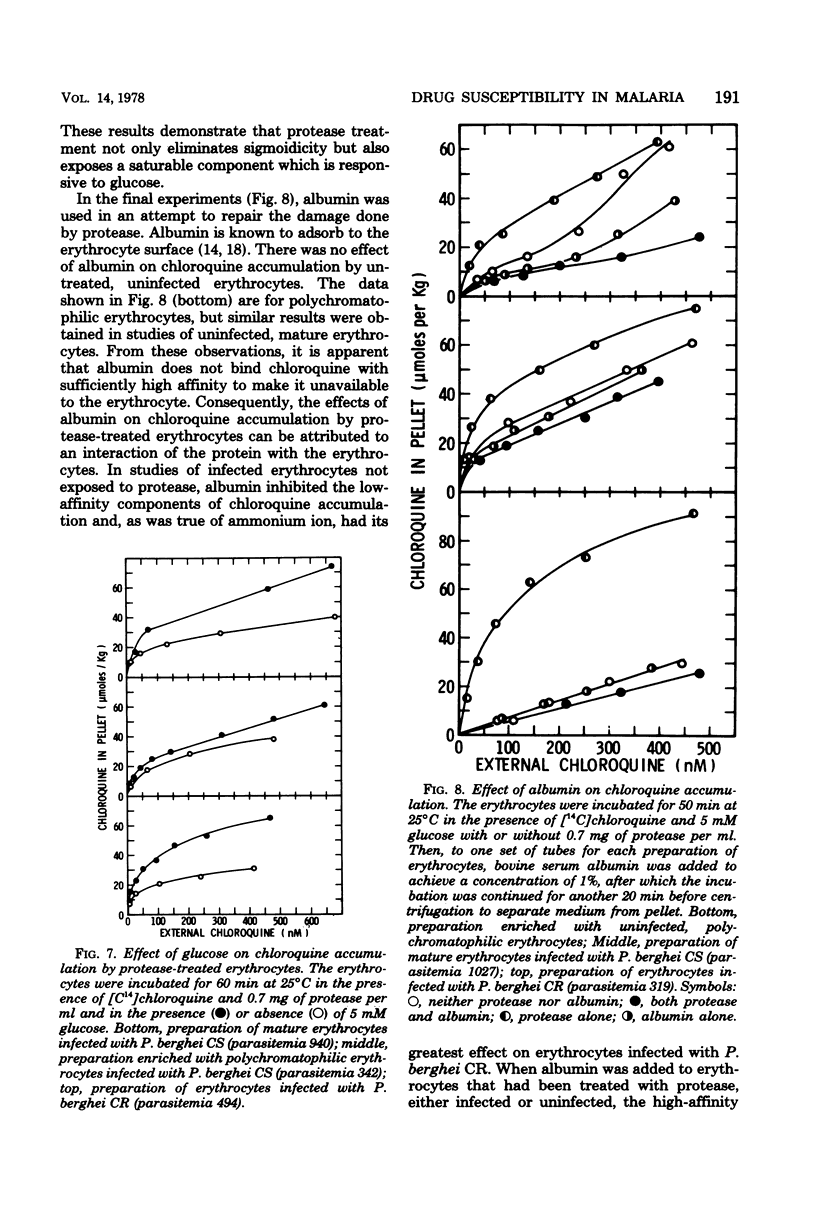
Abstract
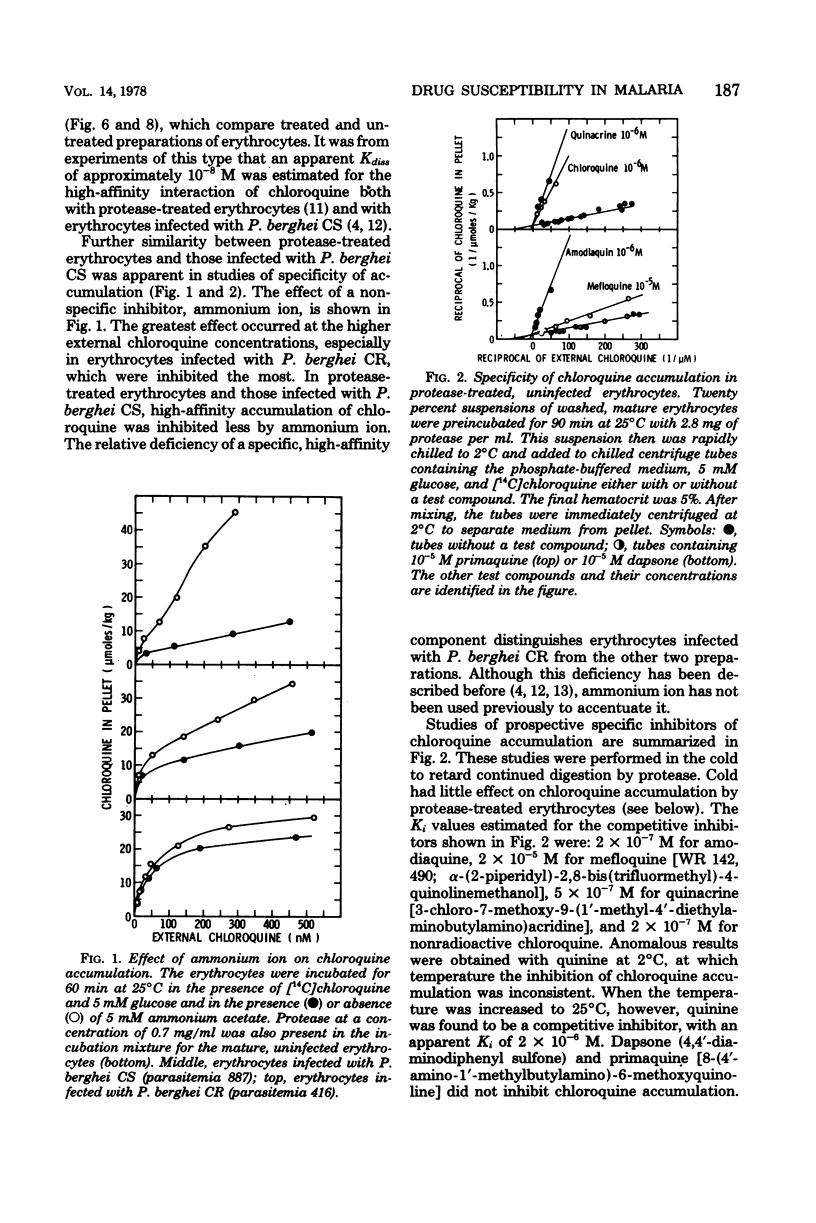
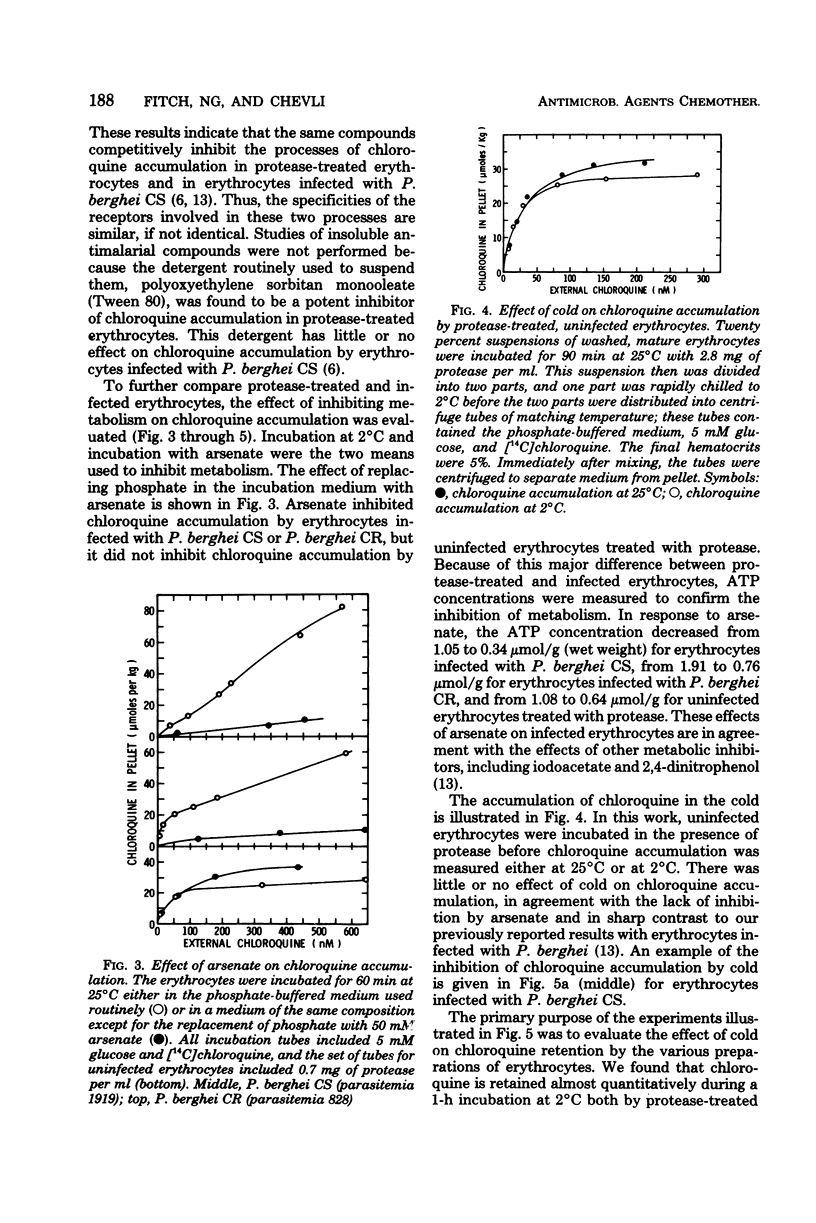
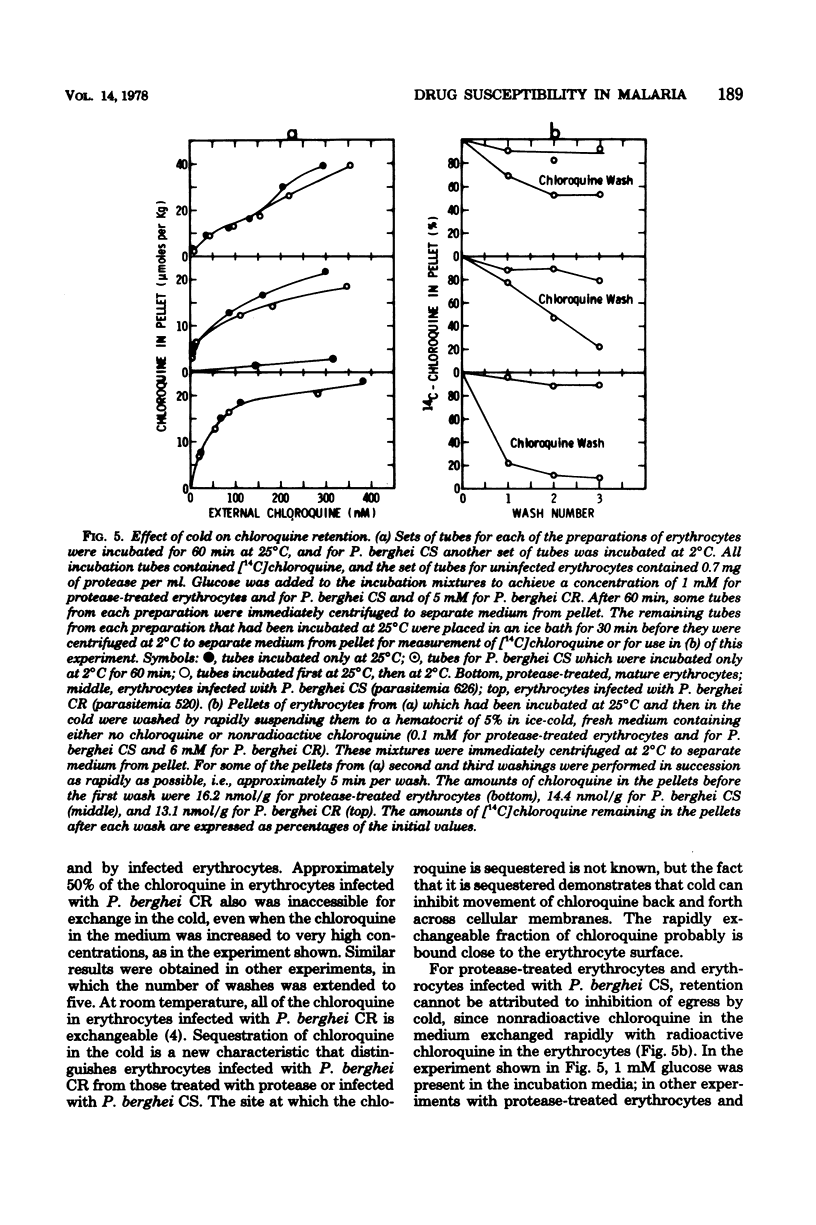
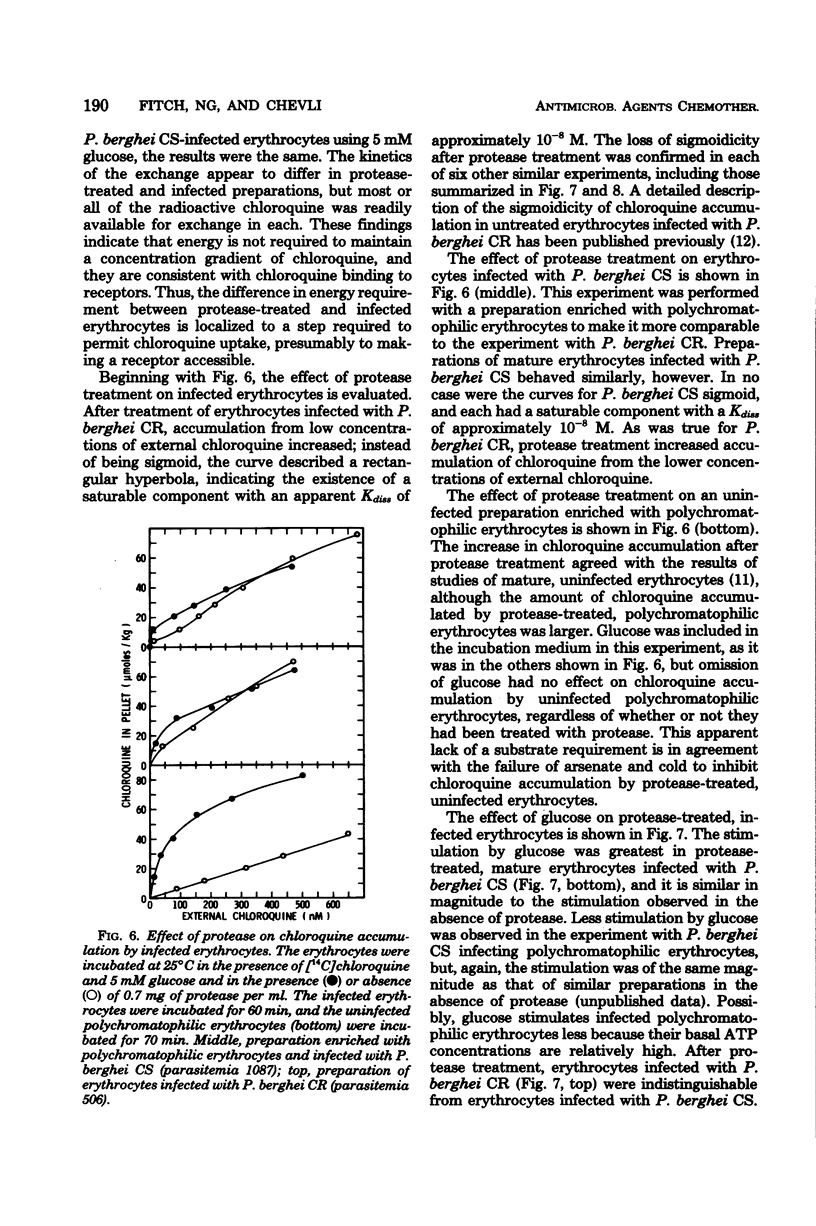
To study the role of the erythrocyte membrane in the process of chloroquine accumulation, surface polypeptides were digested with a nonspecific protease from Streptomyces griseus. This treatment activated a saturable process of chloroquine accumulation with an affinity and a specificity similar to those of mouse erythrocytes infected with Plasmodium berghei CS (chloroquine susceptible). Studies of competitive inhibitors of chloroquine accumulation yielded the following approximate values for Ki: amodiaquine, 2 × 10−7 M; quinacrine, 5 × 10−7 M; quinine, 2 × 10−6 M; and mefloquine, 2 × 10−5 M. Lack of a substrate requirement distinguished this process from the one used by P. berghei and