Summary
Intestinal infection with the parasitic nematode, Trichinella spiralis, provides a robust context for the study of mucosal mast cell function. In rats, mucosal mast cells are exposed to parasites during the earliest stage of infection, affording an opportunity for mast cells to contribute to an innate response to infection. During secondary infection, degranulation of rat mucosal mast cells coincides with expulsion of challenge larvae from the intestine. The goal of this study was to evaluate rat bone marrow-derived mast cells (BMMC) and the rat basophilic leukemia cell line (RBL-2H3) as models for mucosal mast cells, using parasite glycoproteins and antibody reagents that have been tested extensively in rats in vivo. We found that BMMC displayed a more robust mucosal phenotype. Although T. spiralis glycoproteins bound to mast cell surfaces in the absence of antibodies, they did not stimulate degranulation, nor did they inhibit degranulation triggered by immune complexes. Parasite glycoproteins complexed with specific monoclonal IgGs provoked release of RMCPII and β-hexosaminidase from both cell types in a manner that replicated results observed previously in passively immunized rats. Our results document that RBL-2H3 cells and BMMC model rat mucosal mast cells in the contexts of innate and adaptive responses to T. spiralis.
Keywords: nematode, mast cell, antibody, rat
Introduction
Rats and mice are natural hosts for the parasitic nematode, Trichinella spiralis. First-stage larvae (L1) initiate infection in the intestine, colonizing the epithelium where they molt and develop into adults within 3–4 days. Adult worms are expelled between 10 and 15 days post-infection (dpi), concurrent with the development of a pronounced intestinal mastocytosis (1–3). In rats, mast cell protease-II (RMCPII) is released by mucosal mast cells during adult worm expulsion following primary infection (4). Mice deficient in mast cells or lacking the gene for mouse mast cell protease-I (mMCP-1), the protease that is unique to mucosal mast cells of mice, were used to confirm a requirement for mast cells in adult worm expulsion. Both mouse strains demonstrate delayed worm expulsion (5, 6), implicating the mucosal mast cell as a pivotal mediator of immunity.
While a great deal is known about the induction of mastocytosis (7, 8) and the dependence of worm expulsion on mMCP-1 (6, 9), it is less clear whether the role of the mast cell is as an effector, an innate regulator, or both. Mast cell deficient mice are profoundly compromised in their capacity to mount a Th2 response to T. spiralis and mast cells are important sources of TNF-α and IL-4 (10), suggesting a role for the mast cell in the inductive phase of immunity. Following exposure to larval glycoproteins, rat peritoneal mast cells have been shown to release histamine (11), and a hybrid cell that models connective tissue mast cells was reported to release IL-4 and TNF-α (12); however, mucosal mast cells have not been tested for activation in response to parasite products or their immune complexes.
Following secondary intestinal infection with L1, rats demonstrate a dramatic protective immunity that eliminates as many as 99% of larvae from the intestine within hours of infection (13–17). Early reports referred to this immunity as intestinal anaphylaxis (18), and it is well documented that mast cell activation occurs at the time of expulsion (19–21). Antibodies have been shown to mediate this “rapid expulsion” in neonatal rats (22), but an unknown immune factor enables antibodies to be protective for adult rats (23). Mast cells degranulate in neonates and adults during expulsion, releasing RMCPII, which is detected in the sera with 3 hours of challenge (21). Similarly, release of RMCPII is induced by larval challenge in naïve adults and neonates that have been passively immunized with L1-specific IgE or IgG2a; however, we have shown that mediator release is neither required nor sufficient for expulsion (21). Nevertheless, the immediate and dramatic activation of mucosal mast cells during secondary infection with T. spiralis affords a reproducible, natural context for the study of antibody-induced, mucosal mast cell degranulation.
In this study, we evaluated two in vitro models of the rat mucosal mast cell, the RBL-2H3 cell line and bone marrow-derived mast cells (BMMC). We compared the two cell types for responses to both innate and adaptive (antibody-dependent) stimuli. Culture of rat bone marrow cells with IL-3 and SCF yields mast cells that display biochemical and functional properties comparable to intestinal mucosal mast cells (24). BMMC granules contain RMCPII (25) and stain uniformly with Alcian blue, a dye that binds sulphated acid mucopolysaccharides and differentiates mucosal from connective tissue mast cells in rats (26). In these ways, BMMC are a highly relevant model for the study of mucosal mast cells in nematode infections.
Antibodies activate mast cells by aggregating surface Fc receptors. FcεRI is the high affinity receptor for IgE, which triggers rat mast cell degranulation when aggregated with either IgE or IgG2a complexed with antigen (27). Although RBL-2H3 cells have been used extensively in studies of FcεRI function (28), binding and activation of RBL-2H3 and BMMC by other isotypes is less well understood. Previously, we prepared a unique panel of monoclonal IgGs (29), representing all four subclasses and sharing specificity for the same glycan (29–31). These monoclonal antibodies have been thoroughly characterized for their effects on T. spiralis in vivo (21, 32). In the studies reported here, we used this panel of antibodies to compare BMMC and RBL-2H3 cells as models for antibody-mediated mast cell activation.
Our experiments show that BMMC display a strong mucosal phenotype and are phenotypically distinct from RBL-2H3 cells. Neither cell type was induced to release RMCPII or β-hexosaminidase by exposure to soluble products of T. spiralis L1. Antibodies that have been shown to cause RMCPII to be released into the sera of rats during challenge infection also induced degranulation by both cell types in vitro, confirming that BMMC and RBL-2H3 cells are useful models for mucosal mast cells in the context of IgG-mediated activation and parasitic worm infection.
Materials and Methods
Rats
Lewis and AO strain rats (8 to 12 weeks old) were bred and maintained in the vivarium of the Baker Institute for Animal Health. The life cycle of Trichinella spiralis was maintained in rats (33). All rodents were housed in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care and experiments were conducted with the approval of the Cornell University Institutional Animal Care and Use Committee.
Antibodies
Monoclonal antibody AA4 (34, 35) was used to detect the ganglioside GD1b. Tyvelose-specific monoclonal rat antibodies (clones 9D (IgG1), 18H (IgG2a), 10G11 (IgG2b), and 9E6 (IgG2c)) were characterized previously (29). Antibodies were recovered from heat-inactivated ascites fluid (purchased from Harlan, Indianapolis, IN) by precipitation with 40% saturated (NH4)2SO4, as described (36). Monoclonal mouse IgE specific for DNP was purified as described (37), and rat IgG2a anti-DNP was purchased (clone DNP-16; American Research Products, Inc.; Belmont, MA). For preparation of polyclonal IgE, AO rats were infected orally with 2,000 T. spiralis L1 and re-infected 30 days later with the same dose. One week after the second infection, rats were bled by cardiac puncture under deep isoflurane anesthesia. Sera were stored at −80°C until IgE was purified by affinity chromatography using mouse anti-rat IgE antibodies (clones A2 and B5) as described in Bell et al. (23). Antibodies were dialyzed against 0.85% normal saline and stored at −20°C.
