Abstract
Background
Accumulating evidence suggests anatomical and functional differences in connectivity between the anterior and posterior parts of the inferior-parietal lobule (IPL) and the frontal motor areas.
Objective/Hypothesis
This study investigates whether different intra-hemispheric parietal-motor interactions can be observed along the anterior-posterior axis of the IPL in the resting human brain.
Methods
We use a twin coil transcranial magnetic stimulation technique to test intra-hemispheric interactions between three points adjacent to the intra-parietal sulcus (anterior, central, posterior) and the ipsilateral primary motor cortex (M1) at rest in both hemispheres.
Results
We found that stimulation of the anterior IPL resulted in an inhibition of the ipsilateral M1 in both hemispheres. Stimulation of the central and posterior IPL resulted in a facilitatory effect on ipsilateral M1 in the left but not for the right hemisphere. Additionally we show that there is considerable inter-subject variability concerning the optimal parietal facilitatory and inhibitory position.
Conclusions
The IPL has distinct inhibitory and facilitatory connections to the ipsilateral M1. Whereas inhibitory connections are observed in both hemispheres, facilitatory connections are asymmetric. These parietal-motor networks may represent the basis for the functional differences between these regions in reaching and grasping tasks and mirror the functional asymmetry observed in the motor system. From a practical point of view, we note that the inter-subject variability means that future TMS studies of the parietal area might consider a hot-spot localization similar to the procedures commonly used for M1.
Keywords: twin-coil TMS, intraparietal sulcus, primary motor cortex, intra-hemispheric connectivity
Introduction
Fronto-parietal networks are crucial for the precise execution of both reaching and grasping movements (1-3). Functional and anatomical connections between parietal and motor areas have been extensively studied in both monkeys (4) and humans (5) and these studies have concordantly shown distinct connections from anterior and posterior parietal cortex to the motor areas. Anatomically, the anterior part of the inferior-parietal lobule (IPL) is connected to the ventral premotor and prefrontal regions, whereas the posterior IPL is linked to caudal-lateral prefrontal regions (6-8).
Functionally the anterior and posterior portions of the IPL are implicated in different movement circuitry. In humans a twin coil TMS (tcTMS) protocol has been used to show that a precision grip modulated the anterior but not posterior IPL-M1 interaction, whereas a full hand power grip modulated the posterior but not the anterior IPL-M1 interaction (9). The tcTMS protocol applied here has been proven very useful for investigating parietal-motor interactions in humans. For tcTMS a conditioning stimulus (CS) is delivered to a site of interest and followed by a test stimulus (TS) to the primary motor cortex (M1). This protocol has been widely used to investigate different interactions between motor areas (10-12) and in 2007 Koch and colleagues showed that it can also be used to probe parietal-motor connections (13). TcTMS has been used extensively to investigate the time course and locality of parietal-motor interactions during tasks and at rest, as well as parietal-motor interactions in different patient populations (9, 13-19). With the exception of one study that investigated superior parietal – M1 interactions (19), these studies focused on IPL and mostly on the posterior portion of the IPL. They showed that when a conditioning stimulus is given to the posterior IPL 2-8ms prior to the test stimulus over M1 the EMG response triggered by M1 pulse is enhanced, implying a facilitatory connection between the posterior portion of IPL and M1. This facilitatory effect of posterior IPL stimulation has been shown both inter- and intra-hemispherically (16).
Also the anterior IPL has distinct connections to frontal and motor areas. In the original study (13) Koch et al., reported inhibitory aIPL-M1 interactions, but this was only tested in a very small set of participants (n =4) and an inhibitory intra-hemispheric effect has never been replicated in a larger data set. The same group did, however, report inhibitory inter-hemispheric effects in a larger group of participants showing that an anterior IPL conditioning pulse had an inhibitory effect on the contralateral M1 (16). In sum, there is accumulating evidence from tcTMS studies for differential parietal-motor interactions along the IPL but there is little work done on differential interactions within one hemisphere. This can be due to technical reasons: Investigating intra-hemispheric interactions between the anterior portion of IPL and M1 can be difficult, due to restrictions in placing two TMS coils close to each other. Additionally, there are no data on inter-subject variability of parietal-motor networks, or on subjective ‘hot-spots’ for parietal facilitation or inhibition.
