Abstract
The protooncogene C-Myc (Myc) regulates cardiac hypertrophy. Myc promotes compensated cardiac function, suggesting that the operative mechanisms differ from those leading to heart failure. Myc regulation of substrate metabolism is a reasonable target, as Myc alters metabolism in other tissues. We hypothesize that Myc-induced shifts in substrate utilization signal and promote compensated hypertrophy. We used cardiac specific Myc-inducible C57/BL6 male mice between 4–6 months old that develop hypertrophy with tamoxifen (tam) injections. Isolated working hearts and 13Carbon (13C)-NMR were used to measure function and fractional contributions (Fc) to the citric acid cycle by using perfusate containing 13C-labeled free fatty acids, acetoacetate, lactate, unlabeled glucose and insulin. Studies were performed at pre-hypertrophy (3-days tam, 3dMyc), established hypertrophy (7-days tam, 7dMyc) or vehicle control (Cont). Non-transgenic siblings (NTG) received 7-days tam or vehicle to assess drug effect. Hypertrophy was assessed by echocardiograms and heart weights. Western blots were performed on key metabolic enzymes. Hypertrophy occurred in 7dMyc only. Cardiac function did not differ between groups. Tam alone did not affect substrate contributions in NTG. Substrate utilization was not significantly altered in 3dMyc versus Cont. The free fatty acid FC was significantly greater in 7dMyc versus Cont with decreased unlabeled Fc, which is predominately exogenous glucose. Free fatty acid flux to the citric acid cycle increased while lactate flux was diminished in 7dMyc compared to Cont. Total protein levels of a panel of key metabolic enzymes were unchanged; however total protein O-GlcNAcylation was increased in 7dMyc. Substrate utilization changes for the citric acid cycle did not precede hypertrophy; therefore they are not the primary signal for cardiac growth in this model. Free fatty acid utilization and oxidation increase at established hypertrophy. Understanding the mechanisms whereby this change maintained compensated function could provide useful information for developing metabolic therapies to treat heart failure. The molecular signaling for this metabolic change may occur through O-GlcNAcylation.
Keywords: Cardiac hypertrophy, compensated hypertrophy, substrate metabolism, fatty acid oxidation
Introduction
Oxidative and non-oxidative substrate metabolism is altered in hypertrophy and the transition to heart failure; but it is unclear whether this is causal or an epiphenomenon (reviewed in [1–3]). The oncogene c-Myc plays an important role in the development of cardiac hypertrophy [4–12]. Myc also transcriptionally activates genes involved in metabolism across multiple species and models [13]. Myc promotes compensated cardiac hypertrophy in some models although this effect may be dose dependent [6,11,12]. To define Myc’s role in cardiac hypertrophy, Xiao and colleagues developed a cardiac specific Myc-inducible mouse [11]. Myc induction for 7-days led to expression of the hypertrophic gene program in conjunction with increased cardiomyocyte fiber width and heart weight to body weight ratios. Cardiac function remained stable for at least 3 weeks after Myc-induction, demonstrating compensated hypertrophy. With Ahuja et al, we previously showed that Myc induction for 3 days in this mouse model increased the carbohydrate fractional contribution (Fc) to acetyl-CoA while concurrently decreasing the free fatty acid Fc [14]. That study was limited in scope and evaluated only the initial metabolic responses to Myc-induction. However, the results suggested that there may be interaction between compensated hypertrophy initiated by Myc and shifts in substrate metabolism. Therefore, our primary study objective was to test the hypothesis that Myc-induced shifts in substrate utilization signal and promote compensated hypertrophy.
As hypertrophy is near completed by 7 days of Myc-induction [11], we performed extensive metabolic evaluations at 3 and 7 days in this model. The 3 day time point represents the pre-hypertrophy period. Data from this time point indicates whether shifts in substrate utilization precede and are potentially necessary for development of hypertrophy. Experiments performed at 7 days occur after established hypertrophy and investigate whether metabolic changes are important for maintaining compensated function. In this model, we utilized 13Carbon (13C) substrate labeling and isotopomer analysis to determine the Fc of acetyl-CoA to the citric acid cycle from multiple substrates supplied to the heart simultaneously in physiologic concentrations.
We also sought to determine whether Myc augments myocardial beta-O-linked N-acetylglucosamine (O-GlcNAc) levels; a post-translational modification that plays a critical regulatory role in multiple biologic processes [15]. Morrish et al demonstrated that Myc promoted O-GlcNAcylation in association with metabolic shifts in cultured fibroblasts [16]. This suggests a novel mechanism for post-translational regulation by Myc, possibly involving regulation of substrate metabolism. This operative Myc mechanism has not been previously investigated in myocardium. However, O-GlcNAcylation has been shown to promote myocardial fatty acid oxidation in isolated perfused rat hearts [17]. Furthermore, elevated O-GlcNAc levels occur during cardiac hypertrophy and heart failure in both humans and rats [18]. This post-translational modification may regulate myocardial remodeling as well. For instance, activation of the hypertrophy transcription factor NFAT (nuclear factor of activated T-cells) is dependent upon O-GlcNAc signaling [19]. Accordingly, Myc may regulate metabolism and hypertrophy through O-GlcNAc post-translational modification.
Materials and Methods
Animals
For these studies, we utilized cardiac-restricted, Myc-inducible mice generated from a C57/BL6 strain and non-transgenic littermates. The mice were a kind gift from W. Robb MacLellan, M.D. and have been previously described [11,14]. All experiments utilized mice between the ages of 4–6 months. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health (NIH Pub. No. 85–23, revised 1996) and were reviewed and approved by the Office of Animal Care at Seattle Children’s Research Institute.
