Summary
Feeding history and the presence of food dramatically alters chemosensory behaviors. Recent results indicate that internal nutritional state can gate peripheral gustatory and olfactory sensory responses to affect behavior. Focusing primarily on recent work in C. elegans and Drosophila, I describe the neuromodulatory mechanisms that translate feeding state information into changes in chemosensory neuron response properties and behavioral output.
“Come, our stomachs will make what's homely savory”
William Shakespeare (Cymbeline)
Introduction
An animal’s feeding state and food availability can profoundly affect its olfactory and gustatory responses. Thus, while hunger sensitizes our chemosensory abilities to maximize our ability to find food, even the most delectable confections may not tempt us when sated (well, maybe sometimes). Although learning, culture and psychological factors complicate feeding behaviors in humans, in general, feeding state and the presence of food alter dietary choice, food searching and appetitive behaviors across species, driven in part by changes in chemosensory preferences (eg. [1–3]). Thus, the modulation of chemosensory responses as a function of nutritional state is a common feature of nervous systems regardless of their complexity.
In principle, information regarding our feeding state can interface with sensory processing pathways at any level, from peripheral to central brain regions. Indeed, chemical stimulus-evoked activity is altered in an internal energy state-dependent manner in both higher order processing centers as well as at the first synapse between sensory and interneurons in different species (eg. [4–6]). While food-dependent modulation of central neurons can coordinately alter responses to a suite of sensory stimuli, it is now becoming increasingly clear that food and feeding state gate responses in chemosensory cells themselves, thereby modulating specific chemosensory behaviors. Here, I review recent findings in C. elegans and Drosophila regarding modulation of peripheral chemosensory neuron properties by feeding/fasting state and food perception. I refer the reader to recent reviews and articles discussing similar mechanisms in the mammalian olfactory and gustatory systems (eg. [1, 7–11]).
Modulation of chemosensory responses by starvation or satiety
Hungry and satiated animals exhibit markedly distinct responses to attractive or noxious chemicals. Metabolites, neuropeptides, monogenic amines and hormones including dopamine, insulin, serotonin and neuropeptide Y produced by the brain as well as peripheral tissues such as the gut act in a complex manner to inform the body of its nutritional status and energy requirements [12, 13]. Despite the significant differences in nervous system architecture between vertebrates and invertebrates, many (but not all) of the molecules that signal hunger or satiety are conserved and function via similar molecular signaling pathways [14, 15]. Although the roles of these molecules in altering energy homeostasis and behaviors have largely been studied in the central nervous system, recent studies show that peripheral chemosensory neurons may be also be targets, providing a simple mechanism by which feeding status can directly modulate chemosensory responses.
Regulation by Neuropeptide Y (NPY) signaling
Neuropeptide Y (NPY) is a potent orexigenic signal produced in the arcuate nucleus of the hypothalamus in vertebrates [16]. NPY-related peptides and receptors are conserved in invertebrates and have been shown to also play roles in feeding-related behaviors [17, 18]. In C. elegans, animals with reduced or loss of function of the NPY-related peptide receptor NPR-1 exhibit a range of behavioral modifications in the presence of food. For instance, npr-1 mutants move rapidly, avoid high oxygen concentrations and aggregate, behaviors that are exhibited by animals with high NPR-1 activity only when food is limiting or absent [18–20]. Aggregation behaviors are driven in part by altered responses to a complex mixture of small molecule pheromones produced by other individuals [21•, 22•]. Cell-specific rescue experiments together with analyses of pheromone-induced calcium dynamics have now shown that NPR-1 acts in the RMG inter/motor neuron to regulate sensory responses of electrically connected chemosensory neurons to pheromones [21•, 22•]. Thus, food (and other stress information) is integrated by NPR-1 in RMG to indirectly modulate chemosensory neuron responses and allow the circuit to drive distinct behaviors in the presence or absence of food.
Young Drosophila larvae are attracted to sugar, whereas late-stage larvae show sugar aversion prior to pupation. The related neuropeptide F receptor NPFR1 inhibits aversion of sugar in young larvae [23] and acts directly in sugar responsive thoracic sensory neurons to attenuate TRP channel signaling and sugar-induced aversive responses [24]. Interestingly, NPFR1 and insulin have also been shown to act in central circuits to modify responses to noxious stimuli upon starvation [25], suggesting that as in C. elegans, NPFR1 may act indirectly to alter chemosensory responses in a feeding state-dependent manner in Drosophila. Given the central role of NPY-like peptides and receptors in regulating multiple behaviors in addition to feeding, gating of peripheral responses by NPY-mediated neuromodulation of a central circuit may allow fine-tuning of chemosensory responses via integration of multiple internal state cues.
