Abstract
Past research has shown that when given a simultaneous visual-discrimination midsession reversal task, pigeons typically anticipate the reversal well before it occurs and perseverate after it occurs. It appears that they use the estimation of time (or trial number) into the session rather than (or in addition to) the more reliable cue, the outcome from the previous trial (i.e., a win-stay/lose-shift response rule), to determine which stimulus they should choose. In the present research, we investigated several variables that we thought might encourage the pigeons to use a more efficient response strategy. In Experiment 1, we used a treadle stepping response rather than key pecking to test the hypothesis that reflexive key pecking may have biased the pigeons to estimate the time (or trial number) into the session at which the reversal would occur. In Experiment 2 we attempted to make the point of reversal in the session more salient by inserting irrelevant trials with stimuli different from the original discriminative stimuli. and for a separate group, we added a 5 s time-out penalty following incorrect choices. The use of a treadle stepping response did not improve reversal performance and although we found some improvement in reversal performance when the reversal was signaled and when errors resulted in a time out, we found little evidence for performance that approached the win-stay/lose-shift accuracy shown by rats.
Keywords: reversal learning, simultaneous discrimination, spatial discrimination, visual discrimination, win-stay/lose-shift, anticipatory errors, perseverative errors, pigeons
Behavioral flexibility allows organisms to adapt to environmental change quickly while avoiding repeated negative consequences resulting from the use of obsolete strategies. When humans are exposed to a serial reversal task in which the valences of the stimuli in a simultaneous discrimination change abruptly, we often quickly see the development of what might be described as a win-stay/lose-shift response rule. Specifically, following the first instance in which a choice is not reinforced, humans will learn to choose the previously nonreinforced alternative. This type of cognitive flexibility, or ability to change behavior in accordance with changes in the environment, has been suggested to be positively correlated with intelligence (Bitterman, 1965)
Early work investigating behavioral flexibility used reversal learning tasks in which a subject is given a simultaneous discrimination in which responses to a positive stimulus (S+) are reinforced and responses to a negative stimulus (S−) are not. At a point determined by an acquisition criterion or number of training trials the discrimination is reversed (the S+ becomes the S− and the S− becomes the S+). How quickly the subjects learn to respond consistently to the S− following the reversal and the degree to which the number of errors per reversal decrease with successive reversals has been taken as a measure of behavioral flexibility (Bitterman, 1965). Differences found among species in the degree of improvement with successive reversals have been taken as a measure of flexibility (Bitterman & Mackintosh, 1969; Mackintosh, 1969).
The logic involved in the use of improvement in serial reversal learning with increasing reversals as a measure of behavioral flexibility is that it measures the improvement in reversal performance relative to the degree of difficulty of the original discrimination. That is, it controls for the difficulty of the original discrimination which may depend on the sensory apparatus of the species (e.g., pigeons are much more visual than rats). However, there is evidence that procedural variables can affect not only the degree of difficulty of the original discrimination but also the improvement in reversal learning with successive reversals (Warren, 1965).
In a variation of the serial reversal procedure, each session begins with a consistent S+ and S− and the reversal occurs halfway through the session (Rayburn-Reeves, Stagner, Kirk, & Zentall, 2011; see also Cook & Rosen, 2010). The question asked is how animals deal with the fact that the reversal occurs at a predictable point in each session. Interestingly, when this task has been used with pigeons, they begin to anticipate the reversal well before it occurs and once the reversal occurs, they typically continue to perseverate by choosing the former S+.