In this study we used small custom coils and systematically investigated intra-hemispheric parietal-motor connectivity along the intraparietal sulcus. The conditioning effect of three points along the intraparietal sulcus (most anterior, 1cm posterior, 2 cm posterior) on the ipsilateral M1 was tested in both on the left and the right side. Testing networks both on the left and right is important since hemispheric asymmetry in parietal-motor connectivity and parietal function has been reported by several TMS and imaging studies (2, 16, 17, 20, 21). We expected to see an inhibitory parietal-motor connectivity for the anterior spots and an increasingly facilitatory parietal-motor connectivity for the more posterior spots. We also wanted to explore hemispheric asymmetries and inter-personal differences in intra-hemispheric parietal-motor networks.
Materials and Methods
Population
Thirteen healthy volunteers (mean age 26.0 ± 5.1 years, 7 female) took part in the experiment. One participant had to be excluded since space limitations did not allow proper coil placement for the anterior IPL; therefore only twelve participants were included in the analysis.
EMG Setup
Participants were seated in a comfortable armchair with both arms resting on a pillow. Electromyogram (EMG) activity of the right first dorsal interosseus muscle (FDI) was recorded throughout the experiment in a belly-tendon montage using Ag-AgCl surface electrodes. Impedances were kept below 20 kΩ. EMG signals were collected using a Viking IV EMG machine (Nicolet Biomedical, Madison, Wisconsin), bandpass-filtered at 20-2000Hz. The amplified analog outputs from the Viking were digitized at 5 kHz using Labview software (National Instruments, Austin, Texas), and stored for off-line analysis.
Experimental Setup
All participants completed a total of 6 blocks of paired-pulse TMS. For three blocks the M1 coil was placed over the left M1 while the IPL coil was placed over one of three locations along the left IPL (aIPL, cIPL and pIPL). For the other three blocks the M1 coil was placed over the right M1 while the IPL coil was placed over one of three locations along the right IPL (aIPL, cIPL and pIPL). Coil locations, coil placement and coil orientations are shown in Figure 1A and 1C. The order in which the blocks were tested was pseudo-randomized between four different test orders (1.left cIPL-aIPL-pIPL - right cIPL-aIPL-pIPL; 2. right cIPL-aIPL-pIPL -left cIPL-aIPL-pIPL; 3.leftpIPL-cIPL-aIPL -right pIPL-cIPL-aIPL; 4.right pIPL-cIPL-aIPL -left pIPL-cIPL-aIPL). During both experiments participants were completely at rest, with their eyes open and their hands relaxed.
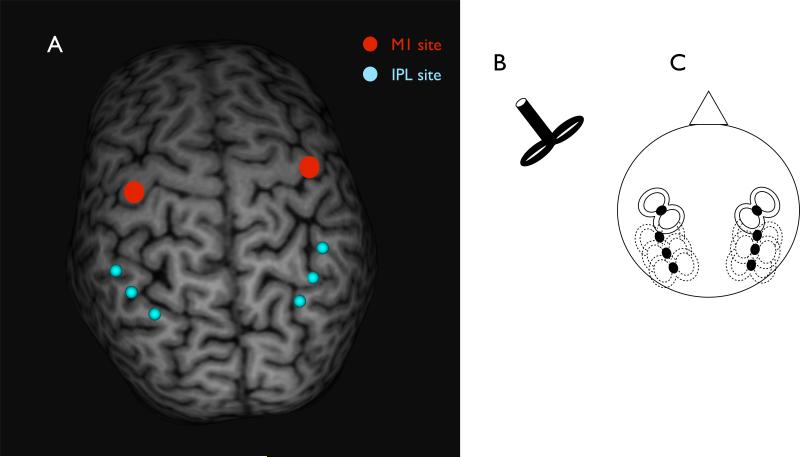
Figure 1.
(A) shows the mean locations for IPL coil positioning (blue), and an example for M1 coil positioning (red), (B) shows an illustration of the coil type that was used and (C) shows a schematic illustration of how the coils were placed.