Regulation by dopaminergic signaling
Although food intake is under complex homeostatic control in vertebrates, the hedonic effects of food can override the body’s caloric requirements. However, internal metabolic state and learning can alter the hedonic value of food stimuli suggesting an interaction between nutritional state and food perception [26, 27]. Midbrain dopaminergic circuits play a crucial role in the cross-talk between homeostatic and hedonic control of eating behaviors [26, 27]. In both flies and worms (see below), food-regulated dopamine signaling also alters chemosensory preferences in part via direct effects on chemosensory neuron responses.
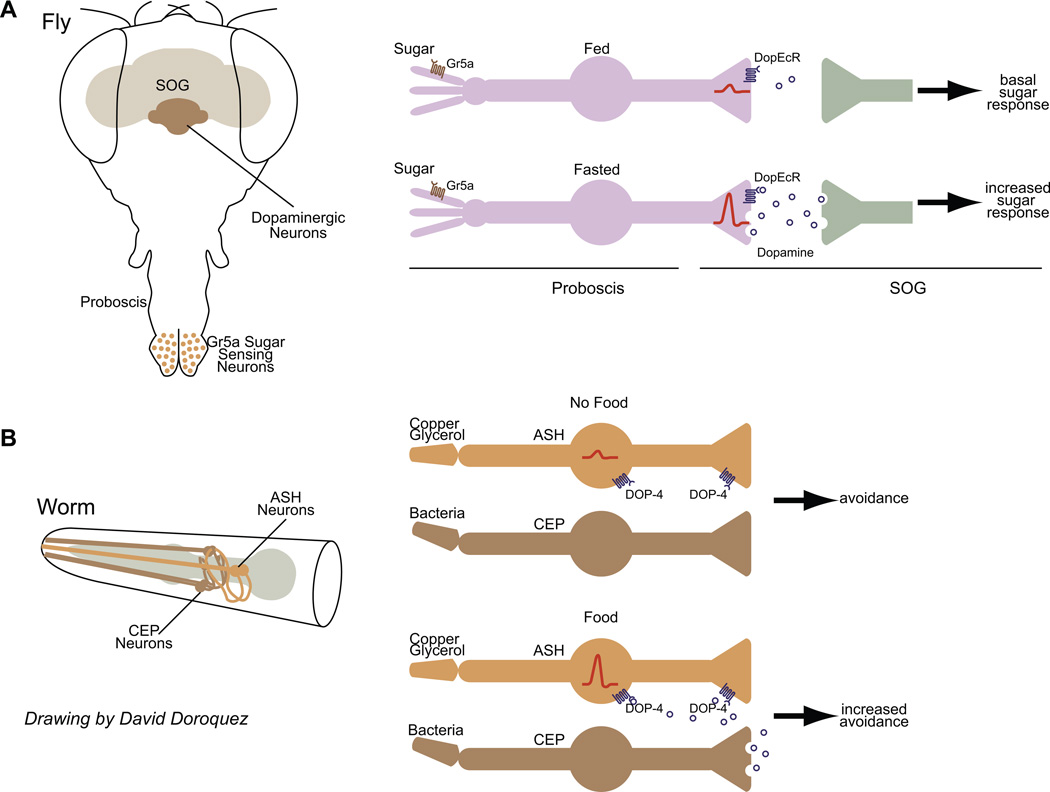
Hungry flies extend their proboscis (the proboscis extension reflex or PER is a commonly used measure of feeding behavior) more frequently when presented with sugar than fed flies [28••, 29••]. Two studies have recently demonstrated a role for dopamine in mediating this starvation-dependent increase in sugar responses [28••, 29••]. Sugar is sensed by Gr5a-expressing gustatory neurons located at the proboscis tip; the termini of these neurons arborize in the subesophageal ganglion (SOG), the primary taste relay center in the brain [30] (Figure 1A). Food deprivation was shown to increase dopaminergic signaling in the SOG [28••, 29••] (Figure 1A). Dopamine in turn acts directly on dopamine receptors in the termini of Gr5a-expressing neurons to increase PER [28••] (Figure 1A). In fact, activation of the single dopaminergic TH-VUM neuron in the SOG was sufficient to increase PER [29••].
Figure 1.
Chemosensory responses are altered by dopaminergic input as a function of feeding state or food availability.
A) (Left) Schematic of fly brain showing location of Gr5a-expressing sugar sensing gustatory neurons in the proboscis; termini of these neurons arborize in the SOG. (Right) Fasted flies show enhanced PER to sugar. Starvation results in increased dopamine release from neurons such as the TH-VUM neuron in the SOG. Dopamine acts via the DopEcR to increase presynaptic calcium dynamics in the termini of Gr5a gustatory neurons in the SOG (red traces). Increased neurotransmission may contribute to enhanced PER. See [28••, 29••] for details.