Rayburn-Reeves et al. (2011) reasoned that the fact that the reversal occurred at the midpoint of the session may have encouraged the pigeons use the passage of time from the start of the session as a cue to reverse (although not a very efficient cue) rather than the feedback from the first error to the original S+. To discourage pigeons from timing, they varied the point of reversal within a session, randomly across sessions. Results indicated that although the pigeons had no training with the reversal exclusively at the midpoint of the session and now the point of the reversal was much less predictable, it appeared that the pigeons continued to use the passage of time as the primary cue to reverse, resulting in still poorer task accuracy. Specifically, when the reversal point occurred early in a test session, the pigeons committed few anticipatory errors but they committed many more perseverative errors. But when the reversal occurred late in a test session, the pigeons committed many more anticipatory errors but few perseverative errors. It appeared as if the pigeons were averaging over all of the reversal points experienced and continued to use a time into the session as a cue to reverse. To make the pigeons’ choice more salient and memorable to them, Rayburn-Reeves, Molet, and Zentall (2011) required pigeons to indicate their choice of stimulus by pecking it 20 times but they found no improvement in either anticipatory or perseverative errors.
Even more interesting, perhaps is the fact that rats are much more efficient at this task than pigeons (Rayburn-Reeves, Stagner, Kirk, & Zentall, in press). When given a spatial version of this task (e.g., pressing the left lever but not the right lever provides a pellet of food for the first 40 trials of each session and then pressing the right lever but not the left lever provides a pellet for the remaining 40 trials). The rats show no evidence of using the passage of time as a cue to reversal and they approach a win-stay/lose-shift response strategy, showing no anticipatory responding and virtually no perseverative responding. Furthermore, the rats transferred readily to varying the point of the reversal within the session, making it unpredictable and after very few sessions to adjust, they transferred to two and then three reversals per session.
One might hypothesize that the spatial discrimination and reversal for the rats was easier to acquire than the visual (color) discrimination and reversal for the pigeons and that difference could have accounted for the species difference. However, when pigeons were trained on a spatial discrimination and reversal, they did no better than the pigeons that were trained on the visual discrimination (Rayburn-Reeves et al., in press).
The purpose of the present experiments was to examine several variables that might encourage pigeons to use the feedback from the preceding trial as a choice cue and rely less on the passage of time as a cue to determine when to reverse their choice. The first variable that we examined was the effect of the difference between the pigeons’ key peck and the rats’ lever press. That is, there is evidence that the pigeon’s key peck response consists of two components, an operant component and a Pavlovian component (Gamzu & Schwartz, 1973). The Pavlovian component is elicited by the signaling value of the stimulus (pecking that occurs with an autoshaping procedure, Brown & Jenkins, 1968). In the rats lever pressing response no such Pavlovian component leading to lever pressing has been found. It may be that those Pavlovian pecks are not as sensitive to the outcome of the preceding trial as are the operant pecks. If so, the difference between rats and pigeons may be in the nature of the response that they make (i.e., the difference between making the response with the beak for pigeons and with the paw for rats). In Experiment 1 we asked if the pigeons’ response consisted of stepping on a treadle, rather than pecking a response key, would it result in a different pattern of anticipatory and perseverative errors.
In Experiment 2 we asked if an irrelevant salient event that signaled the reversal would alter the pattern of anticipatory and perseverative errors. To help answer this question we added four simultaneous discrimination trials involving colors different from the original colors involved in the original discrimination and the reversal. The appearance of trials involving different colors could serve as a signal that the reversal was about to occur and they could have an effect not only on perseverative errors but also on anticipatory errors. They possibly could also encourage the development of a win-stay/lose-shift response rule.
In Experiment 2, we also asked if making errors a bit more aversive would alter the pattern of errors. To make the errors more aversive, a single peck to the incorrect color resulted in the offset of the correct color while the incorrect color remained on for an additional 5 s (a time-out period).
Experiment 1
The purpose of Experiment 1 was to make the method used to test pigeons more similar to that used with rats by Rayburn-Reeves et al. (2011) to determine if the pecking response for pigeons was responsible for the use of temporal cues rather than the outcome from the preceding trial as a cue to switch from choosing one discriminative stimulus to the other. Thus, in Experiment1, we gave pigeons a spatial reversal learning task that required them to step on either a left or right treadle, rather than to peck at a left or right response key, to make their response.
