Abstract
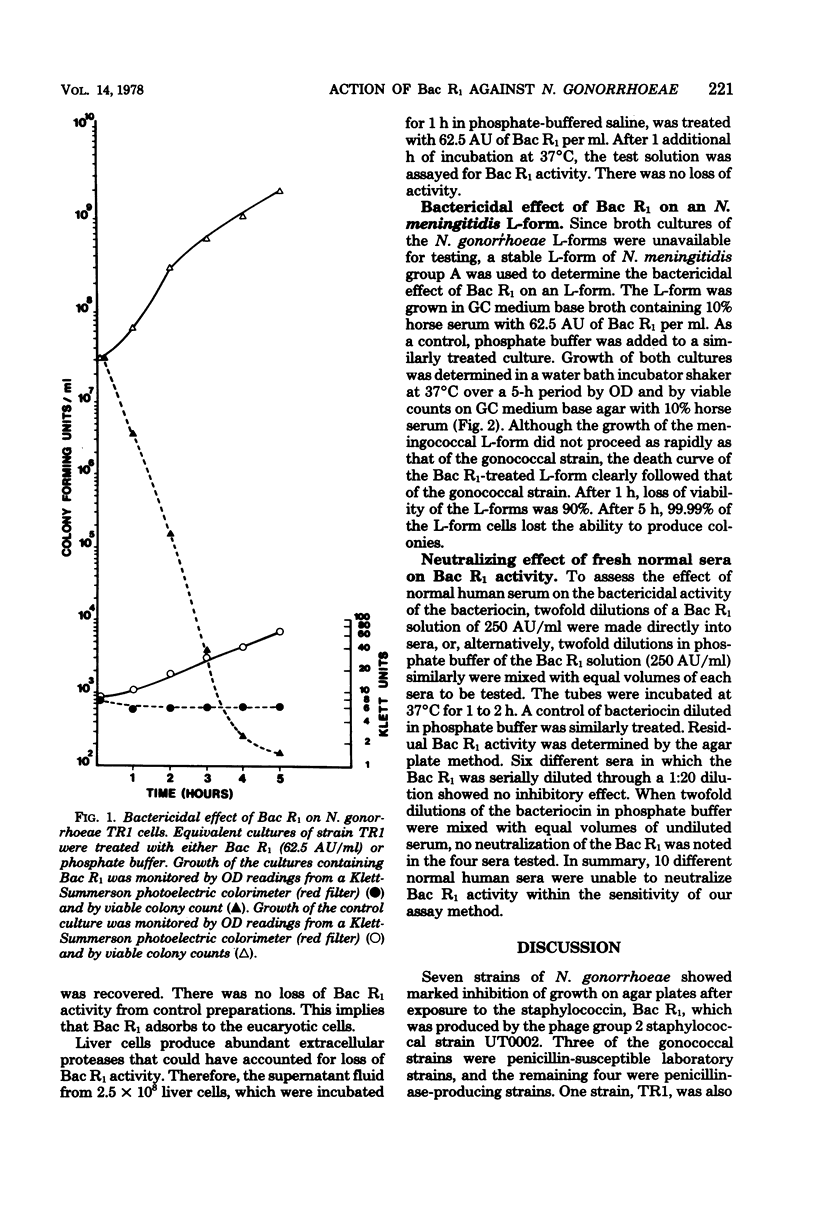
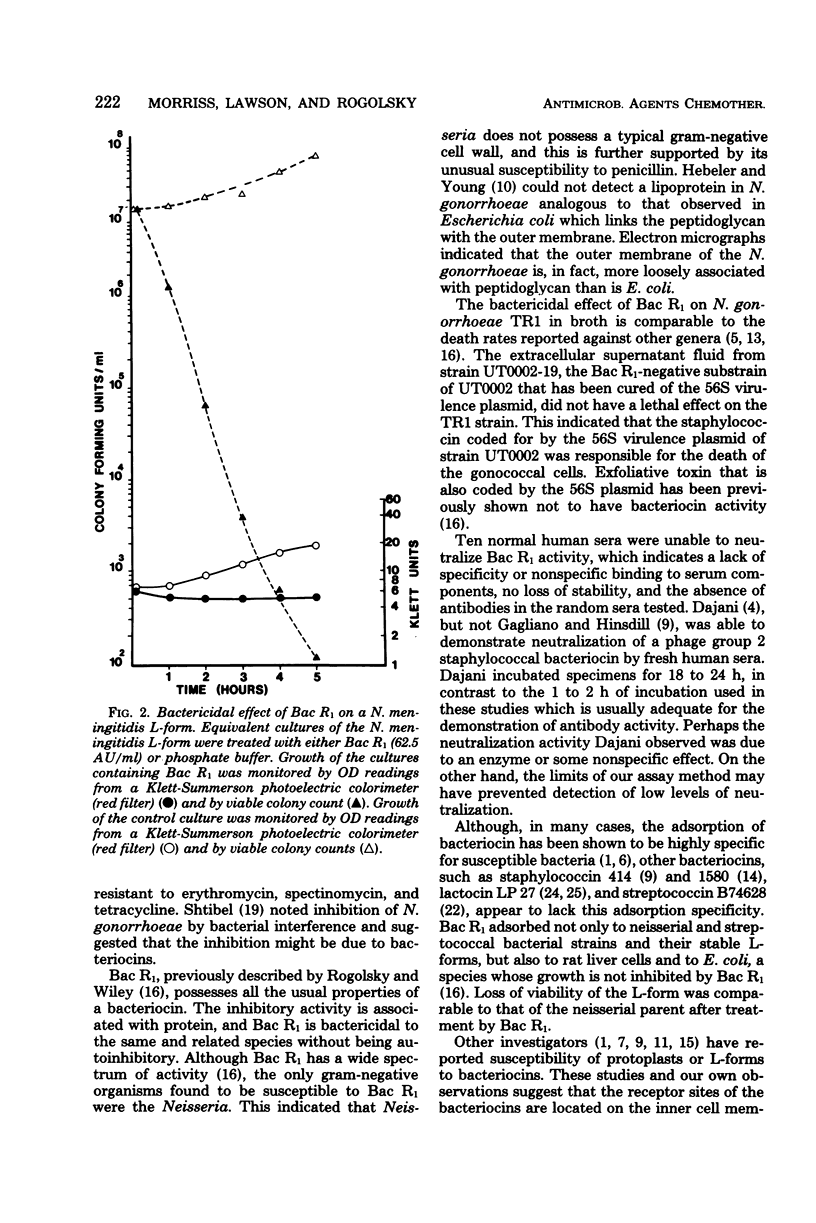
Phage group 2 staphylococcal strain UT0002 contains a large 56S virulence plasmid with genes that code for both exfoliative toxin and a specific staphylococcin termed Bac R1. Four penicillinase-producing strains and three penicillin-susceptible strains of Neisseria gonorrhoeae were killed by Bac R1. After 30 min of growth of the penicillin-resistant TR1 strain in 62.5 arbitrary units of Bac R1 per ml, loss of viability was approximately 90%, and, after 5 h, an approximately 99.99% loss of viability was observed. Lysis did not accompany cell death, and 84% of the Bac R1 added to the growth medium was adsorbed to the gonococcal cells. The extracellular supernatant fluid from a substrain of staphylococcal strain UT0002 cured of the plasmid for Bac R1 production had no lethal effect on the gonococcal strains. Bac R1 was also shown to have bactericidal activity against an L-form of N. meningitidis, indicating that the outer envelope of a neisserial cell is not needed for bacteriocin activity. Ten different normal human sera were unable to neutralize Bac R1 activity. The bacteriocin lacks adsorption specificity. It binds to but does not kill Escherichia coli cells, indicating that the cell envelope of gram-negative organisms can provide protection against the staphylococcin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasio K. L., Soucheck J. A., Sugiyama H. Boticinogeny and actions of the bacteriocin. J Bacteriol. 1971 Jul;107(1):143–149. doi: 10.1128/jb.107.1.143-149.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P., Wendt L., Whitney E., Silver S. Colicin-tolerant mutants of Escherichia coli: resistance of membranes to colicin E1. Science. 1970 May 22;168(3934):998–1000. doi: 10.1126/science.168.3934.998. [DOI] [PubMed] [Google Scholar]