Magnetic stimulation was delivered using two custom-made figure-of-eight coils with handles perpendicular to the coil windings (‘branding iron style’, 70 mm external diameter; Fig. 1B), connected to two high-power Magstim 200 stimulators (Magstim Company Ltd, Whitland, Dyfed, UK). Stimulation of M1 was always applied at the point that evoked the largest MEP in the contralateral FDI (“motor hotspot”). The M1 coil was held tangentially to the scalp, at a 45° angle from the anteroposterior axis. Both the test and the conditioning coil were placed tangentially to the skull and induced a posterior-anterior current flow in the brain. The position of the M1 coil was marked on a tight fitting cap to ensure proper coil placement throughout the experiment. Resting motor threshold of the FDI (RMTFDI) was measured for each subject and for each hemisphere. RMT was defined as the lowest intensity that induced a 50-μV peak-to peak amplitude MEP in at least five out of ten trials. The conditioning coil was positioned over three different positions along the IPL depending on the block. Parietal sites were localized using neuronavigation, the anterior IPL was defined as the junction of post central sulcus and IPL. The central and posterior spot were defined as 1cm and 2 cm posterior to the anterior spot following along the IPL. Neuronavigation (Brainsight, Magstim Company Ltd, Whitland, Dyfed, UK) was used for precise positioning of the conditioning coil. Magnetic resonance imaging (MRI) data specific to each participant was used to ensure correct placement of the coil over the IPL locations. For the MRIs 3D T1-weighted scans were collected using a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence on a 3T GE Excite scanner using an 8-channel receiver only coil (General Electric Medical System, Milwaukee, WI, USA). Each individual MRI was normalized a posteriori onto the Montreal Neurological Institute (MNI) brain template using the same software. IPL stimulation coordinates were then expressed with respect to the MNI standard space. Table 1 shows the mean coordinates for the right and left IPL and the placement of both the TS and CS coil. The center of the coil was positioned over the IPL target spots tangentially to the skull, with the central line of the coil aligned to a straight line drawn by connecting the anterior, central and posterior spot, inducing a posterior-anterior-directed current in the underlying cortical tissue. This orientation was chosen based on previous description (18). TMS-evoked MEPs were recorded for all 6 blocks . The conditioning stimulus was applied at 90% RMTFDI, since this intensity has been shown to be most effective for testing parietal-motor interactions (13) whereas the test stimulus was applied at an intensity set to evoke an MEP of 1 mV over the FDI at rest. Four inter-stimulus intervals (ISI) between conditioning and test pulses were tested for each participant: 2 ms, 4 ms, 6 ms and 8 ms (18). Sixty pulse-pairs were applied per block (12 test pulse only and 12 for each of the four ISIs), pulses were pseudo-randomized and the inter-trial interval between stimuli was set to 5 seconds, resulting in block durations of 5 minutes. During blocks, EMG from FDI was monitored. MEP size was determined by averaging peak-to-peak amplitudes.
Table 1.
shows the mean locations of the anterior, central and posterior IPL stimulation site in both the left and the right hemisphere.
| Left | Right | |
|---|---|---|
| Anterior | x = -40.2 (± 6.3) y = -53.2 (± 5.2) z = 49.3 (± 4.6) |
x = 40.1 (± 3.9) y = -52.7 (± 7.0) z = 49.7 (± 3.8) |
| Central | x = -35.6 (± 5.6) y = -63.8 (± 4.8) z = 48.4 (± 4.7) |
x = 34.9 (± 5.2) y = -64.6 (± 7.4) z = 47.6 (± 5.4) |
| Posterior | x = -27.6 (± 5.2) y = -73.6 (± 5.1) z = 46.0 (± 5.4) |
x = 29.4 (± 5.4) y = -74.1 (± 6.1) z = 45.3 (± 5.8) |
Coordinates are expressed as x, y and z coordinates of the MNI standard space, standard derivations for each coordinate are shown in brackets.
Statistical analysis
All data were checked for normality distribution using the Kolmogorov-Smirnov test. A student's t-test for dependent samples was used to check for differences in RMT between both hemispheres, Second, to check that there was no difference in TS size alone a repeated measures ANOVA with TS a dependent variable and Site and Hemisphere as independent factors was performed. Before testing for the effects that the placement of the conditioning coil had on conditioned MEPs, the conditioned MEPs for each participant were normalized to the test pulse. After normalization a 2 × 3 × 4 repeated ANOVA was calculated with the independent factors Hemisphere (left/right), Stimulation Site (anterior, central, posterior) and ISI (2, 4, 6, 8 ms). Additionally, separate 3 × 4 repeated ANOVAs with the independent factors Stimulation Site (anterior, central, posterior) and ISI (2, 4, 6, 8 ms) were calculated for each hemisphere. All post-hoc comparisons were done using the Fisher's Least Significant Differences (LSD) test. All statistical analyses were performed using Statistica 9.1 (Statsoft, Inc., Tulsa, OK, USA).