Myc Induction
In the transgenic mice, the Myc protein is fused to a mutated estrogen receptor (making it unresponsive to endogenous estrogen) and is made cardiac specific by linking expression to the α-myosin heavy chain promoter. Upon exposure to 4-hydroxytamoxifen (tam, Sigma H-7904), the Myc chimeric protein translocates to the nucleus where it mediates transcriptional events. Tam at a dose of 1 mg in peanut oil injected via an intraperitoneal route is sufficient for Myc nuclear translocation.
Echocardiogram
Serial echocardiograms were performed on a cohort separate from those undergoing working heart experiments. Mice were initially sedated with 3% isoflurane in 21% O2 at a flow of 1 LPM and placed in a supine position at which time the isoflurane is reduced to 1% administered via a small nose cone. ECG leads were placed for simultaneous ECG monitoring during image acquisition. Echocardiographic images were performed with a Vevo 2100 machine using a MS400 transducer (VisualSonics, Inc, Toronto, Canada). M-Mode measurements at the midpapillary level of the left ventricle (LV) were performed at end-diastole (LVEDD) and end-systole (LVESD) to determine LV function via the fractional shortening [(LVEDD-LVESD)/LVEDD * 100] in a parasternal short axis mode. LV posterior wall (LVPW) thickness was measured in a parasternal short axis view. At the end of the echocardiographic studies, myocardial tissue was collected, weighed and stored for western blot studies.
Isolated working heart preparation
Mice were heparinized (5000 U/kg ip) and anesthetized with a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg). After adequate sedation, the aorta was rapidly cannulated with an 18-guage steel cannula and the hearts were administered Custodiol HTK cardioplegia solution (Dr. Franz Kohler Chemie GMBH, Alsback-Hahnlein, Germany) until the hearts were rapidly excised and placed on the perfusion system. The hearts were initially perfused in a Langendorff manner (70 mmHg perfusion pressure) with physiological salt solution (PSS), pH 7.4, containing (in mmol/l) 118.0 NaCl,25.0 NaHCO3, 4.7 KCl, 1.23 MgSO4, 1.2 NaH2PO4, 5.5 D-glucose, and 1.2 CaCl2. All perfusates were equilibrated with 95% O2-5% CO2, passed through filters with 3.0-μm pore size and warmed to maintain heart temperature around 37.5 degrees C. During retrograde perfusion, the left atrium was connected to the preload reservoir by cannulating the pulmonary vein with a 16-gauge steel cannula. An SPR-PV-Catheter (SPR-869 or -839 Millar Pressure-Volume Systems, Millar Instruments, Inc, Houston, TX) was inserted into the left ventricle through the apex for continuous measurement of left ventricular pressure (LVP). Spontaneously beating hearts were then switched to the antegrade work-performing mode with perfusion into the left atrium of semi-recirculating PSS containing the following 13C-labeled substrates in addition to unlabeled glucose (5.5 mmol/l): 1,3-[13C]acetoacetic acid (ACAC, 0.17 mmol/l), L-lactic-3-[13C]acid (Lactate, 1.2 mmol/l), and U-[13C]-long-chain mixed free fatty acids (free fatty acids, 0.35 mmol/l) bound to 0.75% (wt/vol) delipidated bovine serum albumin reconstituted with deionized water. Additional studies were performed with the same labeled substrates with insulin (2 nM) in the perfusate. To establish the origin of the unlabeled fraction, additional hearts were perfused with unlabeled lactate (1.2 nmol/l), 1-[13C]glucose (5.5 mmol/l), 1,3-[13C]ACAC (0.17 mmol/l), U[13C]free fatty acids (0.35 mmol/l) and insulin (2 nM). Details regarding this preparation and labeling strategy have been published previously [20]. In our system, only aortic outflow that bypasses the coronary circulation is recirculated. In the working mode, preload was 12 mmHg and afterload was 50 mmHg. Left atrial inflow was measured with a flow probe (T403; Transonic Systems, Ithaca, NY) and aortic (not including coronary flow) flow was measured via 30 second timed collections. Coronary flow was calculated as the difference between left atrial inflow and aortic flow although this measurement is affected by perfusate leak from the left atrium. Every 10 minutes, left atrial influent and coronary effluent was collected for determination of PO2, PCO2, and pH with an ABL800 blood gas analyzer (Radiometer, Copenhagen, Denmark). Continuously recorded parameters are left ventricular (LV) pressure (mmHg), HR (beats/min), and rate of LV contraction and relaxation (±dP/dt, mmHg/s). Developed pressure (DP) was calculated as the maximum LV pressure subtracted by LV end diastolic pressure. Cardiac work was calculated as the cardiac output times the developed pressure. Myocardial oxygen consumption (MVO2) was calculated as MVO2 = CF × [(PaO2 − PvO2) × (c/760)] × heart weight, where CF is coronary flow (ml·min−1g wet weight−1), (PaO2 - PvO2) is the difference in the partial pressure of oxygen (PO2, mmHg) between perfusate and coronary effluent, and c is the Bunsen solubility coefficient of O2 in perfusate at 37°C (22.7 μl O2·atm−1·ml−1). Cardiac efficiency was defined as cardiac work/MVO2.
Experimental Protocol
To assess whether Tam alone affected myocardial substrate utilization for the citric acid cycle, non-transgenic (NTG) littermates were divided into 2 groups: NTG with 7-days vehicle injections (NTG-Con) or NTG with 7-days of tam injections (NTG-Tam) and subjected to working heart experiments.
Myc-induction for 7-days in this transgenic mouse results in stable cardiac hypertrophy as assessed by hypertrophic gene program expression, increased cardiomyocyte fiber width and heart weight to body weight ratio (HW/BW) [11]. Induction for 3 weeks did not lead to further hypertrophy growth. As stated, the objective of our current study was to test the hypothesis that Myc-induced metabolic changes signal for hypertrophy development and promote compensated hypertrophy. We performed metabolic and functional studies before hypertrophy (Tam injections for 3-days, 3dMyc) and at established hypertrophy (Tam injections for 7-days; 7dMyc). Myc transgenic mice injected with vehicle (peanut oil) for 7-days served as the control group (Cont).