B) (Left) Schematic of worm head showing location of cell bodies and processes of the ASH and CEP sensory neurons (from www.wormatlas.org). (Right) Food acutely enhances ASH-mediated aversive responses. Bacteria are sensed by the mechanosensory CEP neurons which release dopamine to enhance somatic calcium transients in ASH via the DOP-4 D2-like receptor, and increase ASH-driven avoidance behavior. The location of DOP-4 receptors and sites of dopamine release from CEP are unknown. From [45••].
How does dopamine affect gustatory neuron responses? Starvation and dopamine signaling increased stimulus-evoked intracellular calcium dynamics in the terminals of the Gr5a-expressing sugar-responsive neurons in the SOG without affecting sugar-evoked action potentials [28••] (Figure 1A). This suggests that presynaptic release of neurotransmitter by gustatory neurons may be facilitated by starvation/dopamine. However, when flies are starved for longer periods of time (>24h), mechanisms other than dopamine are required to modulate chemosensory responses [28••]. A candidate for mediating this regulation is the takeout (to) molecule since starvation-induced sensitization of sugar responses in gustatory neurons is also abolished in to mutants [31]. Takeout encodes a putative juvenile hormone carrier protein produced in fat tissues and also in olfactory and gustatory cells, is upregulated upon starvation, and regulates feeding and foraging behavior [31, 32]. Although the functions of to remain to be fully elucidated, these observations suggest that distinct phases of the feeding state may be communicated via partly different mechanisms to peripheral chemosensory neurons to continuously reshape their responses.
Regulation by insulin signaling
Circulating levels of insulin reflect internal metabolic state since fasting decreases, and feeding increases insulin levels. Recent genetic and physiological experiments in flies and worms suggest that insulin levels directly affect peripheral chemosensitivity.
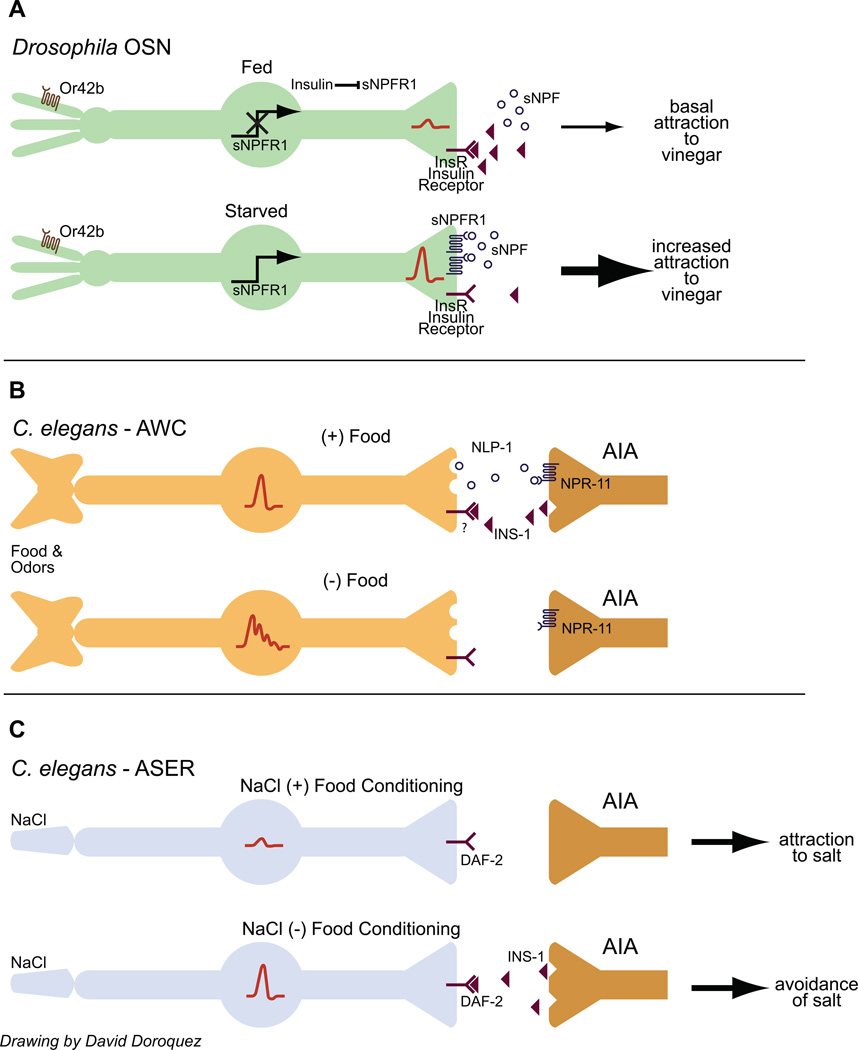
Recent elegant work in Drosophila has described a complex positive feedback loop by which feeding state alters olfactory responses via insulin signaling [33••]. Drosophila is more strongly attracted to vinegar when fasted [33••]. As in the case of feeding state-dependent enhancement of sugar responses, increased search behavior for vinegar is mediated via increased activity of the vinegar-responsive Or42b olfactory sensory neurons (OSNs) [33••] (Figure 2A). This enhancement is facilitated by an autocrine positive feedback loop requiring the sNPF peptide (not to be confused with the NPY-like NPF peptide discussed earlier; [17]) and increased expression of the sNPFR1 receptor in OSNs [33••] (Figure 2A). In this case, however, feeding state regulates the activity of this autocrine mechanism not by dopamine, but by insulin. Root et al found that expression of a constitutively activated insulin receptor in the OSNs downregulates sNPFR1 expression and was sufficient to block hunger-induced enhancement of vinegar responses via decreased sNPF-sNPFR1 autocrine signaling [33••] (Figure 2A). The source of the insulin signal was not identified but is likely to be the insulin producing cells (IPCs) in the fly brain. Intriguingly, expression of insulin-like peptides in the IPCs is itself under positive regulation by sNPF, raising the possibility of a food-driven negative feedback loop regulating food search behavior [34]. The insulin receptor is expressed broadly among OSNs, suggesting that feeding state may have diverse effects on the responses to other odors as well either directly, or indirectly via other neuromodulatory loops.
Figure 2.
Insulin signaling translates feeding state information into changes in chemosensory neuron responses.