Results
The results of a student's t-test showed that there was no significant difference between the resting motor threshold on the right (mean ± std: 47.4 ± 8.9) and on the left (mean ± std: 44.5 ± 6.9) hemisphere (T(11) = -1.76; P = 0.10). To check that TS alone was the same size in all 6 blocks a repeated measures ANOVA was performed.
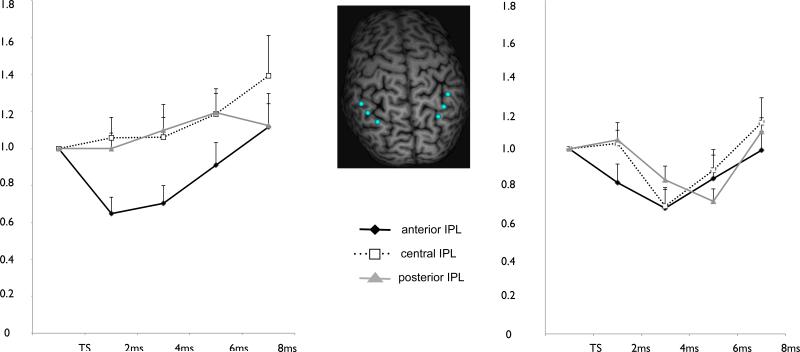
This analysis did not show a significant effect of either Site or Hemisphere when the TS was given alone. Also the Site × Hemisphere interaction was not significant. The 2 × 3 × 4 repeated measures ANOVA showed a significant main effect for hemisphere (F(1) = 5.56; P = 0.038), IPL site (F(2) = 3.86; P = 0.036) and ISI F(3) = 9.18; P < 0.000). For the difference between hemispheres post-hoc testing showed that the average MEP after paired-pulse TMS over the left hemisphere was greater than after paired pulse over the right hemisphere (p = 0.038). For the IPL site, post hoc testing demonstrated that a CS over the anterior IPL resulted in significantly lower MEPs than a CS over the central or posterior IPL (P =0.01 and P =0.04 respectively). Finally post hoc testing showed that the 8ms interval was significantly higher than all other intervals (p = 0.001, p < 0.000 and p =0.002 for comparison with 2, 4, 6 ms respectively). There were no significant interactions observed even though there was a trend for a Hemisphere × ISI interaction (F(3) = 2.06; P = 0.124) and a IPL site × ISI interaction (F(6) = 1.86; P = 0.124). The results are shown in Figure 2. When testing both hemispheres separately, using a 2 × 3 repeated measures ANOVA there was a significant effect for ISI (F(3) = 3.84; P = 0.02) and a borderline significant effect of SITE (F(2) = 3.40; P = 0.05) in the left hemisphere and a significant effect for ISI (F(3) = 6.15; P = 0.002) but not for SITE (F(2) = 0.89; P = 0.42) in the right hemisphere. No significant ISI × SITE interactions were observed (P = 0.16 and P = 0.19 for the left and right hemisphere, respectively). Post-hoc testing showed that for the left hemisphere the 8 ms interval was significantly higher than the 2 and 4 ms intervals (P = 0.004 and P = 0.02, respectively). For the right hemisphere the 8 ms was significantly higher than the 4 and 6 ms interval. Post-hoc tests for the effect of SITE in the left hemisphere did show that a CS at the anterior site significantly lowered MEP compared to the central location (P = 0.02) and showed a trend for lower MEPs when compared to the posterior location (P = 0.06).
Figure 2.
shows the TMS response curves for the left (A) and right (B) hemisphere. Each data point represents the mean ± SEM for either the test stimulus alone or for one of the four paired-pulse ISIs (2, 4, 6, 8 ms). MEPs are normalized to the test stimulus. Data for the anterior IPL is plotted in black, for the central IPL in dotted black and for the posterior IPL in grey.