Method
Subjects
Eight White Carneaux pigeons (Columba livia) ranging in age from 2 to 12 years served as subjects. All subjects had been given experience in a previous study involving a simultaneous color discrimination but they had never been exposed to a discrimination reversal procedure. Subjects were maintained at 85% of their free-feeding weight throughout the experiment, and were individually housed in wire cages with free access to water and grit in a colony room that was maintained on a 12-hr/12-hr light/dark cycle. The pigeons were maintained in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Apparatus
The experiment was conducted in an operant chamber (Coulbourn Instruments, Lehigh Valley, PA) measuring 25.7 cm across the response panel, 33 cm from ceiling to floor, and 31 cm from response panel to the back wall. The chamber had a white houselight, centered on the response panel and located 1.3 cm from the ceiling. A pellet dispenser delivered pellets (45 mg grain-based pigeon pellets, Bioserv, Frenchtown, NJ) to a food well that was centered on the response panel, 5.6 cm from the floor. Two response treadles, 5.08 cm wide and 5.08 cm deep, were located on either side of the food well, located 5.08 cm from the side walls, respectively, and 0.64 cm from the floor. The experimental chamber was located in a small isolated room to reduce extraneous visual and auditory stimulation. The experiment was controlled by a microcomputer and interface located in an adjacent room.
Procedure
Pigeons were initially shaped to step on each treadle by the method of successive approximation. At the start of each experimental session, the house light was illuminated, indicating that both treadles were operable. For half of the subjects, a single response to the left treadle (S1) resulted in the feeder light turning on and a single pellet being delivered to the food well. After 2 s, both the feeder light and house light turned off for a 3-s dark intertrial interval (ITI). If the pigeon chose the right treadle (S2), the house light turned off for a 5-s dark ITI and no food was delivered. Immediately following the ITI, the house light turned on indicating the start of the next trial. After 40 trials, the contingencies were reversed for the last 40 trials such that responses to S2 were reinforced and responses to S1 were no longer reinforced. For the other half of the subjects, choice of the right treadle (S1) and not the left (S2) was reinforced for the first half of each session. Subjects were trained for 50 sessions.
Results and Discussion
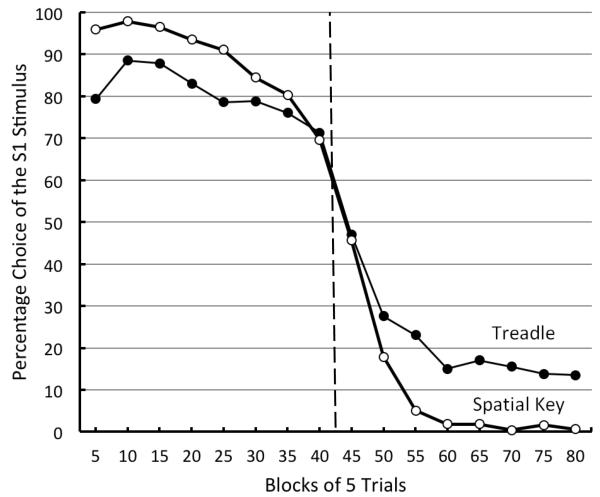
Pigeons reached stable choice accuracy in about 30 sessions of training. Asymptotic performance for Sessions 41-50 can be viewed in Figure 1. Also appearing in Figure 1 are the results of spatial discrimination reversal task using key pecking (from Rayburn-Reeves et al., 2011). In Figure 1, it can be seen that pigeons in Experiment 1 did not perform as well as those in the Rayburn-Reeves et al. (2011) procedure. Relative to pigeons in the spatial key-peck discrimination task, pigeons in the present treadle response study made more anticipatory errors during the first half of Sessions 41-50, and more perseverative errors during the last half of those sessions. Overall, for Sessions 41-50, pigeons in the present experiment were 79.4% correct whereas those in Rayburn-Reeves et al. were at 89.6% correct, t(16) = 3.49, p = .003. In the present experiment, pigeons were choosing S2 before it was correct over 28.8% of the time (Trials 36-40) and they continued to choose S1 53.0% of the time after the reversal (Trials 41-45).
Figure 1.