Antigens
T. spiralis L1 were recovered from rat muscle tissue by digestion with 1% pepsin in acidified water (33). Rats had been infected at least 28 days prior to collection of larvae. Excretory-secretory antigen (ES Ag) was obtained from overnight cultures of L1 as described previously (29). Crude antigen (cAg) was prepared from homogenates of whole L1 as described (38), except that detergent was omitted from the buffer. ES Ag was dialyzed against, and cAg was prepared in, Dulbecco’s PBS (DPBS) and stored at −20°C. BSA was conjugated with an average of 15 DNP groups per molecule (DNP-BSA, multivalent antigen) as described (37), dialyzed against PBS, and stored at 4°C.
Cell culture
Cell culture media and supplements were purchased from Gibco (Grand Island, NY), unless otherwise noted. Monolayer cultures of RBL-2H3 cells (39) were maintained in minimum essential medium (MEM) supplemented with 20% fetal bovine serum (Atlanta Biologicals, Inc.; Lawrenceville, GA) and 50 µg/ml gentamicin sulfate. Cells were grown at 37°C in 5% CO2, passaged by trypsinization weekly, and used in experiments 3–5 days after passage. BMMC were isolated and propagated using a protocol modified from Haig et al. (24). Briefly, female Lewis strain rats were euthanized by CO2 inhalation and cervical dislocation. Bone marrow was flushed from femurs with sterile Hank’s balanced salt solution (HBSS). Recovered cells were washed five times in HBSS, resuspended at 0.33–0.5 × 106 cells/ml, and cultured in Dulbecco’s modification of Eagle’s medium (DMEM) buffered with 1 mM Hepes and supplemented with 20% horse serum, 4 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamicin, 100 ng/ml recombinant rat interleukin-3 (IL-3), and 50 ng/ml recombinant rat stem cell factor (SCF) (Peprotech; Rockyhill, NJ). Cells were incubated at 37°C in 5% CO2 and resuspended (without trypsin) for passage when cell density reached 1 × 106 cells/ml (approximately 3–5 days). Experiments were conducted with cells harvested between 14 and 28 days of culture.
Cytology
BMMC or RBL-2H3 cells were prepared for cytologic examination by centrifuging at 500 rpm for 5 minutes (Shandon Cytospin 2). Slides were air dried, stained with Alcian blue (Sigma; St. Louis, MO) for 10 minutes, and counter-stained with Safranin-O for 10 minutes (Sigma). Coverslips were mounted with glycergel (DakoCytomation,Inc; Carpinteria, CA). A total of 100 cells were evaluated in high power fields (40X) of slides prepared from individual flasks. The percentage of cells stained blue was calculated and the mean value of three flasks reported. Slides were examined on a BX51 microscope and photographed with a DP-12 digital camera system (Olympus, Melville, NY). Total cell numbers were estimated from each of the three flasks using a hemacytometer (Reichert; Buffalo, NY).
Flow cytometry
Rat monoclonal IgG1, IgG2a, IgG2b, IgG2c, and polyclonal IgE were conjugated with Alexa Fluor-488 (Molecular Probes; Eugene, OR). Briefly, immunoglobulins (1.0 mg/ml) were prepared in PBS (pH 8.5) and incubated with Alexa Fluor-488 (100 µg/ml) at room temperature for 4 hours or overnight. Samples were dialyzed in PBS (pH 7.4) and stored with 0.1% sodium azide at 4°C in a light-protected container. Molar dye-to-protein ratios were estimated to be between 3 and 13.
Direct binding of parasite glycoproteins to mast cells was tested by incubating cells for 1 hour on ice with either ES Ag or cAg (10 µg/ml). Because rat IgG2c did not bind to mast cells (shown in Fig. 4A), clone 9E6 (IgG2c)-Alexa 488 (10 µg/ml) was used to detect tyvelose-bearing glycoproteins on the cell surface. Unbound antigen was removed from cells by washing with PBS and the cells were incubated with IgG2c for 30 minutes, washed with PBS three times, and fixed in 2% paraformaldehyde.
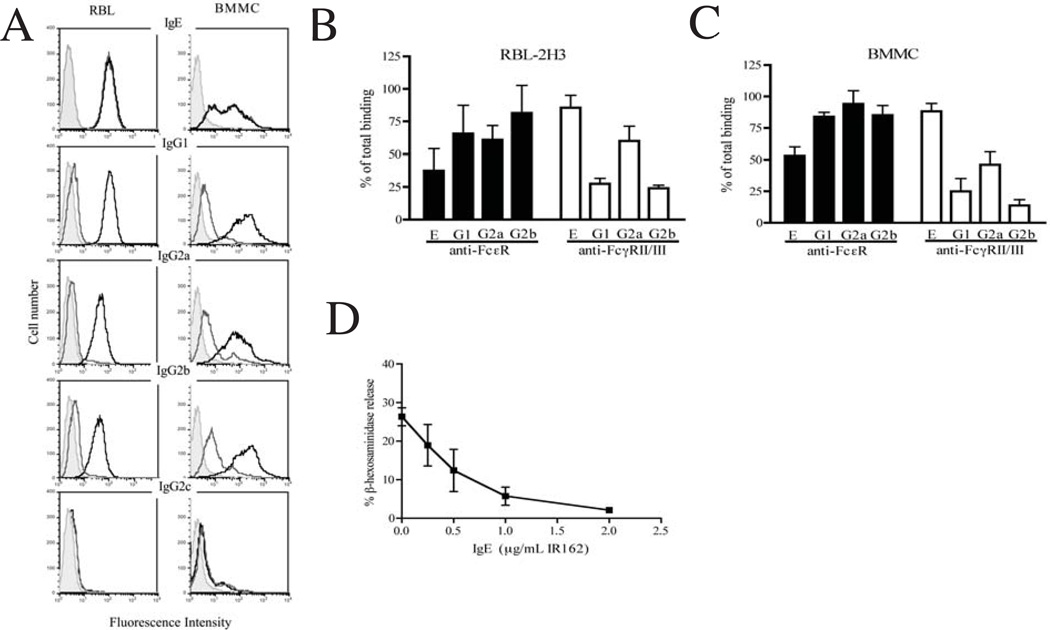
Figure 4.
Detection of binding by immune complexes to RBL-2H3 cells and BMMC. (A) Cells were incubated with Alexa-488 conjugated antibodies of the indicated isotypes (10 µg/ml) complexed with ES Ag (10 µg/ml; black line). Also shown are unstained cells (shaded peak) and cells incubated with Alexa-488 conjugated antibody without antigen (grey line). Experiments were performed three times with similar results. (B, C) Effect of receptor-specific antibodies on GMFI of RBL-2H3 cells (B) or BMMC (C) incubated with immune complexes formed with IgE or IgG. Cells were pre-treated with antibodies specific for FcεRI (black bars) or FcγRII/III (open bars). Values represent the GMFI obtained in the presence of receptor-specific antibodies as a fraction of the GMFI obtained in the absence of receptor-specific antibodies. Bars represent the mean +/− 1 SD from two (RBL-2H3) or three (BMMC) experiments. *p<0.05 compared to immune complexes alone. (D) Inhibition of IgG2a-induced mediator release by pre-treatment of RBL-2H3 cells with monoclonal anti-DNP IgE (clone IR162). Cells were incubated with indicated concentrations of IgE prior to adding complexes formed by anti-tyvelose IgG2a (2 µg/ml) and ES Ag (1 µg/ml). Symbols represent the mean +/− 1 SD of triplicate samples.
