Abstract
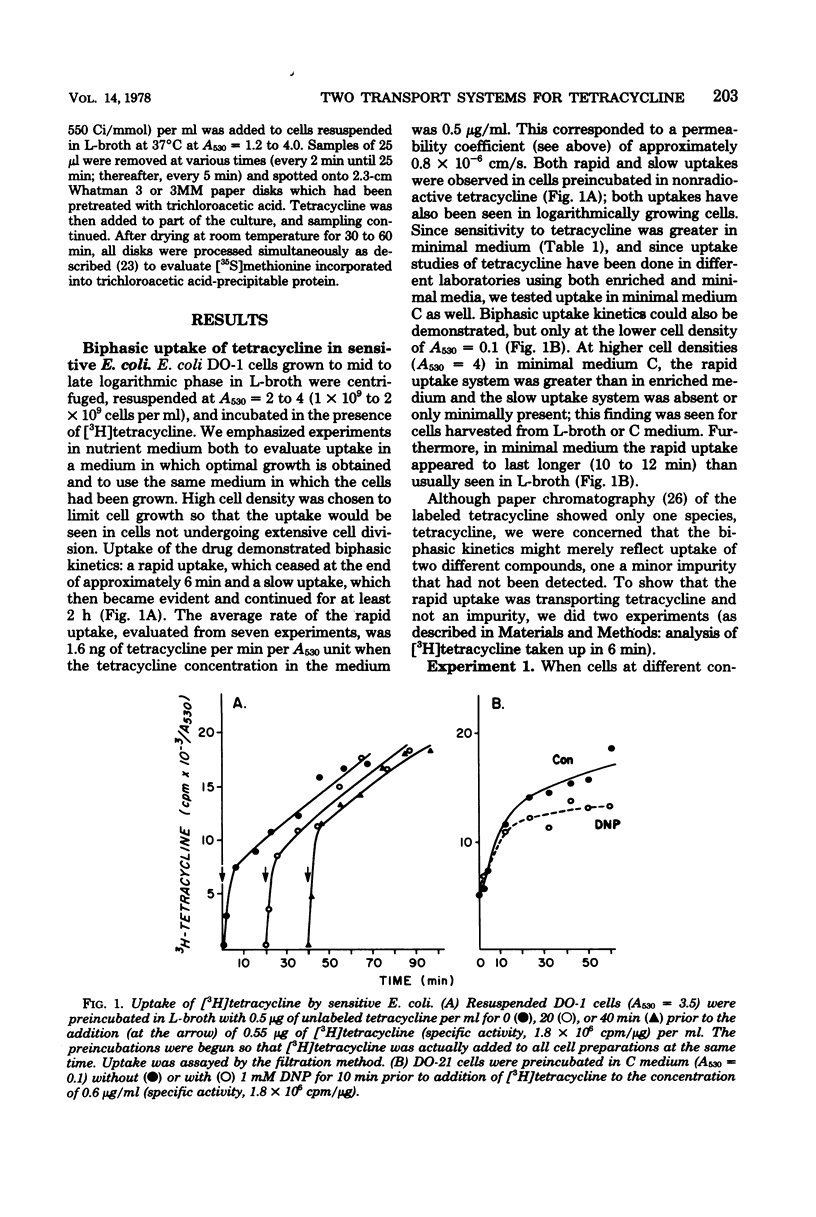
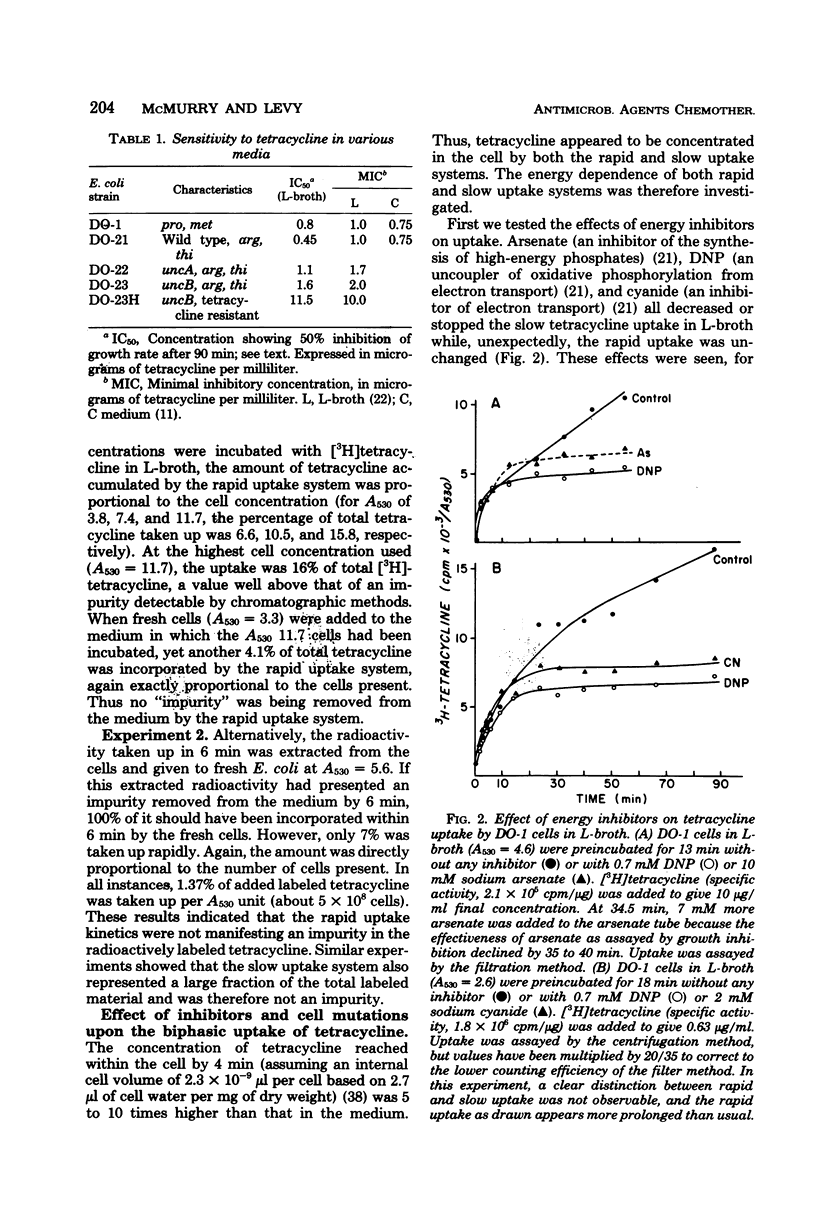
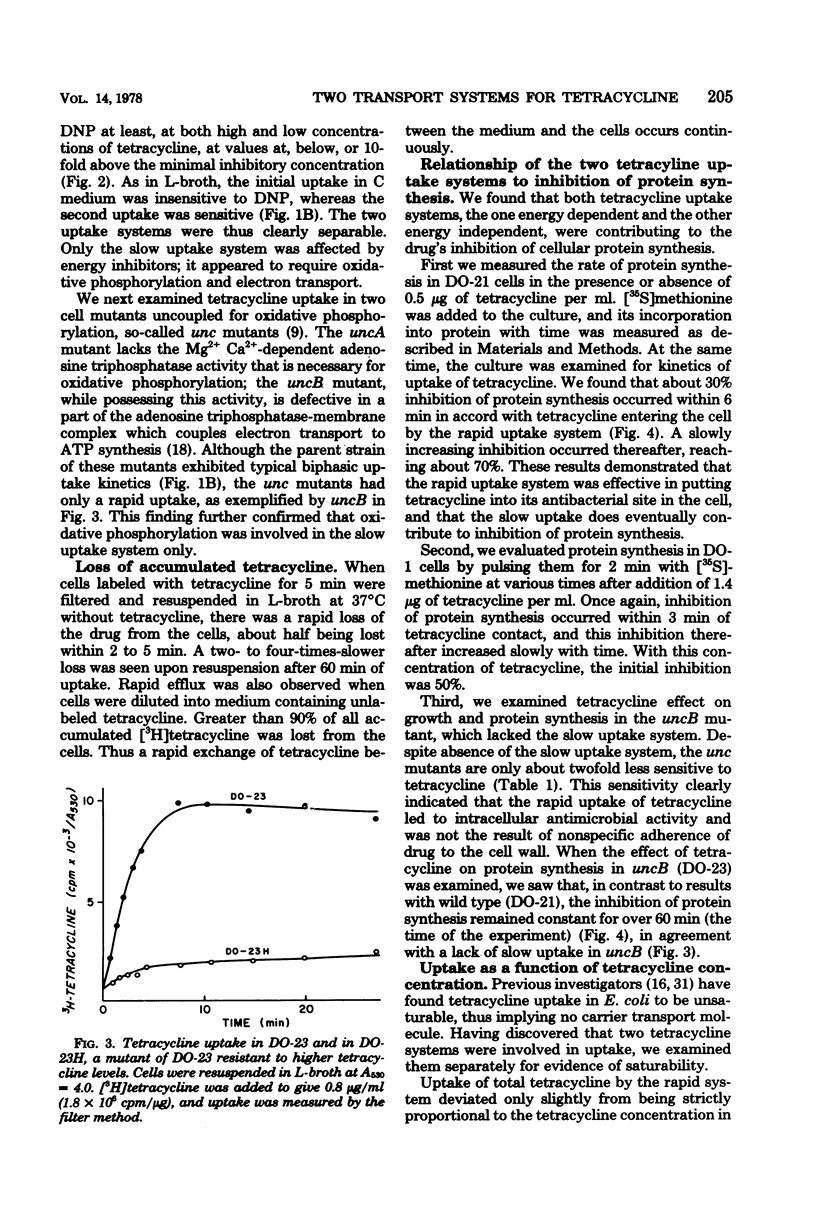
Escherichia coli sensitivity to tetracycline involves transport and accumulation of the antibiotic within the cell by two different uptake systems: an initial rapid uptake, which occurs over the initial 6 min of contact of the cell with tetracycline, and a slower uptake system, which continues indefinitely and whose rate of uptake is 1/10 that of the rapid system. Only the slow uptake system is blocked by inhibitors of energy-driven systems; it appears to be particularly dependent upon energy from oxidative phosphorylation. Although both uptake systems lead to accumulation of intracellular tetracycline and contribute to the cell's sensitivity, the rapid uptake system appears to be the more important. While these studies confirm active transport of tetracycline into the cell, they demonstrate that a critical uptake system which appears insensitive to metabolic inhibitors occurs initially.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIMA K., IZAKI K. ACCUMULATION OF OXYTETRACYCLINE RELEVANT TO ITS BACTERICIDAL ACTION IN THE CELLS OF ESCHERICHIA COLI. Nature. 1963 Oct 12;200:192–193. doi: 10.1038/200192a0. [DOI] [PubMed] [Google Scholar]
- Ball P. R., Chopra I., Eccles S. J. Accumulation of tetracyclines by Escherichia coli K-12. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1500–1507. doi: 10.1016/s0006-291x(77)80148-4. [DOI] [PubMed] [Google Scholar]