We previously demonstrated in a working mouse heart model that perfusate containing insulin increases the unlabeled Fc primarily at the expense of free fatty acid Fc with our substrate labeling protocol [21]. Our current experiments showed significant changes in the free fatty acid Fc between the groups and a modest trend towards increased unlabeled Fc (see results below). To further investigate these findings, we repeated our isolated working heart studies with insulin-containing perfusate. We reasoned that this would increase the probability of detecting important changes in the unlabeled Fc as well as robustly confirm the changes in free fatty acid Fc.
13C Magnetic Resonance Spectroscopy (MRS) and isotopomer analyses
Myocardial tissue was extracted as previously described [20]. Substrate metabolism was established by using 13C-labeled substrates in combination with NMR spectroscopy. Glutamate isotopomer analyses provide Fc of acetyl-CoA to the citric acid cycle from up to three differentially labeled substrates.
Lyophilized heart extracts were dissolved in 99.8% D2O for decoupled 13C NMR spectral acquisition. NMR free-induction decays (FIDs) were acquired on a Varian Direct Drive (VNMRS) 600 MHz spectrometer (Varian Inc., Palo Alto, CA) equipped with a Dell Precision 390 Linux workstation running VNMRJ 2.2C. The spectrometer system was outfitted with a Varian triple resonance salt-tolerant cold probe with a cold carbon preamplifier. A Varian standard one dimensional carbon direct observe sequence with proton decoupling was used to collect data on each sample. Final spectra were accumulations of 4800 individual FIDs. Each FID was induced using a nonselective, 45-degree excitation pulse (7.05 us @ 58 dB), with an acquisition time of 1.3 seconds, a recycle delay of 3 seconds, and a spectral width of 224.1 ppm.
FIDs were baseline corrected, zero-filled, and Fourier transformed. All of the labeled carbon resonances (C1–C5) of glutamate were integrated with the Lorentzian peak fitting subroutine in the acquisition program (NUTS, Acorn NMR, Livermore, CA). The individual integral values were used as starting parameters for the citric acid cycle analysis fitting algorithm tcaCALC, kindly provided by Drs. Mallow and Jeffrey [22]. This algorithm provided the Fc for each substrate in the acetyl-CoA pool entering citric acid cycle. The absolute flux for the citric acid cycle and oxidative flux for individual substrates were calculated as previously described [22,23].
Immunoblotting
Immunoblotting was done as previously described in our laboratory [21]. The primary antibody used in this study for malonyl CoA decarboxylase (MCD) was obtained from Proteintech Group, Inc, Chicago, IL. The primary antibody for FAT/CD36 was obtained from Novus Biologicals, Littleton, CO. The primary antibodies for liver (l)- and muscle (m) carnitine palmitoyltransferase I (CPT-I) and pyruvate dehydrogenase (PDK)-2 and PDK-4 and were obtained as personal gifts from Gebre Woldegiorgis (Oregon Health Sciences University, Beaverton, OR) and Robert Harris (Indiana University School of Medicine, Indianapolis, IN), respectively. The primary antibodies for peroxisome proliferator-activated receptor alpha (PPARα) and peroxisome proliferator-activated receptor gamma co-activator-1 alpha (PGC-1α) were obtained from Santa Cruz Biotechnology, Inc, Santa Cruz, CA. The primary antibody for Acetyl CoA carboxylase (ACC) was purchased from Cell Signaling Technology, Danvers, MA. The molecular weights are as follows (kDa): MCD, 50 kDa; FAT/CD36, 72 kDa; PDK-4, 46 kDa; PDK-2, 42 kDa; PGC-1α, 90 kDa; PPAR α, 55 kDa; mCPT-I, 85 kDa; lCPT-I 90 kDa and ACC, 265 kDa. Total protein O-linked β-N-acetylglucosamine (O-GlcNAc) levels were determined using an antibody (clone CTD110.6) from Covance, Princeton, NJ. Protein extract for total O-GlcNAc levels was obtained from a separate cohort of mice and prepared as previously described [17]. Of note, all immunoblots were normalized to total protein levels assessed from Ponceau S staining, which are shown in their respective figures.
Statistical Analysis
Reported values are means ± standard error (SE) in figures and text. Data were analyzed with a single factor ANOVA for multiple comparisons. If significance was identified by ANOVA (P-value < 0.05); then a two-way unpaired t-test was performed between groups. A paired t-test was solely used to compare serial echocardiographic changes within individual mice after significant changes were determined by ANOVA and two-way unpaired t-test between the groups. Criterion for significance was p < 0.05 for all comparisons.
RESULTS
Morphometric and echocardiographic data
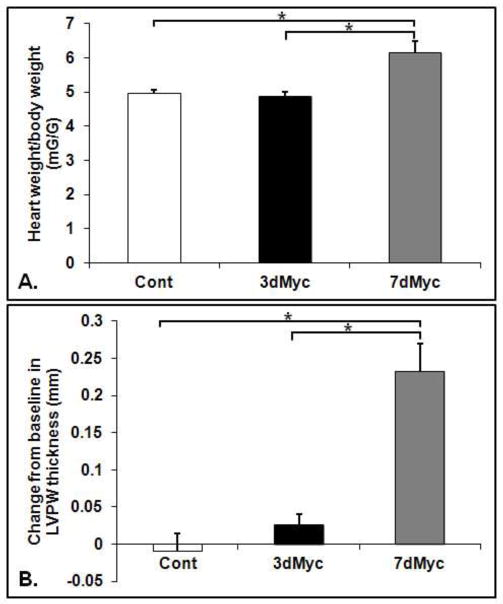
Heart to body weight ratios increased by around 26% in 7dMyc compared to 3dMyc and Cont (Figure 1A, p<0.05 for 7dMyc vs. 3dMyc and 7dMyc vs. Cont). There was no difference between 3dMyc and Cont. The change from baseline in left ventricular posterior wall thickness was significantly greater in 7dMyc versus 3dMyc and Cont (Figure 1B, p<0.05). The value increased from baseline in 7dMyc only (Figure 1B, p< 0.05 by paired t-test). Left ventricular shortening fraction (SF) was unchanged from baseline and similar among all groups. In 7dMyc, the SF was 30.1±1.8% (data not shown). Additional functional measurements were made during the working heart experiments.