Surface binding of immune complexes was detected by suspending BMMC and RBL-2H3 cells in PBS at 0.5 × 106 cells/ml, then incubating with labeled immunoglobulins (10 µg/ml) for 15 minutes prior to adding ES Ag or cAg (10 µg/ml). Cells were incubated on ice for 1 hour, then washed and fixed. In some experiments, RBL-2H3 and BMMC were incubated with antibody AA4 followed by FITC-conjugated anti-mouse antibody. In other experiments, mouse anti-rat FcεRI (clone BC4) or rat anti-mouse FcγRII/III (clone 2.4G2) (BD Biosciences; San Jose, CA) were added to cells 30 minutes prior to addition of antibodies and antigens. Cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences), and data were evaluated using Cell Quest or Flow Jo software. Figures were generated using Flow Jo.
Degranulation assay
Mast cells were cultured overnight in either 48 well (2.5 × 105 cells/well) or 96 well (1.25 × 105 cells/well) plates. After washing twice with Tyrode’s buffer (20 nM HEPES, 135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5.6 mM glucose, 0.1% BSA, and 1.8 mM CaCl2 (pH 7.4)), immunoglobulins diluted in Tyrode’s buffer were added, followed 15 minutes later by the equivalent volume of antigen (62.5 µl for a 96 well or 125 µl for a 48 well). For positive controls, cells were treated with calcium ionophores, ionomycin or A23187 (1 µM; Sigma), in the presence of phorbol ester-12 myristate-13 acetate (PMA; 50 nM; Sigma). Cultures were incubated for 1 hour at 37°C, then collected for analysis. Treatments were tested in triplicate. In some experiments, 1 mM doxantrazole (Sigma), prepared in Tyrode’s buffer, was added together with PMA and ionophore.
Antigens and antibodies were cross-titrated in preliminary experiments and data shown were obtained with concentrations that were found to be optimal. All experiments were replicated.
β-hexosaminidase was measured using a modified colorimetric assay (40) and is reported as optical density or as a percentage of the total enzyme per well. Briefly, an aliquot of assay supernatant was collected from each well, and the remaining buffer and cells extracted (1:1) with 1% Triton X-100 (Sigma). Samples were incubated with 200 ml of 1 mM p-nitrophenyl N-acetyl-β-D-glucosaminide (Sigma) in 0.05 M citrate buffer (pH 4.5) for 1 hour at 37°C. The reaction was stopped with 500 µL of 0.1 M sodium carbonate buffer and absorbance read at 400–405 nm using an Ultraspec 2100 pro spectrophotometer (Amersham Pharmacia Biotech; Piscataway, NY) or an ELISA plate reader (Bio-Tek Instruments; Winooski, VT).
RMCPII was detected by ELISA in culture supernatants and cell lysates according to the manufacturer’s instructions (Moredun Scientific Limited, Mildlothian, Scotland). Concentrations were estimated from a standard curve and reported as a mean for each treatment group. In some experiments, values were reported as a percentage of the total enzyme content of the cells.
Statistical analysis
Data were evaluated by analysis of variance (ANOVA) and Scheffe’s comparison of multiple means using Statistix (Analytical Software; Tallahassee, FL) or GraphPad Prism software. Differences were considered statistically significant when p < 0.05.
Results
Phenotypic comparison of BMMC and RBL-2H3 cells
Cytologic evaluation of cells cultured from bone marrow revealed granules stained with Alcian blue as early as 3 days in culture, and 99% of cells contained granules after 15 days (Fig. 1A). When compared to RBL-2H3 cells, bone marrow cells had larger, more distinct granules that stained with greater intensity (supplemental Fig. 1). These results are consistent with the larger quantities of β–hexosaminidase (Fig. 1B) and RMCPII (Fig. 1C) detected in bone marrow cells after 14 days in culture.
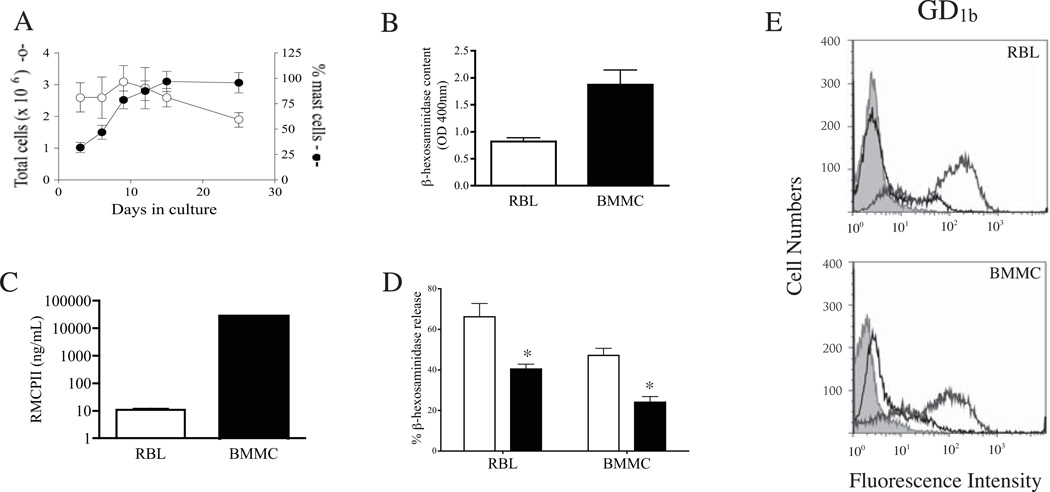
Figure 1.
Properties of BMMC and RBL-2H3 cells. (A) Growth and staining properties of bone marrow cells cultured in the presence of IL-3 and SCF. Number of viable cells (open symbols) and number of cells containing granules detected with Alcian blue (mast cells) (closed symbols) were counted. Values represent the mean +/− 1 SD (n = 3). (B) β-hexosaminidase activity recovered from 1.25 × 105 BMMC (21 days) or RBL-2H3 cells. Values represent the mean +/− 1 SD (n = 12 wells). (C) RMCPII content of 5 × 104 BMMC (20 days) and RBL-2H3 cells. Values represent the mean +/− 1 SD (n= 4 wells). (D) Effect of doxantrazole on β-hexosaminidase release induced by PMA and A23187 in RBL-2H3 and BMMC. Open bars = untreated, black bars = drug treated * p< 0.001 compared with untreated. (E) Flow cytometric detection of GD1b (dark grey line) on RBL-2H3 cells and BMMC. Unstained cells (shaded grey) and nonspecific IgG-FITC (black line) served as negative controls.
Further evidence in support of a mucosal phenotype for bone marrow-derived mast cells was obtained using a pharmacologic inhibitor. Doxantrazole is a mast cell stabilizer that inhibits degranulation by rat mucosal mast cells (41). Treatment of bone marrow or RBL-2H3 cells with 1 mM doxantrazole inhibited release of β-hexosaminidase induced by PMA/A23187 (Fig. 1D).
Monoclonal antibody AA4 detects two α-galactosyl derivatives of the ganglioside GD1b (42) that are unique to mast cells in the rat (43) and are present on all rat mast cells, including BMMC (35). Surface expression of GD1b was approximately 3-fold greater on RBL-2H3 cells than bone marrow cells (GMFI of 90 vs. 33, respectively; Fig. 1E).