Experiment 1. Percentage choice of S1 as a function of 5-trial block number averaged over pigeons for Sessions 41-50 (solid circles) compared with spatial reversal data (open circles) from Rayburn-Reeves et al. (in press) . The dotted line indicates the point at which the reversal occurred in the session.
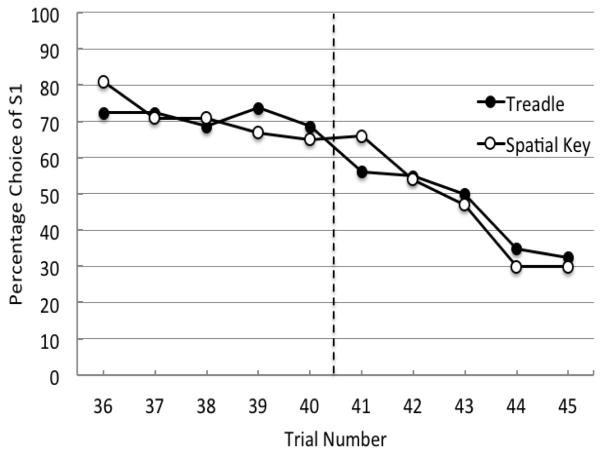
A more detailed presentation of the data appears in Figure 2 in which trial by trial data from the five trials before the reversal (Trials 36-40) and five trials after the reversal (Trials 41-45) are presented for the same data that appear in Figure 1. On Trial 41, the first reversal trial and the first trial which provided feedback about the reversal, pigeons were choosing the previously correct stimulus 56.2% of the time. On Trial 42, the first trial following feedback that the reversal had occurred, pigeons were still choosing the previously correct stimulus 55.0% of the time. Thus, there was very little effect of the feedback from the first reversal trial.
Figure 2.
Experiment 1. Percentage choice of the first correct stimulus (S1) as a function of trial number for trials 36-45, averaged over subjects, for Sessions 41-50 (closed circles) compared with spatial reversal data (open circles) from Rayburn-Reeves et al. (in press). The dotted line indicates the point at which the reversal occurred in the session.
The results of Experiment 1 indicate that having the pigeons respond by stepping on a treadle rather than pecking a response key did not improve choice accuracy on the midsession spatial reversal. In fact, judging from the overall error rate the pigeons had more trouble with the treadle discrimination reversal then with the spatial key reversal.
Experiment 2
As the use of spatial discriminations did not improve reversal accuracy either in Rayburn-Reeves et al. (in press) or in Experiment 1 of the present research, in Experiment 2, we returned to a simultaneous color discrimination reversal procedure. In Experiment 2, in an effort to make the point of the reversal more salient, for one group (irrelevant trials), we inserted four discrimination trials involving a blue/yellow discrimination unrelated to the red/green discrimination used in the first 40 and last 40 trials. Our rationale for inserting irrelevant trials at the point of the reversal is that it might help to signal the reversal and thus, facilitate detection of the change in contingency. Thus, we expected that the insertion of irrelevant trials might reduce the number of perseverative errors. We were also interested in whether the expectation of the appearance of irrelevant trials might reduce the number of anticipatory errors. If the pigeons learn that an irrelevant stimulus discrimination would be presented, they may forgo using the passage of time as a cue and wait for the irrelevant trials to stop choosing S1 and begin choosing S2.
One reason that in previous research with this midsession reversal procedure pigeons continued to use the passage of time as a cue is that the consequences of making an error may not have been sufficiently nonrewarding to discourage errors. With this procedure, errors merely result in termination of the stimuli and a 5-s ITI, prior to the start of the next trial. Previous research with matching-to-sample procedures has shown that if comparison choice errors result in maintaining the stimulus display for several seconds (a form of added time out), acquisition of matching can be facilitated (Strength & Zentall, 1991; Martin & Zentall, 2005). For this reason, in Experiment 2, we added a group for which there was mild negative punishment for errors (the failure of the S− stimulus to turn off for a limited time). Our hypothesis was that this procedure might encourage the pigeons either to be more careful in making their choices or to review incorrect choices after they were made and learn to rely more on their memory for the previous stimulus selected and the outcome obtained as a cue to reverse, rather than on the time from the start of the session. In addition, adding a time out for making errors should make the duration of the trials more variable and thus, should make it more difficult for the pigeons to use the total time from the start of the session to the reversal as a cue to reverse. Thus, in Experiment 2, for the time-out group we added a 5-s time out following each incorrect choice. During the time out the correct stimulus was turned off and the incorrect stimulus remained on for 5 s.