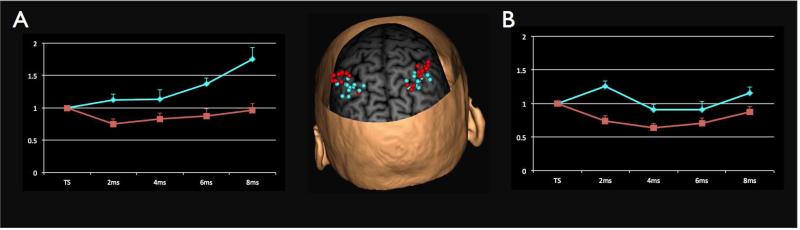
To further describe and visualize interpersonal differences in the parietal-M1 connectivity pattern we selected the IPL facilitatory and inhibitory ‘hotspots’ for each hemisphere and in each participant. (i.e., the two IPL sites with the biggest facilitatory and the biggest inhibitory effect in each hemisphere). Figure 3 shows the MEP conditioned at the IPL hotspots and the individual location of these spots in each participant.
Figure 3.
(middle) shows the inter-individual variation for the preferred inhibitory and excitatory location. The IPL location showing the strongest inhibition for each participant is plotted in red whereas the preferred location for facilitation is plotted in plotted in blue. (A) shows the mean TMS response curves for the preferred inhibitory and facilitatory spots on the left and (B) on the right side. Each data point represents the mean ± SEM for either the test stimulus alone or for one of the four paired-pulse ISIs (2, 4, 6, 8 ms). MEPs are normalized to the test stimulus.
Discussion
We could show that intra-hemispheric parietal-motor connectivity changes along the intraparietal sulcus. A conditioning stimulus over the anterior portion of the ISP had an inhibitory effect on the ipsilateral M1 whereas conditioning stimuli over more posterior portions of the IPL had a facilitatory effect on M1. There was, however, a left-right asymmetry in parietal-motor pathways: The effect of CS coil positioning was more pronounced in the left hemisphere. Only the left side did the central and posterior location show a significant, or close to significant, facilitatory effect compared to the inhibitory effect the anterior site had on M1. Finally, we could demonstrate that there are considerable inter-personal differences in parietal-motor connectivity: The personal ‘hot-spot’ for inhibition and facilitation varied between single subjects, suggesting that the most effective stimulation points within the parietal sulcus can not be solely determined by gross-anatomy. We suggest that a method similar to ‘hotspot’ determination over M1 might be more suitable to stimulate at the most effective site (22)
Intra-hemispheric parietal-motor pathways
The differential effects that stimulation of the anterior and posterior IPL has on the ipsilateral M1 excitability are very similar to the effects that have been demonstrated for inter-hemispherical connections (16). Taken together these data show that the anterior IPL has inhibitory and central and posterior IPL have excitatory connections to both motor cortices at rest. The influence that different sub-regions of the IPL have on M1 excitability might be connected to the different functional roles these areas have in reaching and grasping coordination. Experiments have suggested that the anterior portion of the IPL is important for precise grasping movements (e.g., precision grip) whereas the more caudal regions are implicated with reaching movements (e.g., power grip) (9, 23-26). It is possible that that the fundamental differences in parietal-motor connectivity observed at rest tie in to the functional differences during reaching and grasping, observed in these two areas. A precision grasping movement requires selective activation of specific muscles during the movement, whereas a power grasping requires activation of larger muscle groups. It is therefore plausible that the network controlling precision grip will rely on strong inhibitory connections at rest that can be selectively lifted during periods of movement planning and execution. A power grip network on the other hand might rely on slight facilitation of M1 during rest to quickly and efficiently activate muscles during movement planning and execution. Koch et al. (2010) could show that stimulation of the anterior IPL during precision grip planning had a facilitatory effect on specific muscles (FDI) whereas stimulation of the posterior IPL during power grip planning resulted in facilitation of several recorded muscles (FDI, ADM). Further experiments, comparing parietal-motor networks during motor tasks and rest will be required to fully understand the influence differential parietal-networks at rest have on action preparation and execution. To understand the full pathway of parietal-motor interactions, also premotor regions have to be taken into consideration, since the parietal areas connect to M1 mainly through premotor regions (7, 27, 28). Also premotor regions also have a pivotal role during reaching and grasping movements (29-32). The parietal cortex has differential connections to the premotor regions. Whereas lateral parietal areas close to the anterior intra parietal sulcus are connected to the ventral premotor cortex, parietal areas closer to the central and posterior sections of the intra-parietal sulcus connect to the dorsal premotor cortex and supplementary motor areas (6, 7). This means that the anterior CS site in our experiment is likely to influence M1 via the ventral premotor cortex while the central and posterior CS sites are likely to influence M1 via the dorsal premotor cortex. These differences in parietal-motor pathways could also explain the different effects on M1 excitability observed at different parietal sites. How the different functional roles of the IPL and the premotor cortex relate to the different anatomical parietal-motor pathways is not yet fully understood and more work needs to be done to integrate the functional differences with differential connectivity within the parietal-premotor-motor network.