Figure 1.
Heart weight to body weight ratios (A) and change from baseline in left ventricular posterior wall (LVPW) thickness in mm (B). LVPW was measured via echocardiogram as described in the methods section. Values are means ± SEM. * p<0.05 between indicated groups. Note: p<0.05 versus baseline measurement in 7dMyc only as assessed by paired t-test. n=4–6 per group.
Cardiac function during the working heart experiments
Ex Vivo functional assessments were made during the working heart perfusions. All reported values are after 20 minutes of stable left atrial infusion. First, we measured cardiac function in the Myc-induced mice without insulin in the perfusate (n=5–7 per group, Table 1). +dP/dTmax was significantly greater in 3dMyc compared to Cont (p<0.05) with a trend towards increased +dP/dTmax in 7dMyc versus Cont (p=0.089). Other functional parameters were similar between groups. With insulin, cardiac function, aortic and coronary flow was similar among the groups (n=6–10 per group, Table 2).
Table 1.
Functional measurements during isolated working heart with 13C-labeled substrates without insulin in the perfusate. Values are means ± SEM.
| Cont | 3dMyc | 7dMyc | |
|---|---|---|---|
| Heart rate (BPM) | 384±14 | 373±7 | 396±20 |
| +dP/dTmax (mmHg/sec) | 3779±199 | 4399±87* | 4019±209 |
| −dP/dTmin (mmHg/sec) | −3640±168 | −3770±82 | −3432±157 |
| Power (ml/min)* mmHg | 946±67 | 1088±38 | 1036±154 |
p<0.05 3dMyc versus Cont.
n=5–7 per group.
Table 2.
Functional measurement during isolated working heart with 13C-labeled substrates and insulin-containing perfusate. Efficiency is defined as cardiac power/oxygen consumption. MVO2, Myocardial oxygen consumption.
| Cont | 3dMyc | 7dMyc | |
|---|---|---|---|
| Heart rate (BPM) | 401±16 | 381±14 | 382±10 |
| +dP/dTmax (mmHg/sec) | 4702±166 | 4967±246 | 4842±313 |
| −dP/dTmin (mmHg/sec) | −3892±92 | −4043±144 | −4038±120 |
| Power (ml/min)*mmHg | 1268±91 | 1329±71 | 1267±115 |
| Aortic flow (ml/min) | 11.3±0.6 | 10.5±0.6 | 10.6±0.4 |
| Coronary flow (ml/min) | 3.7±0.3 | 4.9±0.7 | 3.8±0.6 |
| MVO2 (μmol/g wet weight/min) | 7.9±0.1 | 8.0±1.5 | 6.9±0.6 |
| Efficiency (ml*mmHg/μmol O2/g) | 145±8 | 153±10 | 183±21 |
Values are means ± SEM.
n=6–10 per group.
Oxygen consumption was determined after 20 minutes of stable left atrial infusion with the insulin containing perfusate in a subgroup of mice. As shown in Table 2, MVO2 per gram wet heart weight did not differ between groups (n=5 for Cont and 7dMyc, n=3 3dMyc). Cardiac efficiency (defined as work/MVO2) did not differ between groups.
Fc of acetyl-CoA to the citric acid cycle
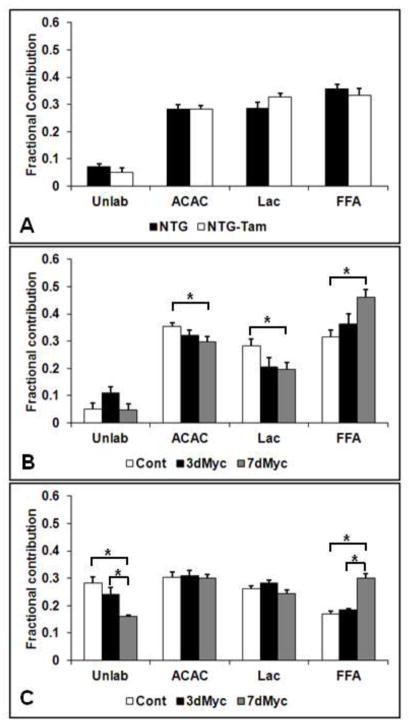
We next determined the Fc of acetyl-CoA to the citric acid cycle for each studied substrate (Figure 2A). First, we established whether Tam alone affects myocardial intermediate metabolism. Tam did not alter substrate utilization patterns in NTG mice. The Fc of free fatty acids, lactate and ACAC (used to represent ketones) were similar to each other at around 30%. Based upon previous experiments with this labeling strategy, the unlabeled fraction is composed of primarily of exogenous unlabeled glucose and endogenous glycogen, although endogenous triglycerides may contribute as well [20].
Figure 2.
Fractional contribution (Fc) to the citric acid cycle for unlabeled substrates (unlab), acetoacetate as ketone (ACAC), lactate (Lac) and free fatty acids (FFA). (A) Non-transgenic mice receiving vehicle (NTG) or tam injections (NTG-Tam) for 7 days. (B) 13C-labeled substrates without insulin. (C) 13C-labeled substrates with insulin. * p<0.05 between indicated groups. There were no significant differences between NTG and NTG-Tam. n=5–9 per group for A, 5–7 per group for B and 7–10 per group for C.