Method
Subjects
Twenty-one White Carneaux pigeons (Columba livia) similar in age and experience to those used in Experiment 1 served as subjects. They were all treated as were the pigeons in Experiment 1.
Apparatus
A standard (LVE/BRS, Laurel, Md.) test chamber was used, with inside measurements 35 cm high, 30 cm long, and 35 cm across the response panel. The response panel in the chamber had a horizontal row of three response keys, 25 cm above the floor. The rectangular keys (2.5 cm high × 3.0 cm wide) were separated from each other by 1.0 cm and behind each key was a 12-stimulus inline projector (Industrial Electronics Engineering, Van Nuys, Calif.) that projected red, yellow, blue, and green (Kodak Wratten Filter Nos. 26, 9, 38, and 60, respectively). In the chamber, the bottom of the center-mounted feeder (filled with Purina Pro Grains) was 9.5 cm from the floor. When the feeder was raised, it was illuminated by a 28 V, 0.04 A lamp. A 28 V 0.1 A houselight was centered above the response panel and an exhaust fan was mounted on the outside of the chamber to mask extraneous noise. A microcomputer in the adjacent room controlled the experiment.
Procedure
For pigeons in the control group (n = 7), red and green hues were illuminated on the left and right response keys randomly from trial to trial to indicate the beginning of a trial. For half of the subjects, a response to the red key (S1) turned off both keys and resulted in 1.5-s access to food, followed by a 3.5-s dark intertrial interval (ITI), whereas a response to green (S2) immediately turned off both stimuli and resulted in a 5-s dark ITI. For the other half of the subjects, choice of the green key (S1) was reinforced not red key (S2). For the first 40 trials of each session, all subjects were trained with S1+/S2−. For Trials 41 to 80, the contingencies were reversed (S2+/S1−).
Subjects in the irrelevant-trials group (n = 8), were treated similarly to pigeons in the control group with the following exception: following Trial 40, there were four irrelevant trials in which blue and yellow hues were randomly presented on the left and right keys and a response to yellow was always reinforced. Following the four irrelevant blue/yellow discrimination trials, the same red/green reversal contingency was in effect as it was for the control group (S2+/S1−) for trials 41-80 of the red-green discrimination.
Subjects in the time out group (n = 6) were treated similarly to pigeons in the control group with the following exception: following an error, the correct stimulus was turned off and the incorrect stimulus remained on for 5 s, after which the incorrect stimulus was turned off and a 5-s ITI began. All subjects were trained for 80 sessions.
Results and Discussion
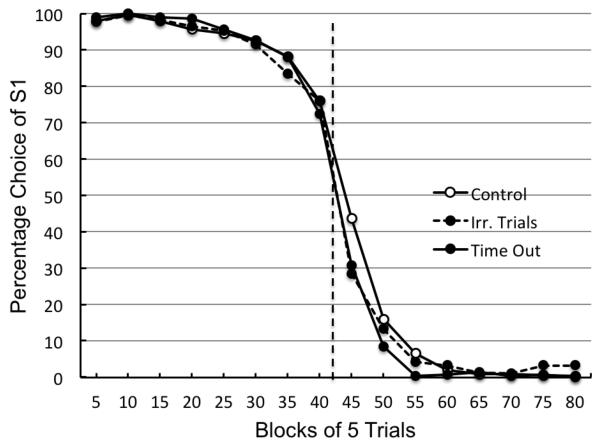
In Experiment 2, the control group performed much like pigeons in the reversal procedure conducted by Rayburn-Reeves et al. (2011). The percentage choice of S1 as a function of block number, pooled over Sessions 71-80, is plotted in Figure 3 in five trial blocks, for all three groups. On Trials 36-40 (the last trials prior to the reversal), the control group chose S1 76% of the time and on Trials 41-45 (the first trials after the reversal) they chose S1 43.7% of the time. The irrelevant trials group chose S1 76% of the time on Trials 36-40 (same as the controls) and they chose S1 28.5% of the time on Trials 41-45, a 15.2% statistically significant difference from controls, t(13) = 3.0, p = .01. The time out group chose S1 72% of the time on Trials 36-40 and 30.7% on trials 41-45, 13% better than the controls but the difference was not quite significant, t(11) = 2.12, p = .06.