- Bavoil P., Nikaido H., von Meyenburg K. Pleiotropic transport mutants of Escherichia coli lack porin, a major outer membrane protein. Mol Gen Genet. 1977 Dec 14;158(1):23–33. doi: 10.1007/BF00455116. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van den Elzen H. M. Streptomycin accumulation in susceptible and resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Jun;9(6):928–938. doi: 10.1128/aac.9.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Escherichia coli K-12 tolF mutants: alterations in protein composition of the outer membrane. J Bacteriol. 1977 May;130(2):781–786. doi: 10.1128/jb.130.2.781-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. Studies on electron transport and energy-linked reactions using mutants of Escherichia coli. Biochim Biophys Acta. 1974 Apr 30;346(1):1–25. doi: 10.1016/0304-4173(74)90010-x. [DOI] [PubMed] [Google Scholar]
- Del Bene V. E., Rogers M. Comparison of tetracycline and minocyclie transport in Escherichia Coli. Antimicrob Agents Chemother. 1975 Jun;7(6):801–806. doi: 10.1128/aac.7.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockter M. E., Magnuson J. A. Characterization of the active transport of chlorotetracycline in staphylococcus aureus by a fluorescence technique. J Supramol Struct. 1974;2(1):32–44. doi: 10.1002/jss.400020105. [DOI] [PubMed] [Google Scholar]