A) Drosophila is more strongly attracted to food odors such as vinegar when starved. Under fed conditions, insulin inhibits expression of the sNPF receptor in Or42b OSNs. Upon starvation, reduced insulin signaling disinhibits sNPFR1 expression and enhances presynaptic calcium influx (indicated by red traces at neuron termini). For details see [33••].
B) Food responses in the AWC olfactory neurons of C. elegans are dampened by INS-1 insulin signaling from the AIA interneurons. Calcium responses are indicated by red traces in the AWC soma. Insulin signaling is promoted by a NLP-1/NPR-11 neuropeptide signaling loop. The temporal relationship between food presence/removal and insulin or NLP-1 production is speculative. For details see [35••]. C) Prolonged exposure to salt in the absence of food switches the response of C. elegans to salt from attraction to aversion. This switch is mediated by INS-1 insulin signaling from the AIA interneurons which increases calcium influx in the ASER salt sensing neurons (red traces in ASER soma). For details see [56••, 58••].
In C. elegans, insulin can dampen responses to food and food-related odors in the AWC olfactory neurons to maximize gain and regulate food search behaviors. NLP-1 neuropeptide signaling from the AWC sensory neuron promotes INS-1 insulin production from the AIA interneurons, direct postsynaptic partners of AWC [35••, 36] (Figure 2B). INS-1 in turn feeds back onto the AWC neurons to inhibit olfactory responses [35••] (Figure 2B). However, since the timescale of release of either NLP-1 or INS-1 with respect to food is not yet known, the exact relationship of this gain control mechanism with starved/fed state remains speculative. Nevertheless, the well-described role of AWC in the response to food-derived odors and in food search behavior [37, 38], suggests that insulin-mediated regulation of AWC response properties has significant implications for the regulation of chemosensory behaviors as a function of feeding state and history.
Changes in gene expression in chemosensory neurons by fasting/feeding
Internal metabolic state has dramatic consequences on multiple aspects of physiology and behavior mediated in part via changes in gene expression in a broad range of tissues and organs [39–42]. A recent study in Drosophila has shown that food deprivation for variable periods of time leads to robust changes in gene expression in the major chemosensory organs [43]. Although the functional consequences of these gene expression changes have not yet been clarified, these observations suggest that feeding state-regulated changes in gene expression may contribute to altered chemosensory neuron responses. Indeed, as described above, sNPFR1 expression is upregulated in fly antennae in the starved state [33] (Figure 2A) and is required for the increased responsiveness of these neurons to chemosensory stimuli.
Acute modulation of chemosensory responses by food
In addition to being gated by prior feeding experience, sensory responses can also be acutely regulated by the presence or absence of food. In this context, I define acute regulation as alteration of sensory responses to a stimulus based upon simultaneous presentation of the stimulus and food. Information about food and a chemical stimulus can be integrated in parallel channels, and this information in turn can modulate behavior either by feedback regulation of sensory neuron activity, or alternatively, by feedforward mechanisms in the circuit. In some cases, however, food odors have been shown to act within the sensory neurons themselves to directly alter sensory transduction as in the case of food-dependent modulation of carbon dioxide responses in Drosophila [44].
