Abstract
Aims
Whether long-term cardiovascular risk is reduced by the Diabetes Prevention Program interventions is unknown. The aim of this study was to determine the long-term differences in cardiovascular disease risk factors and the use of lipid and blood pressure medications by the original Diabetes Prevention Program intervention group.
Methods
This long-term follow-up (median 10 years, interquartile range 9.0–10.5) of the three-arm Diabetes Prevention Program randomized controlled clinical trial (metformin, intensive lifestyle and placebo), performed on 2766 (88%) of the Diabetes Prevention Program participants (who originally had impaired glucose tolerance), comprised a mean of 3.2 years of randomized treatment, approximately 1-year transition (during which all participants were offered intensive lifestyle intervention) and 5 years follow-up (Diabetes Prevention Program Outcomes Study). During the study, participants were followed in their original groups with their clinical care being provided by practitioners outside the research setting. The study determined lipoprotein profiles and blood pressure and medication use annually.
Results
After 10 years’ follow-up from Diabetes Prevention Program baseline, major reductions were seen for systolic (2–3 mmHg) and diastolic (5–6 mmHg) blood pressure, and for LDL cholesterol (0.47–0.54 mmol/l) and triglycerides (0.18–0.32 mmol/l) in all groups, with no between-group differences. HDL cholesterol also rose significantly (0.13–0.16 mmol/l) in all groups. Lipid (P < 0.012) and blood pressure (P < 0.09) medication use, however, were lower for the lifestyle group during the Diabetes Prevention Program Outcomes Study.
Conclusion
Overall, intensive lifestyle intervention achieved, with less medication, a comparable long-term effect on cardiovascular disease risk factors, to that seen in the metformin and placebo groups.
(Clinical Trials Registry No; NCT 00038727).
Keywords: blood pressure, diabetes prevention, lifestyle, lipids, metformin, statins
Introduction
Impaired glucose tolerance carries an increased risk of cardiovascular disease [1] and diabetes [2]. While the Diabetes Prevention Program (DPP) in the USA [3] and similar programmes in China [4], Finland [5] and India [6] have clearly demonstrated that diabetes prevention or delay is possible, the impact of these interventions on cardiovascular risk is less clear. Although the DPP has shown a favourable short-term (mean 3.2 years) effect of lifestyle intervention on reducing cardiovascular risk factors [7] and the incidence of the metabolic syndrome [8], the durability of these benefits and their translation into reduced event rates is not established. The importance of preventing cardiovascular disease in those with early glucose intolerance is underscored by three trials documenting that, in those with diabetes, intensification of glycaemic control may not reduce cardiovascular events overall [9–11] and might even increase mortality [9]. Similarly, in one trial, neither intensification of blood pressure treatment [12] nor the use of a fibrate medication in addition to a statin medication [13] improved cardiovascular morbidity or mortality in those with diabetes.
There have not been sufficient cardiovascular disease events in the DPP Outcomes Study (DPPOS) [14] to permit examination by treatment group. Nonetheless, the potential reasons for the overall low event rates and any variation in risk status merits further examination and the DPPOS permits a number of pertinent analyses. Primarily, the additional 5 years of follow-up enable an assessment of the durability of the beneficial effect of lifestyle on cardiovascular risk factors over a 10-year period despite all groups now receiving a lifestyle intervention and whether the original lifestyle intervention leads to relatively less medication use. These current, more detailed, cardiovascular disease risk factor analyses thus supplement those already published [14], which show a durable 10-year effect of both metformin and intensive lifestyle assignment on diabetes incidence. We also explore the extent of statin use and its impact on lipid concentrations and any effect of diabetes status on the levels of blood pressure and lipid control.
Subjects and methods
Participants
Recruitment and randomization of DPP participants have been described previously [3,8]. DPPOS enrolment was initiated in September 2002 and largely completed by 27 August 2008, the closing date for this report, when 87.8% (2766) of the 3150 surviving DPP participants had enrolled [14]. Median follow-up from randomization to the most recent evaluation was 10.0 (interquartile range 9.0–10.5) years. Intervention in the DPP was delivered in three groups: (1) intensive lifestyle with weight loss goal of 7% of baseline weight through low-fat diet and exercise goal of 150 min per week; (2) metformin 850 mg twice daily and (3) placebo control.