permitted the protease to be used in studies of infected erythrocytes. Protease treatment of erythrocytes infected with P. berghei CR (chloroquine resistant) produced a dramatic transformation. Instead of describing a sigmoid curve, the process of chloroquine accumulation became saturable and substrate dependent, with a Kdiss of approximately 10−8 M; i.e., protease-treated erythrocytes infected with P. berghei CR now behaved similarly to those infected with P. berghei CS. Coating the erythrocyte surface with albumin completely inhibited the protease-activated process of chloroquine accumulation. These findings are presented as evidence that erythrocyte surface components determine the affinity with which chloroquine is accumulated and thereby determine whether or not the malaria parasite will be susceptible to the drug.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. H., Magee L. A. Divalent cation reversal of tetracycline-inhibited respiration of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1973 May;3(5):645–646. doi: 10.1128/aac.3.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W. W., Garan H., Berg H. C. Proteins of the human erythrocyte membrane as modified by pronase. J Mol Biol. 1971 Jun 28;58(3):783–797. doi: 10.1016/0022-2836(71)90040-4. [DOI] [PubMed] [Google Scholar]
- Bodammer J. E., Bahr G. F. The initiation of a "metabolic window" in the surface of host erythrocytes by Plasmodium berghei NYU-2. Lab Invest. 1973 Jun;28(6):708–718. [PubMed] [Google Scholar]
- Fitch C. D., Chevli R., Gonzalez Y. Chloroquine accumulation by erythrocytes: a latent capability. Life Sci. 1974 Jun 16;14(12):2441–2446. doi: 10.1016/0024-3205(74)90140-4. [DOI] [PubMed] [Google Scholar]