The Fc in the Myc-induced mice without insulin is shown in Figure 2B. Compared to Cont, 7dMyc increased free fatty acid Fc by nearly 50% (p<0.05) with a simultaneous decrease in lactate Fc and ACAC Fc (p<0.05 for both). 7dMyc also promoted a trend towards increased free fatty acids Fc versus 3dMyc (p=0.07). The unlabeled Fc trended towards being increased in 3dMyc versus both Cont (p=0.07) and 7dMyc (p=0.06).
The Fc of Myc-induced mice with insulin containing perfusate are displayed in Figure 2C. Of note, we used 1-[13C] glucose and unlabeled lactate in place of L-lactic-3-[13C]acid and unlabeled glucose for a portion of the insulin experiments as noted in Materials and Method. This demonstrated that only a negligible portion of the unlabeled substrate is not from exogenous glucose. Therefore, the unlabeled Fc is indicative of exogenous glucose Fc for these experiments. As expected, insulin increased unlabeled (glucose) at the expense of free fatty acids compared to non-insulin containing perfusate for all groups. There was no change in the unlabeled Fc in 3dMyc versus control. However, under insulin perfusion conditions we detected a decrease in unlabeled Fc in 7dMyc versus Cont with a trend towards being decreased versus 3dMyc (p=0.055). The 7dMyc still had a greater free fatty acid Fc (p<0.05) than both Cont and 3dMyc. There was also a trend towards decreased lactate Fc in 7dMyc versus 3dMyc (p=0.08). Differing from the non-insulin experiments, there were no differences in the ACAC and lactate fractional contributions between 7dMyc and Cont.
Substrate flux
Figure 3 shows the calculated flux rates from the insulin experiments. Since there were no differences in Fc in the 3dMyc group, we only evaluated flux in 7dMyc versus Cont. There was no change in overall citric acid cycle flux. As MVO2 was similar between 7dMyc and Cont, free fatty acid flux rates followed Fc results with a significant greater flux in 7dMyc. Lactate flux was decreased in 7dMyc compared to Cont.
Figure 3.
Flux rates for total citric acid cycle and individual substrates in Cont and 7dMyc. Abbreviations as indicated in Figure 2. * p<0.05 versus Cont. n=5 per group.
Protein expression
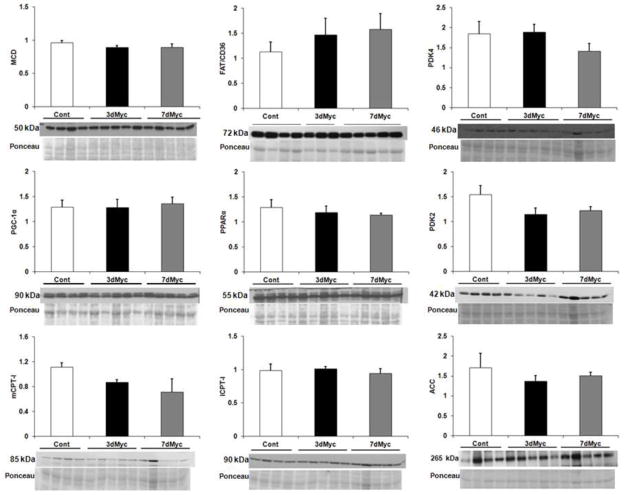
Ahuja et al previously analyzed some proteins regulating glycolysis and fatty acid oxidation [14]. The Myc-induced metabolic changes in established hypertrophy prompted further protein analysis for enzymes involving fatty acid oxidation. Results appear in Figure 4. Myc is primarily a transcriptional regulator, so we focused on total protein levels. There were no changes in protein levels of the fatty acid oxidation transcriptional regulators PPARα and PGC-1α. The fatty acid cellular membrane transport protein FAT/CD36 and mitochondria transporter CPT-I (both mCPT-I and lCPT-I) levels were also unchanged. ACC and MCD affect CPT-I activity through synthesis and breakdown of malonyl CoA, respectively. MCD and ACC proteins levels were similar among groups. PDK-2 and -4 reduce pyruvate entry into the citric acid cycle by inhibiting pyruvate dehydrogenase. PDK-4 and PDK-2 levels were not affected by Myc-induction.
Figure 4.
Expression of proteins involved in the regulation of fatty acid metabolism. All values are arbitrary units normalized to total protein staining shown as Ponceau in the figure. The approximate molecular weight of each protein is noted. n=3–5 per group. PPAR, peroxisome proliferator-activated receptor; PDK, pyruvate dehydrogenase kinase; lCPT-I, liver-specific carnitine palmitoyltransferase I; mCPT-I, muscle-specific CPT-I; MCD, malonyl-CoA decarboxylase; ACC, acetyl-CoA carboxylase. No differences were found between the groups for any protein.
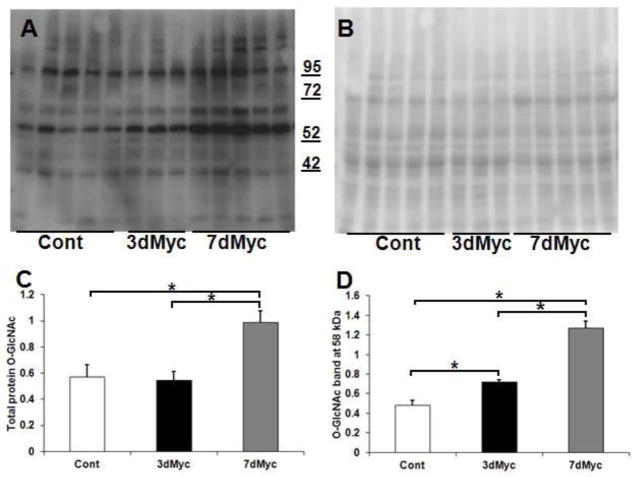
Since Myc did not affect total protein levels of key fatty acid oxidation enzymes, we explored potential mechanisms for post-translational modification [15]. As previously noted, O-GlcNAcylation affects myocardial fatty acid oxidation and is potentially important for the development of hypertrophy [17,19,24]. Total protein O-GlcNAc levels were increased in 7dMyc versus Cont and 3dMyc (p<0.05, Figure 5). Measuring signal intensity along entire lanes can obscure differences in specific bands between our 3 conditions. Accordingly, we specifically evaluated the band at ~ 58 kDa, which qualitatively demonstrated the largest signal difference across groups. The densitometric analyses for this band revealed differences in the O-GlcNAcylation profile between 3dMyc versus Cont. The signal for this band further increased in 7dMyc (Figure 5D). Thus, Myc activation begins to affect protein O-GlcNAcylation prior to the development of hypertrophy.