Transition from the DPP to the DPPOS and changes in the interventions
After being informed of the main DPP results (and a washout study [15] for the placebo and metformin were then unmasked to their treatment assignments), all study participants were offered a group-administered version of the 16-session lifestyle curriculum, under a ’bridge’ protocol [16] conducted from January to July 2002, which was nearly identical in content to the DPP intensive lifestyle intervention, but individualized problem solving and behaviour change support were not provided. Attendances at one or more session were 57% (placebo), 58% (metformin), and 40% (intensive lifestyle intervention) [16].
The DPPOS protocol, initiated in September 2002, offered quarterly lifestyle sessions to all participants and an additional two group classes (BOOST) annually to DPP intensive lifestyle intervention participants. The original metformin group continued to receive unmasked metformin 850 mg twice daily, unless discontinued for safety reasons or the participant had developed diabetes with HbA1c ≥ 53 mmol/mol (7%), requiring management outside of the trial protocol.
Outcome assessment examinations continued on the same annual and semi-annual schedule as in the DPP. During the DPPOS, 56.8% of the metformin participants without diabetes took ≥ 80% of the prescribed metformin dose and 70.1% took metformin in any amount, whereas 3.0% of former placebo without diabetes and 1.2% of intensive lifestyle intervention participants without diabetes took metformin prescribed outside the study. Attendance at quarterly lifestyle sessions did not differ by former treatment group. Attendance at the BOOST sessions for the former intensive lifestyle intervention group averaged 17%.
Measurements
Outcomes for this report consist of blood pressure and lipoprotein concentrations and changes and the proportion of participants meeting diagnostic or treatment guidelines. Blood pressure was measured with a standard mercury manometer with the participant seated in a chair for 5 min prior to the first of two measures separated by 30 s, the mean of which was used for analysis. Mercury manometers were replaced in August 2006 with aneroid (Welch Allyn Tycos 767-series; XXXXX, XXXX, XX, XXXX) after careful assessment of comparability of readings [17]. Hypertension was defined as use of anti-hypertensive medications or systolic/diastolic blood pressure ≥ 140/90 for participants without diabetes or systolic/diastolic blood pressure ≥ 130/80 for those with diabetes.
The Central Biochemistry Laboratory (Northwest Lipid Research Laboratories, University of Washington, Seattle, WA, USA) performed all the laboratory analytical measurements. Total plasma cholesterol and triglycerides were determined enzymatically using methods standardized to the Centers for Disease Control and Prevention Reference Methods [18]. HDL cholesterol analysis was performed after precipitation of apo B-containing lipoproteins with dextran sulphate magnesium [19]. LDL cholesterol was calculated by the Friedewald equation [20]. Lipoprotein fractions were separated using preparative ultracentrifugation by β-quantification for triglycerides of 4.52 mmol/l or greater [20]. A non-equilibrium density-gradient ultracentrifugation method was used to characterize LDL floatation rate [22,23]. The laboratory is a CDC Reference Laboratory for lipids. The assay precision is monitored through the use of quality control materials with rigorously established acceptance/rejection criteria. Per cent mean bias for triglycerides and total cholesterol showed little change between 1999 and 2009, i.e. +0.23% and −3.21%, respectively, while for HDL cholesterol, the corresponding mean bias was +2.98 and −5.21% (see also Supporting Information, Appendix S1). Additionally, large batches of samples with low, medium and high lipid levels are analysed on a monthly basis to monitor for possible long-term drift. Dyslipidaemia is defined as having met any of the three criteria: triglyceride ≥ 1.7 mmol/l, LDL cholesterol ≥ 2.59 mmol/l or use of lipid-lowering medications.
Statistical analyses
Between-group comparisons were performed using the χ2-test of independence for qualitative variables and the ANOVA and Kruskal–Wallis test (as appropriate) for quantitative variables
For analyses of changes over time in quantitative measures, we used the normal errors longitudinal regression model [24] with adjustment for DPP baseline level. Interaction between treatment groups and time was assessed by including an interaction term in the model. We used R2 to assess the contribution of time-varying covariates to the observed increase in HDL [25]. For the analysis of anti hypertension and anti-dyslipidaemic drug use over time, we used generalized estimating equations with repeated measures over time [26] to model the percentage of participants taking medicines. Analyses were performed using SAS versions 8.1 and 9.1 (SAS Institute, Cary, NC, USA).
Although this follow-up study was not anticipated in the original DPP design, we present between-group comparisons of cardiovascular disease risk factors for the total follow-up, which consists of data from three phases of the study: (1) from DPP randomization through the masked phase, (2) the bridge period and (3) the DPPOS follow-up to 27 August 2008. While not independent of the ‘DPP-only’ and ’DPPOS-only’ analyses, these overall analyses were included to describe the cumulative experience of the cohort.