In summary, cells cultured from bone marrow in the presence of SCF and IL-3 displayed a robust mucosal mast cell phenotype, differing from RBL-2H3 cells in polysaccharide and enzyme content, as well as surface ganglioside expression. In subsequent experiments, we assayed cells harvested from bone marrow cultures between 14 and 28 days of culture and refer to these cells as bone marrow-derived mucosal mast cells (BMMC).
Binding and activation of mucosal mast cells by parasite glycoproteins
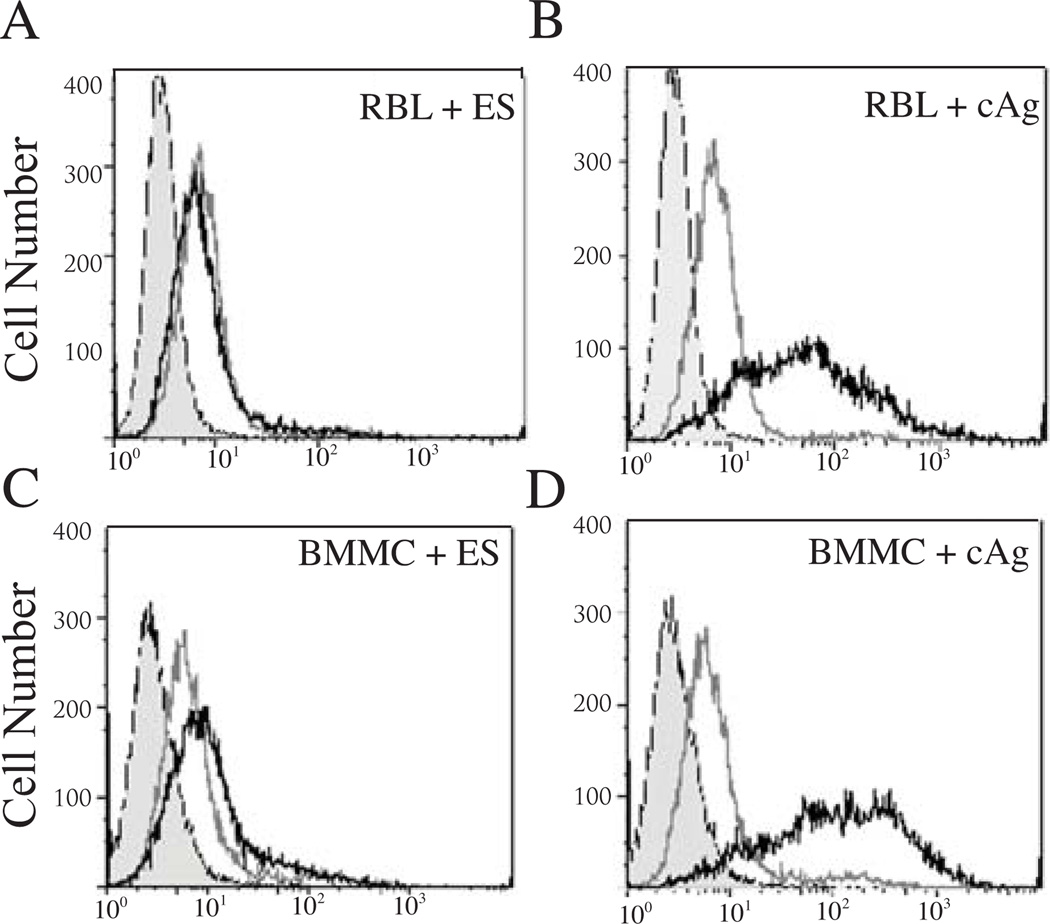
As a first step in determining if degranulation is induced directly by parasite products, we assayed binding of the tyvelose-bearing glycoproteins in ES Ag and cAg to the surfaces of BMMC and RBL-2H3 cells. Binding by tyvelose-bearing glycoproteins was not detected following incubation with ES Ag (Fig 2A, C), while tyvelose-bearing glycoproteins in cAg bound to both cell types (Fig. 2B, D). Despite evidence for surface binding, when cAg and ES Ag were tested in degranulation assays, mediator release was similar to cells treated with buffer alone (Fig. 3 A, C, null treatment).
Figure 2.
Detection of binding by tyvelose-bearing parasite molecules to mast cells. RBL-2H3 cells (A,B) and BMMC (C,D) were incubated with ES Ag (A,C) and cAg (B,D) (10 µg/ml) and tyvelose was detected with IgG2c-Alexa 488 (10 µg/ml) (black line). Also shown are unstained cells (shaded peak) and cells incubated with conjugate alone (grey line).
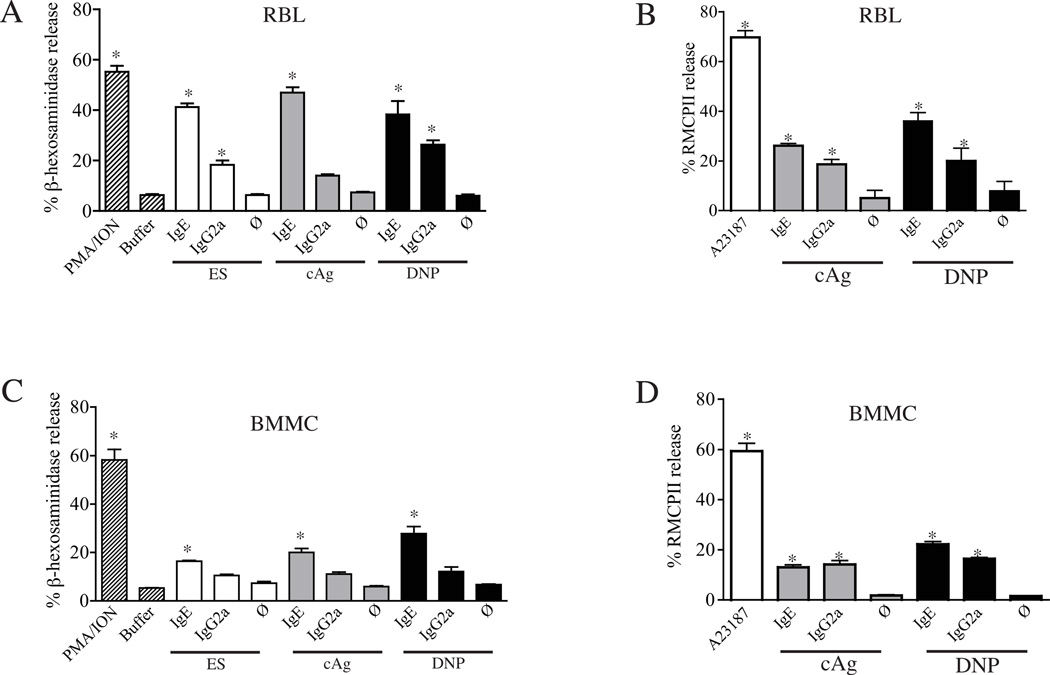
Figure 3.