Figure 3.
Experiment 2. Percentage choice of S1 as a function of block number averaged over pigeons in both the control (open circles), time out group (solid circles) and irrelevant trials group (solid circles, dashed line) for Sessions 71-80. The dotted line indicates the point at which the reversal occurred in the session.
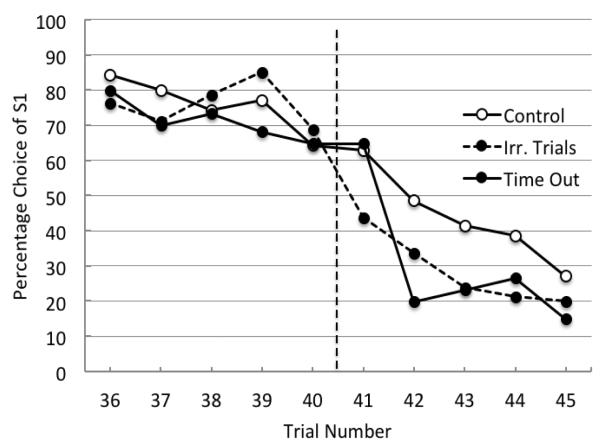
Examination of trial by trial data for the red-green discrimination (Trials 36-45) provides a better comparison of the difference in performance by the groups (see Figure 4). On Trial 41, the first trial of the reversal, choice of S1 for the control group was 62.9% and 65% for the time out group, whereas the irrelevant trial group was choosing S1 only 43.8% of the time. The difference between the control group and the irrelevant-trial group on Trial 41 was statistically significant, t(19) = 2.63, p = .016. Thus, the effect of the four irrelevant trials was to serve as an effective signal for the reversal. When errors in choice of S1 were pooled over the first four postreversal trials the difference between the irrelevant-trial group (43.9%) and control group (37.8%) remained, t(13) = 3.23, p = .007. Thus, the effect of the irrelevant trials was to signal the reversal and they continued to have an effect on errors.
As expected, the time-out effect had little effect on anticipatory errors but it did influence the effect of Trial 41, the first reversal trial, on Trial 42 performance. Importantly, on Trial 42, there was a significant difference in choice of S1 between the time-out group (20.0%) and the control group (48.6%), t(11) = 3.17, p =.009. Thus, although the time-out group could not predict when the reversal would occur, it did show a remarkable 45.0% decline in choice of S1to the first reversal trial.
General Discussion
Pigeons given considerable experience with a midsession task show a surprising pattern of responding. They make an increasing number of anticipatory errors as the reversal approaches and they continue to make perseverative errors once the reversal has occurred. The pattern of errors suggests that the pigeons appear to be timing the occurrence of the reversal rather than (or in addition to) using the feedback (reinforcement and its absence) from the preceding trial(s) as a cue to reverse. Interestingly, when the point of the reversal was made variable such that timing the point of the reversal would be even less efficient, pigeons continued to use the passage of time as a cue to reverse (Rayburn-Reeves et al., in press). These results are even more surprising given the fact that rats show a very different and more efficient human-like win-stay/lose-shift response strategy (Rayburn-Reeves et al., in press). The purpose of the present experiments was to examine several variables that might influence the reversal performance of pigeons on this midsession reversal task.