Results
DPPOS enrolment did not differ significantly by sex or treatment group, but differed by race/ethnicity, age, weight, BMI and diabetes status, being higher among American Indians, those who were older, had lower weight/BMI or had developed diabetes by 1 September 2002 and lower in Hispanic and Asian Americans. Mean age was 50.4 years at DPP baseline for DPPOS enrollees, 68% of whom were women. Table 1 shows the characteristics at key time points during the follow-up period, specifically, DPP baseline, the end of the DPP (i.e. the end of the masked phase) and years 1 and 5 of the DPPOS, which reflects observations after the bridge and after 4 years of group-implemented lifestyle sessions. After 10 years of follow-up (i.e. year 5 of the DPPOS) those in the lifestyle and metformin group retained lower weight, waist circumference and BMI values than those in the placebo group. Moreover, HbA1c and fasting glucose were lowest in the metformin group, while physical activity (P = 0.099) differed little across groups. Smoking, although low overall, was least in the lifestyle group and greatest in the placebo group.
Table 1.
Characteristics at baseline and key time points during follow-up by treatment group
| Characteristics | Visit§ | Placebo | Metformin | Lifestyle | P-value overall |
|---|---|---|---|---|---|
| Number of visits (% of enrolled) | Baseline | 932 | 924 | 910 | |
|
| |||||
| Last DPP annual | 930 (99.8%) | 924 (100%) | 908 (99.8%) | ||
|
| |||||
| DPPOS year 1 | 886 (95%) | 888 (96%) | 862 (95%) | ||
|
| |||||
| DPPOS year 5 | 848 (91%) | 844 (91%) | 827 (91%) | ||
| Weight (kg) | Baseline | 93.9 (92.7–95.2) | 94.0 (92.7–95.3) | 93.5 (92.2–94.8) | 0.851 |
|
| |||||
| Last DPP annual | 94.1 (93.6–94.5) | 92.0 (91.6–92.4) | 89.2 (88.8–89.7) | < 0.001*†‡ | |
|
| |||||
| DPPOS year 1 | 93.4 (92.9–93.9) | 91.6 (91.0–92.1) | 91.4 (90.9–92.0) | < 0.001*† | |
|
| |||||
| DPPOS year 5 | 93.2 (92.6–93.9) | 91.8 (91.2–92.5) | 91.8 (91.1–92.4) | 0.003*† | |
|
| |||||
| Waist (cm) | Baseline | 105.1 (104.2–106.0) | 104.7 (103.8–105.6) | 104.7 (103.8–105.6) | 0.805 |
|
| |||||
| Last DPP annual | 104.6 (104.1–105.0) | 103.1 (102.6–103.5) | 99.9 (99.5–100.4) | < 0.001*†‡ | |
|
| |||||
| DPPOS year 1 | 104.4 (103.8–104.9) | 103.3 (102.8–103.9) | 102.8 (102.2–103.3) | < 0.001† | |
|
| |||||
| DPPOS year 5 | 106.9 (106.3–107.5) | 105.7 (105.1–106.3) | 105.7 (105.1–106.3) | 0.008*† | |
|
| |||||
| BMI (kg/m2) | Baseline | 34.0 (33.6–34.5) | 33.8 (33.4–34.2) | 33.7 (33.3–34.1) | 0.490 |
|
| |||||
| Last DPP annual | 33.9 (33.8–34.1) | 33.2 (33.0–33.3) | 32.2 (32.0–32.4) | < 0.001*†‡ | |
|
| |||||
| DPPOS year 1 | 33.7(33.5–33.9) | 33.0 (32.8–33.2) | 33.0 (32.8–33.2) | < 0.001*† | |
|
| |||||
| DPPOS year 5 | 33.6 (33.4–33.9) | 33.1 (32.9–33.4) | 33.1 (32.9–33.3) | 0.002*† | |
|
| |||||
| HbA1c (mmol/mol) | Baseline | 41 (41–42) | 41 (41–42) | 41 (41–42) | 0.934 |
| HbA1c (%) | 5.92 (5.89–5.96) | 5.92 (5.89–5.95) | 5.92 (5.88–5.95) | ||
|
| |||||
| Last DPP annual | 43 (43–43) | 42 (42–42) | 41 (41–41) | < 0.001*†‡ | |
| 6.07 (6.05–6.10) | 5.97 (5.95–6.00) | 5.88 (5.86–5.91) | |||
|
| |||||
| DPPOS year 1 | 42 (42–43) | 41 (41–41) | 41 (41–41) | < 0.001*† | |
| 6.02 (5.98–6.05) | 5.90 (5.86–5.93) | 5.90 (5.87–5.94) | |||
|
| |||||
| DPPOS year 5 | 42 (41–42) | 40 (40–41) | 41 (40–42) | 0.006* | |
| 5.97 (5.92–6.03) | 5.85 (5.79–5.90) | 5.90 (5.85–5.96) | |||
|
| |||||