- FRANKLIN T. J., GODFREY A. RESISTANCE OF ESCHERICHIA COLI TO TETRACYCLINES. Biochem J. 1965 Jan;94:54–60. doi: 10.1042/bj0940054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. TolF locus in Escherichia coli: chromosomal location and relationship to loci cmlB and tolD. J Bacteriol. 1976 Nov;128(2):604–608. doi: 10.1128/jb.128.2.604-608.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Higginson B. Active accumulation of tetracycline by Escherichia coli. Biochem J. 1970 Jan;116(2):287–297. doi: 10.1042/bj1160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S. M., Tsuchiya T., Rosen B. P. Energy transduction in Escherichia coli: physiological and biochemical effects of mutation in the uncB locus. J Bacteriol. 1978 Jan;133(1):108–113. doi: 10.1128/jb.133.1.108-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högenauer G., Turnowsky F. The effects of streptomycin and tetracycline on codon-anticodon interactions. FEBS Lett. 1972 Oct 1;26(1):185–188. doi: 10.1016/0014-5793(72)80569-6. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Physical and functional characteristics of R-factor deoxyribonucleic acid segregated into Escherichia coli minicells. J Bacteriol. 1971 Oct;108(1):300–308. doi: 10.1128/jb.108.1.300-308.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Pato M. L. Tetracycline inhibits propagation of deoxyribonucleic acid replication and alters membrane properties. Antimicrob Agents Chemother. 1977 Feb;11(2):318–323. doi: 10.1128/aac.11.