- Dajani A. S., Gray E. D., Wannamaker L. W. Effect of Bactericidal Substance from Staphylococcus aureus on Group A Streptococci I. Biochemical Alterations. Infect Immun. 1970 May;1(5):485–490. doi: 10.1128/iai.1.5.485-490.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani A. S. Neutralization of phage type 71 staphylococcal bacteriocin by immune and nonimmune sera. J Infect Dis. 1973 Oct;128(4):494–499. doi: 10.1093/infdis/128.4.494. [DOI] [PubMed] [Google Scholar]
- Dajani A. S., Wannamaker L. W. Kinetic studies on the interaction of bacteriophage type 71 staphylococcal bacteriocin with susceptible bacteria. J Bacteriol. 1973 May;114(2):738–742. doi: 10.1128/jb.114.2.738-742.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison J. S., Kautter J. A. Purification and some properties of two boticins. J Bacteriol. 1970 Oct;104(1):19–26. doi: 10.1128/jb.104.1.19-26.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano V. J., Hinsdill R. D. Characterization of a Staphylococcus aureus bacteriocin. J Bacteriol. 1970 Oct;104(1):117–125. doi: 10.1128/jb.104.1.117-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOVICS G., ALFOLDI L., NAGY E. Mode of action of megacin. J Gen Microbiol. 1959 Aug;21:51–60. doi: 10.1099/00221287-21-1-51. [DOI] [PubMed] [Google Scholar]
- Jaffe H. W., Biddle J. W., Thornsberry C., Johnson R. E., Kaufman R. E., Reynolds G. H., Wiesner P. J. National gonorrhea therapy monitoring study: in vitro antibiotic susceptibility and its correlation with treatment results. N Engl J Med. 1976 Jan 1;294(1):5–9. doi: 10.1056/NEJM197601012940102. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Characteristics of the killing effect of a Staphylococcus epidermidis bacteriocin. Antonie Van Leeuwenhoek. 1974;40(1):177–183. doi: 10.1007/BF00394565. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Mode of action of a Staphylococcus epidermidis bacteriocin. Antimicrob Agents Chemother. 