Figure 5.
Total protein O-linked β-N-acetylglucosamine (O-GlcNAc) levels. A. Total protein O-GlcNAc immunoblot. B. Ponceau S membrane staining. C. Total protein O-GlcNAc quantification in arbitrary units normalized to Ponceau S staining. D. Quantification of O-GlcNAc band at approximately 58 kDa. Of note, the molecular weight latter (in kDa) is shown between A and B. n=3–5 per group. * p<0.05 between indicated groups.
DISCUSSION
Myc regulates cardiac hypertrophy and also affects intermediate metabolism in non-cardiac tissue. Our objective was to test the hypothesis that Myc-induced substrate utilization changes to the citric acid cycle signal for hypertrophy development and promote compensated hypertrophy. Echocardiogram and ex vivo working heart experiments confirmed that this was compensated hypertrophy as function was preserved or slightly improved with Myc-induction. Myc affected myocardial substrate utilization patterns at established hypertrophy (7dMyc), with a marked increase in free fatty acid oxidation with concurrently decreased lactate oxidation.
We chose the 3dMyc group to determine whether substrate utilization changes precede hypertrophy and may therefore signal for its development. Demonstrating a metabolic shift prior to hypertrophic growth is necessary (but not sufficient) to establish a signaling role. The current study justified this time point for pre-hypertrophy metabolic evaluation since the heart weight to body weight ratios are similar to Cont and there was no change in serial LV posterior wall thickness. Metabolic changes are considered part of the hypertrophic process; however altered utilization patterns may also drive hypertrophy development. For example, cardiac specific PPARα overexpression promotes myocardial fatty acid oxidation through transcription of genes involved in this process. These changes lead to hypertrophy, demonstrating that alterations in substrate citric acid cycle contribution can promote hypertrophy development [25]. Given Myc’s ability to regulate substrate metabolism in non-cardiac tissue, we hypothesized that changes in substrate utilization may lead to hypertrophy development in this model. Ahuja et al previously reported that Myc activation for 3 days increased the unlabeled Fc to acetyl-CoA and ultimately the citric acid cycle [14]. However, those experiments included only a small number of mice and were considered preliminary. Our current study with greater numbers (n=6) showed only a modest trend towards increased unlabeled Fc in 3dMyc, which disappeared in follow up studies with insulin-containing perfusate. As no other changes in substrate utilization occurred prior to hypertrophy (i.e. in the 3dMyc versus Cont), oxidative shifts are not the primary signal for cardiac growth in this model. However, oxidative changes may still be required to provide energy and substrates for rapid protein synthesis and growth.
Unlike the 3dMyc, 7dMyc significantly altered substrate contribution to the citric acid cycle. Free fatty acid contribution increased nearly 50% corresponding with decreased exogenous glucose contribution. Absolute flux of free fatty acids and lactate were also changed in established hypertrophy representing oxidative metabolism. Myc-induced hypertrophy maintained compensated function while increasing fatty acid oxidation. This is notable as many investigators are exploring pharmacologic modulation of substrate utilization for heart failure treatment. Typically, however, the therapeutic goal is to inhibit fatty acid oxidation for reasons including that this theoretically requires 11–12% more oxygen for a given amount of ATP produced than glucose oxidation [3]. Trimetazidine is a partial mitochondrial fatty acid β-oxidation inhibitor used to treat chronic stable angina in Europe and Asia. Multiple small, short term studies utilizing trimetazidine to treat heart failure demonstrated improved LV ejection fraction, exercise performance and plasma heart failure biomarkers [26–28]. Similarly, the cardiac specific PPARα overexpressing mice discussed above had decreased cardiac function [25]. Our results do not contradict these studies, but it demonstrates that increased fatty acid oxidation does not uniformly cause functional abnormalities in the hypertrophied myocardium. Future studies are planned to determine the necessity of these metabolic changes for maintaining compensated function in our model. Additionally, understanding the mechanisms whereby fatty acid oxidation is not functionally detrimental in this model would provide useful information for developing metabolic therapies to treat heart failure and diabetic cardiomyopathy which is associated with increased fatty acid oxidation.
Although Myc is upregulated in nearly all types of hypertrophy, the metabolic phenotype in the Myc-inducible mice did not fully recapitulate changes described in physiologic hypertrophy models such pressure overload. As such, our findings are due to Myc-induction and not secondary to hypertrophy. Fatty acid oxidation levels are typically unchanged or mildly diminished in compensated physiologic hypertrophy and decreased in heart failure with physiologic hypertrophy [3,29]. One potential reason for this difference could be that Myc levels are lower in physiologic hypertrophy thereby affecting intermediate metabolism to a lesser degree. Xiao et al [11] showed that Myc mRNA levels in this transgenic mouse are similar to non-transgenic mice after 6 hours of transverse aortic constriction. They did not report protein levels or temporal data, so differences cannot be completely ruled out. Another more likely possibility is that additional metabolic regulation counterbalances Myc. The etiology of decreased fatty acid oxidation in physiologic hypertrophy and heart failure is incompletely understood but it is likely at least partially due to reduced transcriptional activation of genes regulated by peroxisome proliferator-activated receptor alpha (PPARα), peroxisome proliferator-activated receptor gamma co-activator-1 alpha (PGC-1α) and retinoic acid X receptor α (RXRα) [1,3]. These proteins form heterodimers that transcriptionally control fatty acid oxidation encoding genes. We did not find any differences in PPARα or PGC-1 protein levels. In physiologic hypertrophy, changes in PPARα, PGC-1and/or RXRα activity may counteract Myc’s effect on fatty acid oxidation. Myc may prevent a larger reduction in fatty acid oxidation. Future studies are planned using the cardiac specific inducibly inactivate Myc mice (described by Zhong et al [12])to elucidate Myc’s role in metabolic changes from hypertrophy secondary to pressure overload.