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynard A. M., Nellis L. F., Beck M. E. Uptake of 3H-Tetracycline by resistant and sensitive Escherichia coli. Appl Microbiol. 1971 Jan;21(1):71–75. doi: 10.1128/am.21.1.71-75.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynard A. M., Nellis L. F. Uptake of tetracycline by Escherichia coli: lack of binding of tetracycline to the uptake system. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1129–1132. doi: 10.1016/0006-291x(72)90827-3. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Epstein W. Energy coupling to net K+ transport in Escherichia coli K-12. J Biol Chem. 1977 Feb 25;252(4):1394–1401. [PubMed] [Google Scholar]
- Shipley P. L., Olsen R. H. Characteristics and expression of tetracycline resistance in gram-negative bacteria carrying the Pseudomonas R factor RP1. Antimicrob Agents Chemother. 1974 Aug;6(2):183–190. doi: 10.1128/aac.6.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuka I., Kaji H., Kaji A. Binding of specific sRNA to 30S ribosomal subunits: effect of 50S ribosomal subunits. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1483–1490. doi: 10.1073/pnas.55.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Properties of the entry and exit reactions of the beta-methyl galactoside transport system in Escherichia coli. J Bacteriol. 1976 Jun;126(3):1156–1165. doi: 10.1128/jb.126.3.1156-1165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]