Acute modulation via dopaminergic signaling
In C. elegansfood acutely enhances aversion of chemicals such as copper and glycerol [45] via increasing stimulus-evoked intracellular calcium dynamics in the ASH polymodal sensory neurons [45••] (Figure 1B). As in the case of starvation-mediated changes in gustatory responses in flies, food modulates ASH responses via dopaminergic signaling. However, unlike in flies where dopamine release is enhanced upon starvation, dopamine is released in the presence of bacterial food in worms (compare Figures 1A and 1B). Mechanical stimulation of the CEP sensory neurons by bacteria stimulates dopamine release which then feeds back via the DOP-4 D1-like dopaminergic receptors in ASH to enhance somatic calcium responses in response to copper [45••] (Figure 1B).
Whether dopamine affects primary sensory response thresholds or downstream signaling events in ASH in C. elegans is unclear. However, it is evident that dopamine-mediated regulation of ASH responses is quite complex. Food and dopamine enhance chemosensory responses but not nose touch responses in ASH, indicating a modality-specific effect [45••]. Even within the chemosensory modality, different mechanisms are used. Thus, while food also enhances ASH-mediated avoidance of octanol [46•], dopamine actually dampens this response acting via a D2-like receptor [47, 48]. This dampening is mediated by inhibition of serotonergic signaling (see below). These observations point to a clear role for dopaminergic signaling in the modulation of chemosensory sensitivity in response to acute and long-term feeding state in both C. elegans and Drosophila (Figure 1).
Acute regulation via serotonin/octopamine signaling
In C. elegans, serotonin and octopamine play prominent roles in signaling the feeding state in addition to insulin, dopamine and peptides described above. Serotonin mediates the effects of food on locomotion, egg-laying and feeding behaviors, whereas octopamine antagonizes serotonin and mediates a subset of effects of starvation [49]. Food and serotonin have been shown to gate mechanosensory responses in feeding leech via suppression of neurotransmitter release from mechanosensory neurons [50••]. Similarly, serotonin and octopamine gate a subset of ASH-mediated chemosensory responses via cognate ASH-expressed receptors in C. elegans [48, 51, 52].
The effects of different neuromodulators on ASH sensory responses are remarkably stimulus-specific. Thus, food or serotonin enhances ASH-mediated avoidance of octanol whereas dopamine, tyramine, octopamine or starvation decreases this response [46, 48]. In contrast, serotonin does not mediate food-dependent enhancement of copper or glycerol responses in ASH which is instead mediated by dopamine [45••] (Fig. 1B). Thus, different neuromodulatory pathways appear to target distinct signaling pathways within a single sensory neuron type.
Octopamine turns out to have a broader set of targets than just ASH, since it regulates octanol avoidance behavior via modulation of neuropeptide signaling from a distributed set of chemosensory neurons under starvation conditions [53•]. However, whether this remarkably complex signaling network alters sensory neuron response properties upstream (as in the case of dopaminergic modulation of ASH responses to copper) or downstream of calcium responses is not yet known.
Plasticity in chemosensory responses upon prior pairing of food and chemicals
Pairing of food with odors increases the appetitive value of the odors, whereas conversely, pairing of an odor with starvation or another aversive stimulus decreases subsequent responses to the odor in both C. elegans and Drosophila [54, 55]. This behavioral plasticity has been shown to occur in higher integrative centers of the brain [54, 55]. However, at least in one recent report in C. elegans, this plasticity has clearly been shown to arise from changes in chemosensory neuron response thresholds [56••].
While salt is normally attractive to C. elegans, animals will avoid salt upon prior exposure to salt under starvation conditions [57, 58••]. Starvation in the absence of salt does not affect salt responses [57, 58••] suggesting that these stimuli must be paired for the plasticity to occur. Imaging of intracellular calcium dynamics has demonstrated that changes in ASE sensory responses to salt contribute to this ‘salt chemotaxis learning’ [56••] (Figure 2C). This sensory experience-dependent modulation is mediated in part via INS-1 insulin signaling from the AIA interneurons, via the DAF-2 insulin receptor in the right ASE (ASER) neurons [56••, 58••] (Figure 2C). Under these conditions, therefore, insulin appears to be the starvation signal. How food or starvation is sensed in the context of salt to regulate insulin secretion, and whether mechanisms involving modulation of chemosensory responses are generalizable to other forms of foodassociated behavioral plasticity remain to be seen.