Left-right asymmetry of parietal-motor pathways
Whereas the inhibitory effect of anterior IPL stimulation was similar both in the right and left hemisphere we were able to show stronger facilitatory effects of the conditioning stimulus on the left hemisphere. Since all of our participants were right-handed and given their left-hemispheric dominance of many aspects of motor control, including reaching and grasping (33) we propose that differences we observed might be part of a general left-right asymmetry of the motor system. A left-right asymmetry has also been shown for inter-hemispheric parietal-motor connections. Koch et al., (2009) found that stronger intensities over the left IPL were needed to influence the contralateral M1. While stimulating the right IPL with 90% RMT was sufficient to increase contralateral excitability, the stimulation intensity had to be changed to 110% RMT to see the same effect when stimulating the left IPL. Since we did not test higher stimulation intensities we cannot comment if the facilitatory effect in the right hemisphere would have increased at higher intensities. Generally, both our and Koch et al's study show that at low stimulation intensities the dominant motor cortex receives stronger facilitatory projections from the central and posterior parietal cortices. Asymmetries have also been demonstrated between left and right parietal cortices (17) showing an inhibitory connection from the right to the left IPL but not the other way around. It is therefore possible that the observed differences in our study and are due to the right-hand dominance of our participants and the greater demands that are put on the left parietal-motor network. Future experiments will have to show if left-handers display the reversed pattern. It is conceivable, however that those effects might be less strong in a left-handed group since they often show weaker hemispheric dominance (33).
Inter-subject variability in parietal-motor connections
Averaged across participants we could show that the most anterior spot had the greatest inhibitory, and the central - posterior spots the greatest facilitatory, response. Even though these locations gave reliable and reproducible results across subjects we observed considerable variability on a single subject level; even though almost all participants showed facilitation at one and inhibition at another of our three stimulation sites, the preferred ‘hot-spot’ for both varied considerably as shown in figure 3. We propose that there might be optimal ‘target-areas’ in the parietal cortex that are difficult to localize with anatomical information alone. Similar to finding the ‘motor-hotspot’ in M1 there might be areas of best parietal-motor connectivity for both the inhibitory and the facilitatory network that could be determined by a test procedure similar to the one applied to find the M1 hot spot (22): By systematically moving the parietal coil in small steps (0.5 cm) along the IPS while recording some parietal conditioned MEPs at each spot before beginning the experiment researchers could further improve the localization of their stimulation site. Small changes in optimal coil placement that cannot be accounted for by purely anatomy-based localization techniques might be responsible for the relatively large variance in tcTMS studies and could potentially also explain the slight inconsistencies in the conditioning intervals found to be most effective in different studies (4 and 15ms in (13); 6, 8 and 12ms in (16), 2 and 8ms in (18), 8ms in the presented work). Since TMS always affects larger neuronal assembles it is also possible that excitatory and inhibitory effects are present at all three parietal sites with only the net-effect of excitation/inhibition differing along the anterior-posterior axis. Intermixed inhibitory and excitatory effects would naturally also contribute to inter-subject variance observed in this study.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Program. We are grateful to Nguyet Dang for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Financial Interests and Potential Conflicts Of Interest.
None of the authors reported biomedical financial interests or potential conflicts of interest.