| Fasting glucose (mmol/l) | Baseline | 5.9 (5.9–6.0) | 5.9 (5.9–5.9) | 5.9 (5.9–5.9) | 0.512 |
|
| |||||
| Last DPP annual | 6.2 (6.2–6.3) | 5.9 (5.9–6.0) | 5.9 (5.9–6.0) | < 0.001*† | |
|
| |||||
| DPPOS year 1 | 6.2 (6.2–6.3) | 5.9 (5.9–6.0) | 6.0 (6.0–6.1) | < 0.001*† | |
|
| |||||
| DPPOS year 5 | 6.4 (6.3–6.5) | 6.1 (6.0–6.2) | 6.2 (6.1–6.3) | < 0.001* | |
|
| |||||
| Modified Activity Questionnaire— physical activity (met h/week) | Baseline | 17.4 (15.4–19.3) | 16.4 (15.0–17.8) | 15.5 (14.1–17.0) | 0.29 |
|
| |||||
| Last DPP annual | 17.0 (15.9–18.2) | 17.5 (16.4–18.6) | 21.5 (20.3–22.6) | < 0.001†‡ | |
|
| |||||
| DPPOS year 1 | 16.3 (15.1–17.5) | 17.1 (15.8–18.3) | 18.6 (17.3–19.8) | 0.04 | |
|
| |||||
| DPPOS year 5 | 15.6 (14.4–16.7) | 14.5 (13.3–15.6) | 16.3(15.1–17.5) | 0.10 | |
|
| |||||
| Smoking (%) | Baseline | 8.3 | 6.4 | 5.2 | 0.03 |
|
| |||||
| Last DPP annual | 8.1 | 6.3 | 5.2 | 0.05 | |
|
| |||||
| DPPOS year 1 | 7.2 | 6.0 | 5.0 | 0.15 | |
|
| |||||
| DPPOS year 5 | 6.5 | 4.3 | 3.6 | 0.02† | |
Data are presented as mean (95% CI) or per cent for all characteristics except for number of visits.
P-value from treatment group comparison using ANOVA is not adjusted for multiple comparisons, with pairwise significance indicated by:
(P < 0.01 for placebo vs. metformin);
(P < 0.01 for placebo vs. lifestyle);
(P < 0.01 for metformin vs. placebo).
The visits represent baseline when randomization occurred; last DPP annual with average follow-up of 2.9 years (end of masked phase of the study); DPPOS year 1 with average follow-up of 5 years (start of follow-up study after bridge when all groups were offered group-implemented lifestyle curriculum); and DPPOS year 5 with average follow-up of 9 years (end of first phase of DPPOS after 4 years of group-implemented quarterly lifestyle sessions).
DPP, Diabetes Prevention Program; DPPOS, Diabetes Prevention Program Outcomes Study.
Table 2 shows absolute risk factor levels. The highly significant lower blood pressures seen at the end of the DPP in the lifestyle group were no longer apparent at DPPOS year 5. Blood pressure decreased remarkably in all groups from DPP baseline, with mean levels approximating 121 mmHg systolic and 73 diastolic mmHg by DPPOS year 5. LDL cholesterol did not differ significantly by treatment group at any time point; however, the baseline imbalances in HDL cholesterol (lower) and triglycerides (higher) in the placebo group were eliminated at DPPOS year 5. As with blood pressure, all groups showed considerable improvements in LDL cholesterol, triglycerides and HDL cholesterol during the DPPOS. These approximated a 0.52-mmol/l fall in both LDL cholesterol and triglycerides and a 0.13-mmol/l rise in HDL cholesterol. Non-HDL cholesterol showed a similar fall in all groups, but again no group differences were seen. The greater LDL particle size noted at the end of the DPP in the intensive lifestyle intervention, compared with the other groups, was no longer seen in DPPOS year 5, as it had fallen in both lifestyle and metformin groups in contrast with the placebo group, which remained stable. At DPPOS year 5, blood pressure medication use differed modestly but not significantly by treatment group (P = 0.09), being lowest in the lifestyle group (41%) and highest in the metformin group (52%). Lipid medication use, however, was significantly different (P = 0.01) by DPPOS year 5, being lowest in the lifestyle group (32%).