Conclusions
A clear theme running through the above discussion is the remarkable complexity of mechanisms by which internal nutritional state information is transmitted to the chemosensory system to change behavior. However, this is likely only the proverbial tip of the iceberg. What is the reason for this complexity? For one, nutritional state-dependent chemosensory gating is not a binary ON-OFF switch. Instead, responses are likely to be precisely calibrated according to not just whether the animal has eaten or not, but by what they have eaten and when. Behavioral responses also change depending on how long animals have been deprived of food. Thus, complex neuromodulatory pathways may be required to translate multiple aspects of the food response to the periphery. In addition, food signals must be integrated in the context of other external and internal cues such as stress, emotions, sleep and prior experience to regulate chemosensory behaviors; precise integration of these cues may require multiple, interconnected neuromodulatory pathways. A major challenge for the future will be to decode exactly which aspects of the internal state map to changes in chemosensory responses, and how these changes in turn map to alterations in behavior.
Highlights.
Feeding state and food alter chemosensory behaviors
Chemosensory neuron response properties are altered by feeding/fasting
Feeding state information is transmitted via conserved neuromodulatory pathways
Acknowledgements
I am grateful to David Doroquez for providing the illustrations. I thank Cori Bargmann, Denise Ferkey, Leslie Griffith, Bill Schafer and Kristin Scott for discussion, and Cori Bargmann, Leslie Griffith and Kristin Scott for comments on the manuscript. This work is funded by the NSF (IOS 0842452) and the NIH (GM081639).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aime P, Duchamp-Viret P, Chaput MA, Savigner A, Mahfouz M, Julliard AK. Fasting increases and satiation decreases olfactory detection for a neutral odor in rats. Behav Brain Res. 2007;179:258–264. doi: 10.1016/j.bbr.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Colbert HA, Bargmann CI. Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans. Learn Mem. 1997;4:179–191. doi: 10.1101/lm.4.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- 4.Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J Neurophysiol. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- 5.Pager J, Giachetti I, Holley A, Le Magnen J. A selective control of olfactory bulb electrical activity in relation to food deprivation and satiety in rats. Physiol Behav. 1972;9:573–579. doi: 10.1016/0031-9384(72)90014-5. [DOI] [PubMed] [Google Scholar]
- 6.Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mousley A, Polese G, Marks NJ, Eisthen HL. Terminal nerve-derived neuropeptide Y modulates physiological responses in the olfactory epithelium of hungry axolotls (Ambystoma mexicanum) J Neurosci. 2006;26:7707–7717. doi: 10.1523/JNEUROSCI.1977-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacroix MC, Badonnel K, Meunier N, Tan F, Schlegel-Le Poupon C, Durieux D, Monnerie R, Baly C, Congar P, Salesse R, Caillol M. Expression of insulin system in the olfactory epithelium: First approaches to its role and regulation. J Neuroendocrinol. 2008;20:1176–1190. doi: 10.1111/j.1365-2826.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 9.Savigner A, Duchamp-Viret P, Grosmaitre X, Chaput M, Garcia S, Ma M, Palouzier-Paulignan B. Modulation of spontaneous and odorant-evoked activity of rat olfactory sensory neurons by two anorectic peptides, insulin and leptin. J Neurophysiol. 2009;101:2898–2906. doi: 10.1152/jn.91169.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niki M, Yoshida R, Takai S, Ninomiya Y. Gustatory signaling in the periphery: Detection, transmission, and modulation of taste information. Biol Pharm Bull. 2010;33:1772–1777. doi: 10.1248/bpb.33.1772. [DOI] [PubMed] [Google Scholar]
- 11.Shin YK, Egan JM. Roles of hormones in taste signaling. Results Probl Cell Differ. 2010;52:115–137. doi: 10.1007/978-3-642-14426-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur J Neurosci. 2009;30:1688–1696. doi: 10.1111/j.1460-9568.2009.06963.x. [DOI] [PubMed] [Google Scholar]
- 13.Magni P, Dozio E, Ruscica M, Celotti F, Masini MA, Prato P, Broccoli M, Mambro A, More M, Strollo F. Feeding behavior in mammals including humans. Ann NY Acad Sci. 2009;1163:221–232. doi: 10.1111/j.1749-6632.2008.03627.x. [DOI] [PubMed] [Google Scholar]
- 14.Nassel DR, Winther AM. Drosophilaneuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Luedtke S, O'Connor V, Holden-Dye L, Walker RJ. The regulation of feeding and metabolism in response to food deprivation in Caenorhabditis elegans. Invert Neurosci. 2010;10:63–76. doi: 10.1007/s10158-010-0112-z. [DOI] [PubMed] [Google Scholar]
- 16.Mercer RE, Chee MJ, Colmers WF. The role of NPY in hypothalamic mediated food intake. Front Neuroendocrinol. 2011;32:398–415. doi: 10.1016/j.yfrne.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Nassel DR, Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling? Peptides. 2011;32:1335–1355. doi: 10.1016/j.peptides.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 18.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response inC. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 19.Rogers C, Persson A, Cheung B, de Bono M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation inC. elegans. Curr Biol. 2006;16:649–659. doi: 10.1016/j.cub.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Cheung BH, Cohen M, Rogers C, Albayram O, de Bono M. Experience-dependent modulation ofC. elegans behavior by ambient oxygen. Curr Biol. 2005;15:905–917. doi: 10.1016/j.cub.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jang H, Kim K, Neal SJ, Macosko EZ, Kim D, Butcher RA, Zeiger DM, Bargmann CI, Sengupta P. Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron. 2012 doi: 10.1016/j.neuron.2012.06.034. In press. These above two studies show that NPR-1 activity in the RMG interneuron alters pheromone responses in the ASK and ADL chemosensory neurons. RMG is connected to ASK, ADL and additional sensory neurons in a hub-and-spoke gap junction network, suggesting that NPR-1 integrates multiple internal and external cues to modulate RMG and connected sensory neuron activity.