Regulation of intermediate metabolism
Surprisingly, the substrate utilization changes do not appear to be due to transcription and/or translation of fatty acid oxidation genes. Ahuja et al previously demonstrated unchanged gene expression of the fatty acid oxidation transcription factors PGC-1α, PGC-1β, PGC-1 related coactivator, PPARα, estrogen-related receptor α, and nuclear respiratory factor [14]. Medium chain acyl-CoA dehydrogenase (MCAD) and carnitine palmitoyl-transferase 1 (CPT-1) RNA levels were decreased [14], although our study demonstrated that this does not reduce fatty acid oxidation. As noted earlier, protein levels of the transcription factors PPARα and PGC-1α were unchanged confirming that global transcription of metabolic genes is unlikely. Thereafter we explored total protein levels of specific enzymes. Fatty acid oxidation regulation occurs at multiple steps from cellular uptake to mitochondrial transport to β-oxidation [3] but we did not find protein changes that would augment fatty acid oxidation such as in mCPT-I, lCPT-I, FAT/CD36, MCD and ACC. PDK2 and PDK4 inhibit pyruvate dehydrogenase and therefore glucose and lactate oxidation. This could reciprocally promote fatty acid utilization according to the Randle hypothesis. However, we did not find changes in either PDK isoform. Overall, Myc did not augment fatty acid oxidation through transcription or translation of the commonly investigated regulatory enzymes. Further work is necessary to determine whether other enzymes are transcriptionally affected by Myc.
Many metabolic enzymes undergo post-translational modification altering their activity [3]. Myc is primarily a transcriptional regulator, but recent studies have demonstrated transcription-independent functions [16,30]. O-GlcNAc post-translational modification was increased by Myc-induction in our study. Recent work suggests O-GlcNAc promotes myocardial fatty acid oxidation [17,24]. Laczy and colleagues perfused isolated rat hearts with glucosamine to acutely increase total protein O-GlyNAcylation under normoxic condition [17]. Glucosamine caused a dose dependent increase in O-GlcNAc levels, which was associated with increased palmitate oxidation. This metabolic change was attributed to increased FAT/CD36 membrane translocation and fatty acid cellular uptake. FAT/CD36 immunoprecipitation demonstrated evidence of O-GlcNAc modification. We are currently evaluating whether Myc activation increases FAT/CD36 membrane levels in our model.
O-GlcNAc
In addition to regulating cardiac substrate metabolism, O-GlcNAcylation may promote hypertrophy in our model. Facundo and colleagues recently demonstrated that O-GlcNAc signaling is necessary for NFAT-mediated hypertrophy [19]. NFAT activation from phenylephrine was blunted by O-GlcNAc inhibition in neonatal rat cardiac myocytes. Further, O-GlcNAc augmentation increased NFAT activation and nuclear translocation independent of hypertrophic stimuli. In our study, the O-GlcNAc band near 58 kDa was greater in 3dMyc compared to Cont demonstrating that the increase in O-GlcNAcylation begins prior to hypertrophy. Because of the nonspecific nature of the O-GlcNAc antibodies, additional work is required to identify O-GlcNAcylated proteins within the ~58 kDa band and at other molecular weights that may promote the development of hypertrophy in our model. Interestingly, Morrish et al demonstrated that inhibiting the pathway for producing O-GlcNAc reduced growth in Myc-expressing cultured fibroblast [16]. We plan future studies to elucidate Myc’s role in myocardial O-GlcNAcylation.
Summary
Myc is an important regulator of cardiac hypertrophy. In the current study, we show that Myc affects substrate utilization for the citric acid cycle. This process does not precede hypertrophy and therefore does not signal for hypertrophy development. With established compensated hypertrophy, Myc-induction increased fatty acid utilization suggesting a potential mechanistic role. Based upon our results, further studies are warranted to determine whether fatty acid oxidation promotes functional compensation in the model as well as Myc’s role in metabolic changes from physiologic hypertrophy.
Highlights.
C-Myc activation affects myocardial substrate utilization for the TCA cycle.
Fatty acid oxidation increases with established compensated cardiac hypertrophy.
Substrate oxidation is not altered prior to the development of hypertrophy.