The first variable considered was the difference in response topography used by pigeons and rats. Pigeons use their beak to respond (their primary means of eating), whereas rats use their paw. We tested the hypothesis that pigeons may be able to make more efficient choices if they were required to make a response that had less of a Pavlovian component (e.g., treadle stepping). In Experiment 1 we found no evidence that the pigeons were more effective in using their choice and its outcome on the most recent trial(s) as a basis of their choice on the next trial when the required response was treadle stepping than it was when the required response was key pecking.
In Experiment 2, we signaled the reversal for one group by presenting the pigeons with four trials involving an irrelevant discrimination. Relative to a control group that did not receive those irrelevant trials, the four irrelevant trials resulted in fewer perseverative errors on the first reversal trial and the benefit of those irrelevant trials persisted for several additional trials.
In Experiment 2, we also tried to make errors more salient and perhaps more aversive by extending the trial by 5 s following an error. The effect of extending the trial following an error significantly improved the pigeons’ ability to use the feedback from the first reversal trial as a cue to reverse.
In the present research we have referred to the pigeons’ use of time into the session as the cue that they use to determine when to reverse their choice but we have acknowledged as well that they may have used an estimation of the number of trials experienced to decide when to reverse. One way to distinguish between these two alternatives would be to train the pigeons with a 5-s ITI and then test them with longer and shorter ITIs. If the pigeons were using time into the session as a cue to reverse, anticipation errors should occur earlier in the session when the test ITIs were shorter and they should occur later in the session when the test ITIs were longer. Alternatively, if the pigeons were estimating the number of trials into the session on which to base their decision to reverse, manipulation of the duration of the ITI should have little effect on where in the session anticipation error appear.
It has been suggested that better performance on reversal tasks is a measure of the intelligence of the species (Bitterman, 1965), but it is likely that there are other contributing factors associated with reversal learning. Perhaps the reduced sensitivity to the outcome of a preceding trial by pigeons is related to their foraging ecology. Pigeons often travel quite far to find a patch of food (e.g., a field of grain) but it is likely that the patch will not be quickly depleted, so returning to that patch may be a predisposed behavior. This tendency together with a tendency toward neophobia may make pigeons predisposed to stay rather than switch (see, e.g., Zentall, Steirn, & Jackson-Smith, 1990). Rats, on the other hand, tend to forage in smaller patches that deplete faster and thus, they may be predisposed to shift (see, e.g., Olton & Samuelson, 1976). The predisposition to stay within a large patch may also have resulted in a tendency for pigeons to be less sensitive to nonreinforcement than rats. It is likely that if a pigeon is unable to find food in a particular patch for a short time it may not be sufficient grounds to move to a new patch. Thus, relative to rats, pigeons may be predisposed to accumulate further evidence for the depletion of a patch before moving to a new patch. Such predispositions could account for the more rapid switching behavior by rats than pigeons, leading to faster reversal learning, but it would not account for the anticipatory errors made by pigeons with the present midsession reversal task. It may be, however, that the relative insensitivity to nonreinforcement has led pigeons to use other cues to determine when to switch to the alternative discriminative stimulus. It appears that pigeons first learn that one alternative is an effective source of food early in a session, whereas the other alternative is an effective source of food late in a session. This initial learning may encourage the pigeons to use the passage of time as the basis for when to switch. And even though it may not be the most efficient strategy, it works well enough to maintain it.
An alternative hypothesis for why pigeons do not perform more optimally with this task, even with salient cues identifying the reversal and with a time out following an error as in Experiment 2 of the present study, is that they have a problem remembering not only the results of their choice (reinforcement or its absence) but also the stimulus that they chose. That is, in a sense, this task can be thought of as a delayed biconditional matching task, with the sample being the successive compound consisting of the stimulus chosen on the preceding trial and the outcome resulting from the choice, the 5-s ITI being the delay, and the choice on the following trial being the comparison choice. If the pigeon makes an error, it must remember what it did and what was the result of its choice over the 5-s ITI. If the pigeon’s problem is one of memory for the prior stimulus chosen and the outcome (i.e., the sample), if one shortened the ITI one should find that more optimal performance would result.