- 23.Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Li M, Shen P. A G-protein-coupled neuropeptide Y-like receptor suppresses behavioral and sensory response to multiple stressful stimuli inDrosophila. J Neurosci. 2010;30:2504–2512. doi: 10.1523/JNEUROSCI.3262-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Zhao Z, Shen P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nat Neurosci. 2005;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- 26.Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Curr Opin Neurobiol. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figlewicz DP, Sipols AJ. Energy regulatory signals and food reward. Pharmacol Biochem Behav. 2010;97:15–24. doi: 10.1016/j.pbb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inagaki HK, Ben-Tabou de-Leon S, Wong AM, Jagadish S, Ishimoto H, Barnea G, Kitamoto T, Axel R, Anderson DJ. Visualizing neuromodulation in vivo: Tango-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. Responses to sugar are enhanced by starvation in flies. This study uses an elegant inducible reporter strategy to demonstrate that starvation increases dopaminergic signaling in the SOG of Drosophila. Dopamine then acts on the termini of sugar-sensing gustatory neurons in the SOG to increase presynaptic calcium influx and sugar responses upon starvation.
- 29. Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. This study also demonstrates that increased dopaminergic signaling in the SOG underlies enhanced sugar responses upon starvation. This work further shows that activity of the TH-VUM dopaminergic SOG neuron is feeding state-dependent, and that activation of this neuron is sufficient to enhance sugar responses.
- 30.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in theDrosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Meunier N, Belgacem YH, Martin JR. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J Exp Biol. 2007;210:1424–1434. doi: 10.1242/jeb.02755. [DOI] [PubMed] [Google Scholar]
- 32.Sarov-Blat L, So WV, Liu L, Rosbash M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell. 2000;101:647–656. doi: 10.1016/s0092-8674(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 33. Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. Starvation enhances food search behavior in Drosophila. The authors describe a neuropeptide regulatory loop involving insulin and sNPF that mediates starvation-regulated enhancement of food search behavior via facilitation of presynaptic calcium influx in olfactory neurons.
- 34.Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, Jung SA, Kim AK, You KH, Tatar M, Yu K. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- 35. Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–621. doi: 10.1038/nn.2526. This paper shows that the AWC food-sensing neurons release NLP-1 which acts on the NPR-11 receptor in the AIA interneurons to modulate INS-1 insulin release. INS-1 in turn feeds back to dampen AWC olfactory responses and alter food-seeking behaviors.