Antibody-dependent mediator release by RBL-2H3 cells and BMMC. Incubation of RBL-2H3 (A, B) and BMMC (C, D) with immune complexes formed by tyevelose or DNP-specific IgG2a or IgE with ES Ag, cAg, or DNP-BSA. Antibodies (10 µg/ml) were complexed with antigens (2 µg/mL for anti-DNP IgE and IgG2a, and 1 µg/mL for all others), and binding and activation were tested on RBL-2H3 cells. Release of β-hexosaminidase (A, C) or RMCPII (B, D) was measured and expressed as a percentage of the total cell content. *p<0.05 compared with buffer or antigen alone (ø). Values graphed are means +/− 1 SD (n=3).
Binding of mast cells by immune complexes
Immune complexes formed with ES Ag and tyvelose-specific IgE, IgG1, IgG2a, IgG2b bound to both BMMC and RBL-2H3 cells (Fig. 4A, black lines). There was very little surface binding to RBL-2H3 by any of the IgG isotypes in the absence of antigen (Fig. 4A, dark grey lines). A discrete small peak was evident in BMMC preparations incubated with IgG1, IgG2a and IgG2b in the absence of antigen, indicating the presence of cells bearing high affinity IgG receptors or, alternatively, revealing binding to low affinity receptors of small amounts of aggregated IgG that may have been present in conjugate preparations. Such binding was not evident on RBL-2H3 cells.
Immune complexes formed with IgG1, IgG2a or IgG2b yielded high geometric mean fluorescence intensities (GMFI) on both cell types, indicating that these isotypes bound more efficiently to cell surfaces when aggregated, which is consistent with the presence of low-affinity IgG receptors. Complexes formed with IgG2c bound to neither cell type.
Treatment of cells with IgE or complexes formed with IgE generated identical histograms (Fig. 4A, dark grey and black lines are superimposed), which implies that monomeric IgE bound to the high affinity FcεRI. Although IgE binding to RBL-2H3 cells revealed a single population with a high GMFI, two populations of BMMC were observed. RBL-2H3 cells incubated with IgE had a 4-fold greater GMFI than BMMC (125 versus 30, Fig. 4A). The reduced density of IgE binding suggests that BMMC may be less efficiently activated by IgE, a prediction that was confirmed in degranulation assays (Fig. 3).
Compared with IgE binding, BMMC were more uniformly bound by IgG complexes, and the GFMI of IgG was consistently higher on BMMC compared to RBL-2H3. For example, when incubated with IgG2b complexes, BMMC had a 3.6-fold greater GMFI compared with RBL-2H3 cells (155 versus 43; Fig. 4A).
Mast cell binding of IgE-Ag complexes was blocked by antibody to FcεRI but not FcγRII/III (Fig. 4B,C), confirming that binding was specific for the high affinity FcεRI. Binding by IgG1, IgG2a, and IgG2b complexes was variably blocked by anti-FcγRII/III on both cell types, indicating that IgGs were binding to a low affinity FcγR. Although unlikely, this blocking may have occurred via competition from mouse IgG aggregates in the anti-FcγR preparation; however, that mechanism also would be compatible with a low affinity IgG receptor and would not change the interpretation of the result. The absence of a high affinity FcγR was documented when rat IgG1, IgG2a and IgG2b failed to bind cell surfaces in the absence of antigen (Fig. 4A).
Taken together, the results suggest that RBL-2H3 and BMMC cells display surface FcεRI as well as low-affinity IgG receptors. Pre-treatment of cells simultaneously with both receptor-specific antibodies was more effective in blocking IgG2a complexes (not shown), suggesting that this isotype bound to both receptor types. Increasing concentrations of non-specific, monomeric IgE (clone IR162) inhibited degranulation induced by IgG2a complexes (Fig. 4D), confirming that degranulation induced by IgG2a was mediated by FcεRI on RBL-2H3 cells.
Degranulation induced by immune complexes
Immune complexes were tested for their capacities to induce degranulation by measuring β–hexosaminidase and RMCPII release (Fig. 3). Cross-titrations of antibodies and antigens were performed, and results are shown for the concentrations that were determined to be optimal for causing mediator release. Both tyvelose-specific and DNP-specific IgE and IgG2a antibodies caused RBL-2H3 and BMMC degranulation; however, IgE was more potent (Fig. 3). Although IgG1 appeared to bind both cell types in a manner similar to IgG2a (Fig. 4A), it failed to stimulate significant degranulation in RBL-2H3 cells or BMMC (2.4 and 7.6%, respectively). These results were confirmed with a second monoclonal anti-tyvelose IgG1, clone 6D9 (29) (data not shown). Similarly, IgG2b bound to both cell types, yet failed to induce significant degranulation responses (1.9 and 6.1% for RBL and BMMC, respectively). Thus, antibodies shown to bind FcR failed to activate mast cells, suggesting that the low affinity Fcγ involved might be an inhibitory receptor. We attempted to confirm this by co-cross-linking IgG2a complexes with IgG2b in degranulation assays; however, degranulation was not inhibited (not shown).
In order to determine whether the source of antigen influenced activation of mast cells, we tested two different parasite antigen preparations in parallel with the well-characterized monoclonal anti-DNP IgE and IgG2a in complexes with DNP-BSA. Antigen and antibody were cross-titrated in preliminary experiments, and results are shown for concentrations that were found to be optimal. IgE complexed with any of the three antigens stimulated significant release of β–hexosaminidase in both RBL-2H3 (Fig. 3A) and BMMC (Fig. 3C), although the magnitude of the response was lower in BMMC. IgG2a complexed with ES Ag or with DNP-BSA induced significant release of β–hexosaminidase in RBL-2H3 cells; release by BMMC was reproducibly elevated, yet not statistically significant. In contrast, release of RMCPII in response to either IgG2a and IgE was significant for both cell types with either antigen (Fig. 3B,D).
When treated with 50 nM PMA and 1 µM calcium ionophore A23187 or ionomycin, BMMC responded as robustly as RBL-2H3 cells (Fig. 3). Thus, while BMMC are capable of strong degranulation responses to stimulation with PMA and ionophores, they appeared to be somewhat refractory to IgG complexes and responded more weakly to IgE than RBL-2H3 cells.