1972 Dec;2(6):456–463. doi: 10.1128/aac.2.6.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Nature and properties of a Staphylococcus epidermidis bacteriocin. J Bacteriol. 1972 Oct;112(1):243–250. doi: 10.1128/jb.112.1.243-250.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogolsky M., Wiley B. B., Glasgow L. A. Phage group II staphylococcal strains with chromosomal and extrachromosomal genes for exfoliative toxin production. Infect Immun. 1976 Jan;13(1):44–52. doi: 10.1128/iai.13.1.44-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtibel R. Inhibition of growth of N. gonorrhoeae by bacterial interference. Can J Microbiol. 1976 Oct;22(10):1430–1436. doi: 10.1139/m76-212. [DOI] [PubMed] [Google Scholar]
- Smarda J., Havelková M. The effect of colicin G on the spheroplasts of Proteus mirabilis. Folia Microbiol (Praha) 1970;15(2):122–124. doi: 10.1007/BF02880094. [DOI] [PubMed] [Google Scholar]
- Sparling P. F. Antibiotic resistance in Neisseria gonorrhoeae. Med Clin North Am. 1972 Sep;56(5):1133–1144. doi: 10.1016/s0025-7125(16)32339-2. [DOI] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocin of a group B streptococcus: partial purification and characterization. Antimicrob Agents Chemother. 1975 Jun;7(6):764–772. doi: 10.1128/aac.7.6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti G. C., Hinsdill R. D. Isolation and characterization of a bacteriocin from a homofermentative Lactobacillus. Antimicrob Agents Chemother. 1973 Oct;4(4):487–494. doi: 10.1128/aac.4.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti G. C., Hinsdill R. D. Production and mode of action of lactocin 27: bacteriocin from a homofermentative Lactobacillus. Antimicrob Agents Chemother. 1975 Feb;7(2):139–145. doi: 10.1128/aac.7.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]