Myocardial O-GlcNAcylation was increased by c-Myc.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute Grant K08-HL-092333 to A. K. Olson. A portion of the research was performed using EMSL, a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lionetti V, Stanley WC, Recchia FA. Modulating fatty acid oxidation in heart failure. Cardiovasc Res. 2011 May 1;90(2):202–9. doi: 10.1093/cvr/cvr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolwicz SC, Jr, Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovasc Res. 2011 May 1;90(2):194–201. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005 Jul;85(3):1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 4.Dzimiri N, Al-Bahnasi K, Al-Halees Z. Myocardial hypertrophy is not a prerequisite for changes in early gene expression in left ventricular volume overload. Fundam Clin Pharmacol. 2004 Feb;18(1):39–44. doi: 10.1046/j.0767-3981.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- 5.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–43. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HG, Chen Q, Wolfram JA, Richardson SL, Liner A, Siedlak SL, et al. Cell cycle reentry and mitochondrial defects in myc-mediated hypertrophic cardiomyopathy and heart failure. PLoS One. 2009;4(9):e7172. doi: 10.1371/journal.pone.0007172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadoshima J, Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993 Sep;73(3):413–23. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 8.Starksen NF, Simpson PC, Bishopric N, Coughlin SR, Lee WM, Escobedo JA, et al. Cardiac myocyte hypertrophy is associated with c-myc protooncogene expression. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8348–50. doi: 10.1073/pnas.83.21.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taketani S, Sawa Y, Ichikawa H, Ohtake S, Nishimura M, Kawaguchi N, et al. Change of c-Myc expression and cardiac hypertrophy in patients with aortic valve replacement. Ann Thorac Surg. 2001 Apr;71(4):1154–9. doi: 10.1016/s0003-4975(00)02656-4. [DOI] [PubMed] [Google Scholar]
- 10.Taketani S, Sawa Y, Taniguchi K, Mitsuno M, Kawaguchi N, Onishi S, et al. C-Myc expression and its role in patients with chronic aortic regurgitation. Circulation. 1997 Nov 4;96(9 Suppl):II-83–7. discussion II-7–9. [PubMed] [Google Scholar]
- 11.Xiao G, Mao S, Baumgarten G, Serrano J, Jordan MC, Roos KP, et al. Inducible activation of c-Myc in adult myocardium in vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circ Res. 2001 Dec 7;89(12):1122–9. doi: 10.1161/hh2401.100742. [DOI] [PubMed] [Google Scholar]
- 12.Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, et al. Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. Embo J. 2006 Aug 23;25(16):3869–79. doi: 10.1038/sj.emboj.7601252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006 Aug;16(4):253–64. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Ahuja P, Zhao P, Angelis E, Ruan H, Korge P, Olson A, et al. Myc controls transcriptional regulation of cardiac metabolism and mitochondrial biogenesis in response to pathological stress in mice. J Clin Invest. 2010 May 3;120(5):1494–505. doi: 10.1172/JCI38331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darley-Usmar VM, Ball LE, Chatham JC. Protein O-linked beta-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function. J Mol Cell Cardiol. 2012 Mar;52(3):538–49. doi: 10.1016/j.yjmcc.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrish F, Isern N, Sadilek M, Jeffrey M, Hockenbery DM. c-Myc activates multiple metabolic networks to generate substrates for cell-cycle entry. Oncogene. 2009 Jul 9;28(27):2485–91. doi: 10.1038/onc.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laczy B, Fulop N, Onay-Besikci A, Des Rosiers C, Chatham JC. Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation. PLoS One. 2011;6(4):e18417. doi: 10.1371/journal.pone.0018417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, et al. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genomics. 2012 Feb 1;44(2):162–72. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- 19.Facundo HT, Brainard RE, Watson LJ, Ngoh GA, Hamid T, Prabhu SD, et al. O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2012 May;302(10):H2122–30. doi: 10.1152/ajpheart.00775.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyyti OM, Olson AK, Ge M, Ning XH, Buroker NE, Chung Y, et al. Cardioselective dominant-negative thyroid hormone receptor (Delta337T) modulates myocardial metabolism and contractile efficiency. Am J Physiol Endocrinol Metab. 2008 Aug;295(2):E420–7. doi: 10.1152/ajpendo.90329.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyyti OM, Ledee D, Ning XH, Ge M, Portman MA. Aging impairs myocardial fatty acid and ketone oxidation and modifies cardiac functional and metabolic responses to insulin in mice. Am J Physiol Heart Circ Physiol. 2010 Sep;299(3):H868–75. doi: 10.1152/ajpheart.00931.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malloy CR, Jones JG, Jeffrey FM, Jessen ME, Sherry AD. Contribution of various substrates to total citric acid cycle flux and anaplerosis as determined by 13C isotopomer analysis and O2 consumption in the heart. MAGMA. 1996 Mar;4(1):35–46. doi: 10.1007/BF01759778. [DOI] [PubMed] [Google Scholar]
- 23.Krueger JJ, Ning XH, Argo BM, Hyyti O, Portman MA. Triidothyronine and epinephrine rapidly modify myocardial substrate selection: a (13)C isotopomer analysis. Am J Physiol Endocrinol Metab. 2001 Nov;281(5):E983–90. doi: 10.1152/ajpendo.2001.281.5.E983. [DOI] [PubMed] [Google Scholar]
- 24.Lauzier B, Merlen C, Vaillant F, McDuff J, Bouchard B, Beguin PC, et al. Post-translational modifications, a key process in CD36 function: lessons from the spontaneously hypertensive rat heart. J Mol Cell Cardiol. 2011 Jul;51(1):99–108. doi: 10.1016/j.yjmcc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002 Jan;109(1):121–30. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belardinelli R, Purcaro A. Effects of trimetazidine on the contractile response of chronically dysfunctional myocardium to low-dose dobutamine in ischaemic cardiomyopathy. Eur Heart J. 2001 Dec;22(23):2164–70. doi: 10.1053/euhj.2001.2653. [DOI] [PubMed] [Google Scholar]
- 27.Di Napoli P, Di Giovanni P, Gaeta MA, D’Apolito G, Barsotti A. Beneficial effects of trimetazidine treatment on exercise tolerance and B-type natriuretic peptide and troponin T plasma levels in patients with stable ischemic cardiomyopathy. Am Heart J. 2007 Sep;154(3):602, e1–5. doi: 10.1016/j.ahj.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, et al. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol. 2006 Sep 5;48(5):992–8. doi: 10.1016/j.jacc.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 29.Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem. 2001 Nov 30;276(48):44390–5. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- 30.Conacci-Sorrell M, Ngouenet C, Eisenman RN. Myc-nick: a cytoplasmic cleavage product of Myc that promotes alpha-tubulin acetylation and cell differentiation. Cell. 2010 Aug 6;142(3):480–93. doi: 10.1016/j.cell.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]