Impact of parasite glycoproteins on antibody-induced mediator release
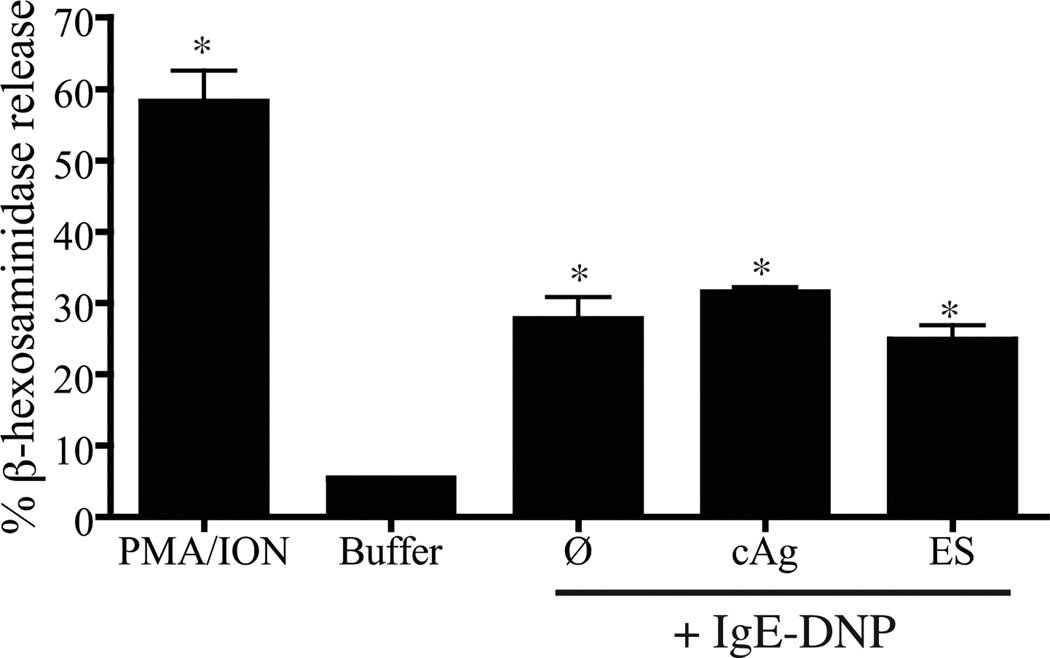
To determine whether the response of BMMC to immune complexes was negatively influenced by the presence of parasite antigens, we incubated cells with ES Ag or cAg (10 µg/ml) before stimulation with IgE-DNP complexes. Neither preparation altered release of β-hexosaminidase (Fig. 5), supporting the conclusion that under these conditions, soluble components of T. spiralis L1 ES Ag or homogenates do not inhibit degranulation.
Figure 5.
Influence of T. spiralis antigens on the induction of mediator release by IgE. BMMC were incubated with ES Ag or cAg (10 µg/ml) before addition of monoclonal anti-DNP IgE (10 µg/ml) and DNP-BSA (1 µg/ml). Values are reported as the mean +/− 1 SD (n = 3). *p<0.05 compared with cells treated with buffer.
Discussion
It has been documented previously that BMMC replicate many features of mast cells recovered from rat intestines (24, 25, 44, 45). Cross-linking of receptor-bound IgE stimulates the release of RMCPII, β-hexosaminidase, and leukotriene C4 (44), as well as TNF-α (25). Activation of BMMC by IgG has not been investigated and direct comparison of BMMC with other mast cell models has not been reported. Our results show that by several criteria, BMMC differ from RBL-2H3 cells. Specifically, BMMC produce greater quantities of RMCPII and β-hexosaminidase, and are more variable in their expression of surface receptors. In response to stimulation with PMA and ionophores, BMMC and RBL-2H3 cells released similar proportions of their stores of RMCPII and β-hexosaminidase, confirming that BMMC granules are competent for exocytosis.
Although it has been established that mucosal mast cells and their specific proteases play key roles in expulsion of adult T. spiralis from the intestine during primary infection, it is less clear whether the mast cell is functioning as an effector cell that clears parasites or as an innate regulator of intestinal immunity. Our investigation of an innate role was prompted by the knowledge that during invasion of the host epithelium, T. spiralis larvae disgorge tyvelose-bearing glycoproteins derived from their own intestinal stichocytes (46, 47). In addition, tyvelose-bearing surface glycoproteins are shed at the time of the first molt, approximately 8 hours post-oral infection (48, 49). The presence of larval glycoproteins in the intestinal epithelium places them in proximity to mucosal mast cells during the first few hours of intestinal infection. Thus, there is potential for larvae to influence mast cell activity in an innate immune context.
We have reported previously that a primary infection of rats with T. spiralis does not induce release of RMCPII in amounts sufficient to detect in blood at 0.5, 1, 1.5, 3 or 24 hours following oral infection (21). These findings are consistent with those of the in vitro studies described in this report, specifically, ES Ag or homogenates of L1 failed to stimulate degranulation of mast cells in vitro. Other investigators have reported that tyvelose-bearing glycoproteins that are affinity purified from cAg trigger significant cytokine and histamine release from a rat-derived hybrid cell line that models connective tissue mast cells (11, 12). In our experiments, some tyvelose-bearing glycans in cAg bound directly to mast cells; however, binding did not induce degranulation, nor did the presence of cAg alter the responsiveness of mast cells to IgE complexed with a different antigen. The excreted and secreted glycoproteins of L1 are highly relevant to parasite establishment, yet the major species of ES Ag, the tyvelose-bearing glycoproteins, did not bind to mast cells. The difference in binding activity between cAg and ES Ag may be explained by the more heterogeneous array of tyvelose-bearing glycoproteins in larval homogenates (38), or the possibility that concentration and dialysis of ES Ag may have depleted tyvelose-bearing molecules that were retained in the homogenates. It is important to note that although our detection method for direct binding of L1 products to mast cells was limited to tyvelose-bearing glycans, degranulation assays showed that none of the contents of ES products or whole larval homogenates induced mast cells to degranulate. The contradiction between our finding and those of previous reports may be due to differences in antigen preparation, to intrinsic properties of cells being assayed in each study, or to the different assays used to assess cellular responsiveness.
In the second aim of the study, we assessed the response of BMMC and RBL-2H3 cells to binding by immune complexes, specifically those formed by IgG. The monoclonal IgG antibodies tested represent all four IgG subclasses, have been shown previously to share specificity for novel glycans (30, 31), and have been characterized extensively in passive immunization studies (21, 29, 32). The antibodies are specific for tyvelose, a di-deoxyhexose that modifies tri- and tetra-antennary N-linked glycans on many glycoproteins in ES products of T. spiralis L1 (50). Monoclonal antibodies specific for tyvelose form large immune complexes (51), which would be expected to be highly efficient in cross-linking FcR. In some experiments, we used the more heterogeneous T. spiralis crude antigen (cAg), which is also rich in tyvelose-bearing glycoproteins.
By using flow cytometry to assay cell surface binding by IgG, our findings extend those of previous studies that reported degranulation as the readout for binding (27, 43, 52). We found that complexes formed by three of the four IgG isotypes bound to mast cells. Complexes formed with IgG2c did not bind. Uncomplexed IgGs did not bind, indicating that the high affinity FcγRI was not present. High affinity FcεRI and the low affinity Fcγ receptors bind IgGs only when they are complexed with antigen. RBL-2H3 cells display FcεR (28) and also the low affinity, inhibitory receptor FcγRII (53). Although results of competition experiments supported the presence of FcγRII or FcγRIII on RBL-2H3 and BMMC, our attempts to demonstrate activity for an inhibitory FcγRII were unsuccessful.
Immune complexes formed with IgG2a induced degranulation by both cell types, consistent with our previous findings in rats that were passively immunized with the same monoclonal IgG2a and then challenged with T. spiralis L1 (21). Although binding and activation of RBL-2H3 cells by IgG2a was previously documented in several studies, contradictory results have been reported for IgG1 (27, 52). We found that the binding properties of IgG1 and IgG2b were similar to those of IgG2a, yet only IgG2a induced mediator release. In vivo, IgG1 induces a weak release of RMCPII in passively immunized rats that is reproducible but not statistically significant (21). Further investigation of a possible discrepancy between the in vivo and in vitro activities of rat IgG1 is of interest because it is closely related to IgG2a; genetic evidence suggests that the two subclasses evolved following a gene duplication event (54). Such information could shed light on the specific genetic changes that affect the effector functions of these two closely-related subclasses. Furthermore, mast cells play a role in the pathogenesis of many allergies and autoimmune diseases. Discerning the basis for variation in mast cell activation by IgG2a, IgG1, and IgG2b may provide valuable insight into the therapeutic potential of blocking antibodies in inflammatory processes.