References
- 1.Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18(7):314–20. [PubMed] [Google Scholar]
- 2.Iacoboni M. Visuo-motor integration and control in the human posterior parietal cortex: evidence from TMS and fMRI. Neuropsychologia. 2006;44(13):2691–9. doi: 10.1016/j.neuropsychologia.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44(13):2594–606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308(5722):662–7. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 5.Koch G, Rothwell JC. TMS investigations into the task-dependent functional interplay between human posterior parietal and motor cortex. Behav Brain Res. 2009;202(2):147–52. doi: 10.1016/j.bbr.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr., et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15(6):854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 7.Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228(1):105–16. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 8.Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(Pt 3):630–53. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 9.Koch G, Cercignani M, Pecchioli C, Versace V, Oliveri M, Caltagirone C, et al. In vivo definition of parietal-motor connections involved in planning of grasping movements. Neuroimage. 2010;51(1):300–12. doi: 10.1016/j.neuroimage.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14(6):1444–53. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- 11.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–46. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37(6):703–13. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- 13.Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, et al. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27(25):6815–22. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, et al. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J Neurosci. 2008;28(23):5944–53. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch G, Oliveri M, Cheeran B, Ruge D, Lo Gerfo E, Salerno S, et al. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008;131(Pt 12):3147–55. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch G, Ruge D, Cheeran B, Fernandez Del Olmo M, Pecchioli C, Marconi B, et al. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J Physiol. 2009;587(Pt 17):4281–92. doi: 10.1113/jphysiol.2009.174086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch G, Cercignani M, Bonni S, Giacobbe V, Bucchi G, Versace V, et al. Asymmetry of parietal interhemispheric connections in humans. J Neurosci. 2011;31(24):8967–75. doi: 10.1523/JNEUROSCI.6567-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karabanov AN, Jin SH, Joutsen A, Poston B, Aizen J, Ellenstein A, et al. Timing-dependent modulation of the posterior parietal cortex-primary motor cortex pathway by sensorimotor training. J Neurophysiol. 2012 doi: 10.1152/jn.01049.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziluk A, Premji A, Nelson AJ. Functional connectivity from area 5 to primary motor cortex via paired-pulse transcranial magnetic stimulation. Neurosci Lett. 2010;484(1):81–5. doi: 10.1016/j.neulet.2010.08.025. Epub 2010/08/17. [DOI] [PubMed] [Google Scholar]
- 20.Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15(6):681–95. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci. 2001;4(6):656–61. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- 22.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivier E, Davare M, Andres M, Fadiga L. Precision grasping in humans: from motor control to cognition. Curr Opin Neurobiol. 2007;17(6):644–8. doi: 10.1016/j.conb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Begliomini C, Wall MB, Smith AT, Castiello U. Differential cortical activity for precision and whole-hand visually guided grasping in humans. Eur J Neurosci. 2007;25(4):1245–52. doi: 10.1111/j.1460-9568.2007.05365.x. [DOI] [PubMed] [Google Scholar]
- 25.Castiello U. The neuroscience of grasping. Nat Rev Neurosci. 2005;6(9):726–36. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- 26.Karnath HO, Perenin MT. Cortical control of visually guided reaching: evidence from patients with optic ataxia. Cereb Cortex. 2005;15(10):1561–9. doi: 10.1093/cercor/bhi034. [DOI] [PubMed] [Google Scholar]
- 27.Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6(3):342–53. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 28.Battaglia-Mayer A, Caminiti R, Lacquaniti F, Zago M. Multiple levels of representation of reaching in the parieto-frontal network. Cereb Cortex. 2003;13(10):1009–22. doi: 10.1093/cercor/13.10.1009. [DOI] [PubMed] [Google Scholar]
- 29.Davare M, Kraskov A, Rothwell JC, Lemon RN. Interactions between areas of the cortical grasping network. Curr Opin Neurobiol. 2011;21(4):565–70. doi: 10.1016/j.conb.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008;586(Pt 11):2735–42. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davare M, Andres M, Clerget E, Thonnard JL, Olivier E. Temporal dissociation between hand shaping and grip force scaling in the anterior intraparietal area. J Neurosci. 2007;27(15):3974–80. doi: 10.1523/JNEUROSCI.0426-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davare M, Montague K, Olivier E, Rothwell JC, Lemon RN. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009;45(9):1050–7. doi: 10.1016/j.cortex.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin K, Jacobs S, Frey SH. Handedness-dependent and -independent cerebral asymmetries in the anterior intraparietal sulcus and ventral premotor cortex during grasp planning. Neuroimage. 2011;57(2):502–12. doi: 10.1016/j.neuroimage.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]