Of course, it may be simpler to attribute perseverative S1 errors to within-session interference from the previous S1−reinforced trials. And although one might hypothesize that anticipatory S2 errors could be attributed to inter-session interference from the last 40 (S2− reinforced) trials from the previous session, one would expect those errors to come earlier in the following session, rather than as the pigeons approached the end of the S1−reinforced trials. Instead, it appears that the pigeons were estimating the time to the reversal point in the session. It may be, however, that errors made shortly before the midpoint of the session contributed to perseverative errors because, as noted earlier, the pigeons would have had to have remembered not only the stimulus that had been selected but also the consequences (reinforcement or its absence) of having selected that stimulus if the preceding trial would be useful as a cue to guide choice on the current trial. In any case, if the determinants of this suboptimal performance by pigeons can be identified, it should lead to be better understanding of their natural predisposed behavior as well as the pigeon’s flexibility in dealing with this form of reversal learning.
Figure 4.
Experiment 2. Percentage choice of S1 as a function of trial number for trials 36-45 averaged over pigeons in both the control (open circles), time out group (solid circles) and irrelevant trials group (solid circles, dashed line) for Sessions 71-80. The dotted line indicates the point at which the reversal occurred in the session.
Acknowledgments
This research was supported by National Institute of Mental Health Grant 63726 and by National Institute of Child Health and Development Grant 60996.
References
- Bitterman ME. Phyletic differences in learning. American Psychologist. 1965;20:396–410. doi: 10.1037/h0022328. [DOI] [PubMed] [Google Scholar]
- Bitterman ME, Mackintosh NJ. Habit-reversal and probability-learning: Rats, birds and fish. In: Gilbert RM, Southerland NS, editors. Animal Discrimination Learning. Academic Press; New York: 1969. pp. 163–185. [Google Scholar]
- Brown P, Jenkins H. Autoshaping of the pigeon’s key peck. Journal of the Experimental Analysis of Behavior. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RG, Rosen HA. Temporal control of internal states in pigeons. Psychonomic Bulletin & Review. 2010;17:915–922. doi: 10.3758/PBR.17.6.915. [DOI] [PubMed] [Google Scholar]
- Gamzu E, Schwartz B. The maintenance of key pecking by stimulus-contingent and response-independent food presentation. Journal of the Experimental Analysis of Behavior. 1973;19:65–72. doi: 10.1901/jeab.1973.19-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. Comparative studies of reversal and probability learning: Rats, birds, and fish. In: Gilbert RM, Southerland NS, editors. Animal Discrimination Learning. Academic Press; New York: 1969. pp. 137–162. [Google Scholar]
- Martin TI, Zentall TR. Post-choice information processing by pigeons. Animal Cognition. 2005;8:273–278. doi: 10.1007/s10071-005-0254-2. [DOI] [PubMed] [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of places passed: Spatial memory in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:97–116. [Google Scholar]
- Rayburn-Reeves RM, Molet M, Zentall TR. Simultaneous discrimination reversal learning in pigeons and humans: anticipatory and perseverative errors. Learning & Behavior. 2011;39:125–137. doi: 10.3758/s13420-010-0011-5. [DOI] [PubMed] [Google Scholar]
- Rayburn-Reeves RM, Stagner JP, Kirk CR, Zentall TR. Reversal learning in rats (rattus norvegicus) and pigeons (columba livia): Qualitative differences in behavioral flexibility. Journal of Comparative Psychology. doi: 10.1037/a0026311. in press. [DOI] [PubMed] [Google Scholar]
- Strength V, Zentall TR. Matching and oddity learning in pigeons: Effects of penalty time for incorrect responding. Animal Learning & Behavior. 1991;19:49–57. [Google Scholar]
- Warren JM. The comparative psychology of learning. Annual Review of Psychology. 1965;16:95–118. doi: 10.1146/annurev.ps.16.020165.000523. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Steirn JN, Jackson-Smith P. Memory strategies in pigeons’ performance of a radial-arm-maze analog task. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:358–371. [Google Scholar]