In summary, immune complexes formed with IgE and IgG2a induced both cell types to degranulate in a manner that replicated mucosal mast cell function during challenge infection of immune rats. Larval products failed to induce degranulation independently of antibody and did not inhibit mast cell activation by immune complexes, supporting the conclusion that T. spiralis larvae do not influence mucosal mast cells in an innate context. Our results reveal important functional similarities and differences between two models of the rat mucosal mast cell and provide further evidence that BMMC are valuable tools for the study of mast cell function during parasitic worm infection.
Supplementary Material
Acknowledgements
We thank the animal care staff at the Baker Institute for Animal Health for their expertise, Dr. Hugh Miller (University of Edinburgh) for mouse anti-rat RMCPII and advice, and Dr. Norah Smith (Cornell University) for assistance and advice. This work was supported by grants AI 14490 (to JAA) and T32 RR0759 (support for SMT) from the National Institutes of Health.
Footnotes
Disclosures: None.
References
- 1.Alizadeh H, Wakelin D. Genetic factors controlling the intestinal mast cell response in mice infected with Trichinella spiralis. Clin Exp Immunol. 1982;49:331–337. [PMC free article] [PubMed] [Google Scholar]
- 2.Ruitenberg EJ, Elgersma A, Kruizinga W. Intestinal mast cells and globule leucocytes: role of the thymus on their presence and proliferation during a Trichinella spiralis infection in the rat. Int Arch Allergy Appl Immunol. 1979;60:302–309. doi: 10.1159/000232355. [DOI] [PubMed] [Google Scholar]
- 3.Miller HR, Jarrett WF. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971;20:277–288. [PMC free article] [PubMed] [Google Scholar]
- 4.Woodbury RG, Miller HR, Huntley JF, Newlands GF, Palliser AC, Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. Nature. 1984;312:450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- 5.Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun. 1983;41:445–447. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 8.Helmby H, Grencis RK. IL-18 regulates intestinal mastocytosis and Th2 cytokine production independently of IFN-gamma during Trichinella spiralis infection. J Immunol. 2002;169:2553–2560. doi: 10.4049/jimmunol.169.5.2553. [DOI] [PubMed] [Google Scholar]
- 9.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ierna MX, Scales HE, Saunders KL, Lawrence CE. Mast cell production of IL-4 and TNF may be required for protective and pathological responses in gastrointestinal helminth infection. Mucosal Immunol. 2008;1:147–155. doi: 10.1038/mi.2007.16. [DOI] [PubMed] [Google Scholar]
- 11.Arizmendi-Puga NG, Enciso JA, Ortega-Pierres G, et al. Trichinella spiralis: histamine secretion induced by TSL-1 antigens from unsensitized mast cells. Exp Parasitol. 2006;114:67–76. doi: 10.1016/j.exppara.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Niborski V, Vallee I, Fonseca-Linan R, et al. Trichinella spiralis: stimulation of mast cells by TSL-1 antigens trigger cytokine mRNA expression and release of IL-4 and TNF through an Ig-independent pathway. Exp Parasitol. 2004;108:101–108. doi: 10.1016/j.exppara.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Bell RG, McGregor DD, Despommier DD. Trichinella spiralis: mediation of the intestinal component of protective immunity in the rat by multiple, phase-specific, antiparasitic responses. Exp Parasitol. 1979;47:140–157. doi: 10.1016/0014-4894(79)90068-7. [DOI] [PubMed] [Google Scholar]
- 14.Castro GA, Badial-Aceves F, Adams PR, Copeland EM, Dudrick SJ. Response of immunized, parenterally nourished rats to challenge infection with the nematode, Trichinella spiralis. J Nutr. 1976;106:1484–1491. doi: 10.1093/jn/106.10.1484. [DOI] [PubMed] [Google Scholar]
- 15.Love RJ, Ogilvie BM, McLaren DJ. The immune mechanism which expels the intestinal stage of Trichinella spiralis from rats. Immunology. 1976;30:7–15. [PMC free article] [PubMed] [Google Scholar]
- 16.McCoy RO. Rapid loss of Trichinella larvae fed to immune rats and its bearing on the mechanism of immunity. American Journal of Hygiene. 1940;32:105. [Google Scholar]
- 17.Alizadeh H, Wakelin D. Comparison of rapid expulsion of Trichinella spiralis in mice and rats. Int J Parasitol. 1982;12:65–73. doi: 10.1016/0020-7519(82)90097-2. [DOI] [PubMed] [Google Scholar]
- 18.Hessel J, Ramaswamy K, Castro GA. Reduced hexose transport by enterocytes associated with rapid, noninjurious rejection of Trichinella spiralis from immune rats. J Parasitol. 1982;68:202–207. [PubMed] [Google Scholar]
- 19.Harari Y, Castro GA. Simulation of parasite-induced gut hypersensitivity: implications for vaccination. Immunology. 1989;66:302–307. [PMC free article] [PubMed] [Google Scholar]
- 20.Moqbel R, Wakelin D, MacDonald AJ, King SJ, Grencis RK, Kay AB. Release of leukotrienes during rapid expulsion of Trichinella spiralis from immune rats. Immunology. 1987;60:425–430. [PMC free article] [PubMed] [Google Scholar]
- 21.Blum LK, Thrasher SM, Gagliardo LF, Fabre V, Appleton JA. Expulsion of secondary Trichinella spiralis infection in rats occurs independently of mucosal mast cell release of mast cell protease II. J Immunol. 2009;183:5816–5822. doi: 10.4049/jimmunol.0900944. [DOI] [PubMed] [Google Scholar]
- 22.Appleton JA, McGregor DD. Rapid expulsion of Trichinella spiralis in suckling rats. Science. 1984;226:70–72. doi: 10.1126/science.6474191. [DOI] [PubMed] [Google Scholar]
- 23.Bell RG, Appleton JA, Negrao-Correa DA, Adams LS. Rapid expulsion of Trichinella spiralis in adult rats mediated by monoclonal antibodies of distinct IgG isotypes. Immunology. 1992;75:520–527. [PMC free article] [PubMed] [Google Scholar]
- 24.Haig DM, Huntley JF, MacKellar A, et al. Effects of stem cell factor (kit-ligand) and interleukin-3 on the growth and serine proteinase expression of rat bone-marrow-derived or serosal mast cells. Blood. 1994;83:72–83. [PubMed] [Google Scholar]