- 36.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Transact R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 37.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. Dissecting a neural circuit for food-seeking behavior in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- 38.Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans . Proc Natl Acad Sci USA. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinke I, Schutz CS, Katzenberger JD, Bauer M, Pankratz MJ. Nutrient control of gene expression in Drosophila: Microarray analysis of starvation and sugar-dependent response. EMBO J. 2002;21:6162–6173. doi: 10.1093/emboj/cdf600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujikawa K, Takahashi A, Nishimura A, Itoh M, Takano-Shimizu T, Ozaki M. Characteristics of genes up-regulated and down-regulated after 24 h starvation in the head of Drosophila. Gene. 2009;446:11–17. doi: 10.1016/j.gene.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Sokolovic M, Sokolovic A, Wehkamp D, Ver Loren van Themaat E, de Waart DR, Gilhuijs-Pederson LA, Nikolsky Y, van Kampen AH, Hakvoort TB, Lamers WH. The transcriptomic signature of fasting murine liver. BMC Genomics. 2008;9:528. doi: 10.1186/1471-2164-9-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jovanovic Z, Tung YC, Lam BY, O'Rahilly S, Yeo GS. Identification of the global transcriptomic response of the hypothalamic arcuate nucleus to fasting and leptin. J Neuroendocrinol. 2010;22:915–925. doi: 10.1111/j.1365-2826.2010.02026.x. [DOI] [PubMed] [Google Scholar]
- 43.Farhadian SF, Suarez-Farinas M, Cho CE, Pellegrino M, Vosshall LB. Post-fasting olfactory, transcriptional, and feeding responses in Drosophila. Physiol Behav. 2012;105:544–553. doi: 10.1016/j.physbeh.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 45. Ezcurra M, Tanizawa Y, Swoboda P, Schafer WR. Food sensitizes C. elegans avoidance behaviours through acute dopamine signalling. EMBO J. 2011 doi: 10.1038/emboj.2011.22. Avoidance of copper and glycerol is mediated by the ASH polymodal sensory neurons; this aversion response is enhanced in the presence of food. The authors show that dopamine released by the CEP mechanosensory neurons in the presence of food acts directly on ASH to increase stimulus-evoked calcium influx and enhance aversive responses.
- 46. Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci USA. 2004;101:15512–15517. doi: 10.1073/pnas.0403369101. Avoidance of low concentrations of the aversive chemical 1-octanol is enhanced in the presence of food. This work demonstrates that this increased avoidance is mediated by the ASH sensory neurons and serotonin. The study also shows that different sets of chemosensory neurons are recruited to mediate avoidance in the presence or absence of food.
- 47.Ezak MJ, Ferkey DM. The C. elegans D2-like dopamine receptor DOP-3 decreases behavioral sensitivity to the olfactory stimulus 1-octanol. PLoS ONE. 2010;5:e9487. doi: 10.1371/journal.pone.0009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wragg RT, Hapiak V, Miller SB, Harris GP, Gray J, Komuniecki PR, Komuniecki RW. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci. 2007;27:13402–13412. doi: 10.1523/JNEUROSCI.3495-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 50. Gaudry Q, Kristan WB., Jr Behavioral choice by presynaptic inhibition of tactile sensory terminals. Nat Neurosci. 2009;12:1450–1457. doi: 10.1038/nn.2400. Leech do not respond to mechanical stimuli when feeding. This study shows that food suppresses synaptic transmission from a group of pressure-sensitive mechanosensory neurons via serotonergic modulation to decrease tactile responses.
- 51.Harris GP, Hapiak VM, Wragg RT, Miller SB, Hughes LJ, Hobson RJ, Steven R, Bamber B, Komuniecki RW. Three distinct amine receptors operating at different levels within the locomotory circuit are each essential for the serotonergic modulation of chemosensation in Caenorhabditis elegans. J Neurosci. 2009;29:1446–1456. doi: 10.1523/JNEUROSCI.4585-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris G, Mills H, Wragg R, Hapiak V, Castelletto M, Korchnak A, Komuniecki RW. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J Neurosci. 2010;30:7889–7899. doi: 10.1523/JNEUROSCI.0497-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mills H, Wragg R, Hapiak V, Castelletto M, Zahratka J, Harris G, Summers P, Korchnak A, Law W, Bamber B, Komuniecki R. Monoamines and neuropeptides interact to inhibit aversive behaviour in Caenorhabditis elegans. EMBO J. 2012;31:667–678. doi: 10.1038/emboj.2011.422. This study describes the complex and distributed chemosensory neuron and neuropeptidergic signaling network that regulates octanol avoidance behavior in the presence or absence of food.
- 54.Ardiel EL, Rankin CH. An elegant mind: Learning and memory in Caenorhabditis elegans. Learn Mem. 2010;17:191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- 55.Busto GU, Cervantes-Sandoval I, Davis RL. Olfactory learning in Drosophila. Physiology (Bethesda) 2010;25:338–346. doi: 10.1152/physiol.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oda S, Tomioka M, Iino Y. Neuronal plasticity regulated by the insulin-like signaling pathway underlies salt chemotaxis learning in Caenorhabditis elegans. J Neurophysiol. 2011;106:301–308. doi: 10.1152/jn.01029.2010. Preexposure of C. elegans to salt in the absence of food switches the normally attractive salt response to avoidance. This study together with ••58 below describe the physiological and genetic mechanisms by which insulin signaling from the AIA interneurons alters ASER gustatory neuron responses to salt to mediate this behavioral plasticity.
- 57.Saeki S, Yamamoto M, Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol. 2001;204:1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- 58. Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, Iino Y. The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron. 2006;51:613–625. doi: 10.1016/j.neuron.2006.07.024. See comment associated with ••56 above.