- Fitch C. D., Chevli R., Gonzalez Y. Chloroquine-resistant Plasmodium falciparum: effect of substrate on chloroquine and amodiaquin accumulation. Antimicrob Agents Chemother. 1974 Dec;6(6):757–762. doi: 10.1128/aac.6.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D. Chloroquine resistance in malaria: a deficiency of chloroquine binding. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1181–1187. doi: 10.1073/pnas.64.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D. Chloroquine susceptibility in malaria: dependence on exposure of parasites to the drug. Life Sci. 1977 Nov 15;21(10):1511–1514. doi: 10.1016/0024-3205(77)90207-7. [DOI] [PubMed] [Google Scholar]
- Fitch C. D. Linkage of chloroquine resistance in Plasmodium berghei to infection of immature erythrocytes of mice. Life Sci. 1977 Apr 1;20(7):1281–1284. doi: 10.1016/0024-3205(77)90503-3. [DOI] [PubMed] [Google Scholar]
- Fitch C. D., Yunis N. G., Chevli R., Gonzalez Y. High-affinity accumulation of chloroquine by mouse erythrocytes infected with Plasmodium berghei. J Clin Invest. 1974 Jul;54(1):24–33. doi: 10.1172/JCI107747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Macomber P. B., O'Brien R. L., Hahn F. E. Chloroquine: physiological basis of drug resistance in Plasmodium berghei. Science. 1966 Jun 3;152(3727):1374–1375. doi: 10.1126/science.152.3727.1374. [DOI] [PubMed] [Google Scholar]
- Rehfeld S. J. The interaction of albumin and concanavalin A with normal and sickle human erythrocytes. Biochem Biophys Res Commun. 1975 Sep 16;66(2):586–591. doi: 10.1016/0006-291x(75)90550-1. [DOI] [PubMed] [Google Scholar]
- Schmidt L. H., Vaughan D., Mueller D., Crosby R., Hamilton R. Activities of various 4-aminoquinolines against infections with chloroquine-resistant strains of Plasmodium falciparum. Antimicrob Agents Chemother. 1977 May;11(5):826–843. doi: 10.1128/aac.11.5.826. [DOI] [PMC free article] [PubMed] [Google Scholar]