- 25.MacDonald AJ, Pick J, Bissonnette EY, Befus AD. Rat mucosal mast cells: the cultured bone marrow-derived mast cell is biochemically and functionally analogous to its counterpart in vivo. Immunology. 1998;93:533–539. doi: 10.1046/j.1365-2567.1998.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enerback L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66:303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- 27.Benhamou M, Berenstein EH, Jouvin MH, Siraganian RP. The receptor with high affinity for IgE on rat mast cells is a functional receptor for rat IgG2a. Mol Immunol. 1994;31:1089–1097. doi: 10.1016/0161-5890(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 28.Holowka D, Sil D, Torigoe C, Baird B. Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunological reviews. 2007;217:269–279. doi: 10.1111/j.1600-065X.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 29.Appleton JA, Schain LR, McGregor DD. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology. 1988;65:487–492. [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis LA, McVay CS, Probert MA, Zhang J, Bundle DR, Appleton JA. Terminal beta-linked tyvelose creates unique epitopes in Trichinella spiralis glycan antigens. Glycobiology. 1997;7:383–390. doi: 10.1093/glycob/7.3.383. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Appleton J, Ling C-C, Bundle DR. Synthesis of disaccharide congeners of the Trichinella spiralis glycan and binding site mapping of two monoclonal antibodies. Canadian Journal of Chemistry. 2002;80:1141–1161. [Google Scholar]
- 32.Carlisle MS, McGregor DD, Appleton JA. The role of the antibody Fc region in rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1991;74:552–558. [PMC free article] [PubMed] [Google Scholar]
- 33.Crum ED, Despommier DD, McGregor DD. Immunity to Trichinella spiralis. I. Transfer of resistance by two classes of lymphocytes. Immunology. 1977;33:787–795. [PMC free article] [PubMed] [Google Scholar]
- 34.Basciano LK, Berenstein EH, Kmak L, Siraganian RP. Monoclonal antibodies that inhibit IgE binding. The Journal of biological chemistry. 1986;261:11823–11831. [PubMed] [Google Scholar]
- 35.Jamur MC, Grodzki AC, Moreno AN, Swaim WD, Siraganian RP, Oliver C. Immunomagnetic isolation of rat bone marrow-derived and peritoneal mast cells. J Histochem Cytochem. 1997;45:1715–1722. doi: 10.1177/002215549704501215. [DOI] [PubMed] [Google Scholar]
- 36.Carlisle MS, McGregor DD, Appleton JA. The role of mucus in antibody-mediated rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1990;70:126–132. [PMC free article] [PubMed] [Google Scholar]
- 37.Posner RG, Lee B, Conrad DH, Holowka D, Baird B, Goldstein B. Aggregation of IgE-receptor complexes on rat basophilic leukemia cells does not change the intrinsic affinity but can alter the kinetics of the ligand-IgE interaction. Biochemistry. 1992;31:5350–5356. doi: 10.1021/bi00138a015. [DOI] [PubMed] [Google Scholar]
- 38.Appleton JA, Usack L. Identification of potential antigenic targets for rapid expulsion of Trichinella spiralis. Mol Biochem Parasitol. 1993;58:53–62. doi: 10.1016/0166-6851(93)90090-k. [DOI] [PubMed] [Google Scholar]
- 39.Barsumian EL, Isersky C, Petrino MG, Siraganian RP. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981;11:317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz LB, Austen KF, Wasserman SI. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J Immunol. 1979;123:1445–1450. [PubMed] [Google Scholar]
- 41.Shanahan F, Lee TD, Bienenstock J, Befus AD. Mast cell heterogeneity: effect of anti-allergic compounds on neuropeptide-induced histamine release. International archives of allergy and applied immunology. 1986;80:424–426. doi: 10.1159/000234092. [DOI] [PubMed] [Google Scholar]
- 42.Guo NH, Her GR, Reinhold VN, Brennan MJ, Siraganian RP, Ginsburg V. Monoclonal antibody AA4, which inhibits binding of IgE to high affinity receptors on rat basophilic leukemia cells, binds to novel alpha-galactosyl derivatives of ganglioside GD1b. J Biol Chem. 1989;264:13267–13272. [PubMed] [Google Scholar]
- 43.Oliver C, Sahara N, Kitani S, Robbins AR, Mertz LM, Siraganian RP. Binding of monoclonal antibody AA4 to gangliosides on rat basophilic leukemia cells produces changes similar to those seen with Fc epsilon receptor activation. J Cell Biol. 1992;116:635–646. doi: 10.1083/jcb.116.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacDonald AJ, Haig DM, Bazin H, McGuigan AC, Moqbel R, Miller HR. IgE-mediated release of rat mast cell protease II, beta-hexosaminidase and leukotriene C4 from cultured bone marrow-derived rat mast cells. Immunology. 1989;67:414–418. [PMC free article] [PubMed] [Google Scholar]
- 45.Lee HN, Kim CH, Song GG, Cho SW. Effects of IL-3 and SCF on Histamine Production Kinetics and Cell Phenotype in Rat Bone Marrow-derived Mast Cells. Immune Netw. 2010;10:15–25. doi: 10.4110/in.2010.10.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ManWarren T, Gagliardo L, Geyer J, McVay C, Pearce-Kelling S, Appleton J. Invasion of intestinal epithelia in vitro by the parasitic nematode Trichinella spiralis. Infect Immun. 1997;65:4806–4812. doi: 10.1128/iai.65.11.4806-4812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Despommier DD. In: Trichinella and Trichinosis. WC C, editor. New York: Plenum Press; 1983. pp. 75–151. [Google Scholar]
- 48.Philipp M, Parkhouse RM, Ogilvie BM. Changing proteins on the surface of a parasitic nematode. Nature. 1980;287:538–540. doi: 10.1038/287538a0. [DOI] [PubMed] [Google Scholar]
- 49.Otubu OE, Carlisle-Nowak MS, McGregor DD, Jacobson RH, Appleton JA. Trichinella spiralis: the effect of specific antibody on muscle larvae in the small intestines of weaned rats. Exp Parasitol. 1993;76:394–400. doi: 10.1006/expr.1993.1048. [DOI] [PubMed] [Google Scholar]
- 50.Reason AJ, Ellis LA, Appleton JA, et al. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis. Glycobiology. 1994;4:593–603. doi: 10.1093/glycob/4.5.593. [DOI] [PubMed] [Google Scholar]
- 51.McVay CS, Bracken P, Gagliardo LF, Appleton J. Antibodies to tyvelose exhibit multiple modes of interference with the epithelial niche of Trichinella spiralis. Infect Immun. 2000;68:1912–1918. doi: 10.1128/iai.68.4.1912-1918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Philips JR, Brouwer W, Edwards M, Mahler S, Ruhno J, Collins AM. The effectiveness of different rat IgG subclasses as IgE-blocking antibodies in the rat basophil leukaemia cell model. Immunol Cell Biol. 1999;77:121–126. doi: 10.1046/j.1440-1711.1999.00801.x. [DOI] [PubMed] [Google Scholar]
- 53.Bocek P, Jr., Draberova L, Draber P, Pecht I. Characterization of Fc gamma receptors on rat mucosal mast cells using a mutant Fc epsilon RI-deficient rat basophilic leukemia line. Eur J Immunol. 1995;25:2948–2955. doi: 10.1002/eji.1830251035. [DOI] [PubMed] [Google Scholar]
- 54.Bruggemann M, Free J, Diamond A, Howard J, Cobbold S, Waldmann H. Immunoglobulin heavy chain locus of the rat: striking homology to mouse antibody genes. Proc Natl Acad Sci U S A. 1986;83:6075–6079. doi: 10.1073/pnas.83.16.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.