Table 2.
Mean blood pressure and lipid levels at baseline and key time points during follow-up by treatment groups
| Characteristics | Visit§ | Placebo | Metformin | Lifestyle | P-value |
|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | Baseline | 123 (122–124) | 124 (123–125) | 124 (123–125) | 0.65 |
| Last DPP annual | 123 (122–124) | 123 (122–124) | 120 (120–121) | < 0.001†‡ | |
| DPPOS year 1 | 123 (122–124) | 124 (123–125) | 122 (121–122) | 0.001‡ | |
| DPPOS year 5 | 121 (120–122) | 121 (120–122) | 121 (120–122) | 0.72 | |
| Diastolic blood pressure (mmHg) | Baseline | 78 (77–79) | 78 (77–79) | 78 (78–79) | 0.58 |
| Last DPP annual | 76 (76–77) | 76 (76–77) | 74 (74–75) | < 0.001†‡ | |
| DPPOS year 1 | 75 (75–76) | 75 (75–76) | 74 (74–75) | 0.02 | |
| DPPOS year 5 | 73 (72–74) | 73 (72–74) | 73 (72–73) | 0.93 | |
| HDL cholesterol (mmol/l) | Baseline | 1.17 (1.14–1.19) | 1.19 (1.19–1.22) | 1.19 (1.19–1.22) | 0.01 |
| Last DPP annual | 1.17 (1.17–1.19) | 1.19 (1.19–1.22) | 1.22 (1.22–1.22) | < 0.001‡ | |
| DPPOS year 1 | 1.22 (1.22–1.24) | 1.24 (1.22–1.24) | 1.24 (1.22–1.24) | 0.63 | |
| DPPOS year 5 | 1.32 (1.32–1.35) | 1.32 (1.32–1.35) | 1.32 (1.32–1.35) | 0.97 | |
| Non-HDL cholesterol (mmol/l) | Baseline | 4.1 (4.0–4.1) | 4.1 (4.0–4.1) | 4.1 (4.0–4.1) | 0.89 |
| Last DPP annual | 4.0 (4.0–4.1) | 4.0 (3.9–4.0) | 3.9 (3.9–4.0) | 0.06 | |
| DPPOS year 1 | 3.9 (3.8–3.9) | 3.8 (3.7–3.8) | 3.8 (3.7–3.9) | 0.23 | |
| DPPOS year 5 | 3.5 (3.4–3.5) | 3.4 (3.4–3.5) | 3.4 (3.4–3.5) | 0.60 | |
| LDL cholesterol (mmol/l) | Baseline | 3.2 (3.2–3.3) | 3.2 (3.2–3.3) | 3.3 (3.2–3.3) | 0.69 |
| Last DPP annual | 3.2 (3.2–3.3) | 3.2 (3.1–3.2) | 3.2 (3.2–3.3) | 0.18 | |
| DPPOS year 1 | 3.1 (3–3.1) | 3 (3–3.1) | 3.1 (3–3.1) | 0.34 | |
| DPPOS year 5 | 2.7 (2.7–2.8) | 2.7 (2.6–2.7) | 2.7 (2.7–2.8) | 0.41 | |
| LDL size (floatation rate × 10†‡) | Baseline | 263 (261–265) | 266 (264–268) | 265 (263–267) | 0.06 |
| Last DPP annual | 265 (264–267) | 267 (265–268) | 270 (269–272) | < 0.001†‡ | |
| DPPOS year 1 | 262 (261–264) | 264 (263–266) | 264 (262–265) | 0.31 | |
| DPPOS year 5 | 265 (264–267) | 265 (264–267) | 267 (265–268) | 0.31 | |
| Triglycerides(mmol/l) | Baseline | 1.66 (1.6–1.72) | 1.57 (1.51–1.62) | 1.57 (1.53–1.63) | 0.026 |
| Last DPP annual | 1.51 (1.48–1.56) | 1.53 (1.49–1.56) | 1.37 (1.33–1.4) | < 0.001†‡ | |
| DPPOS year 1 | 1.45 (1.41–1.49) | 1.44 (1.4–1.48) | 1.4 (1.37–1.44) | 0.13 | |
| DPPOS year 5 | 1.4 (1.37–1.44) | 1.41 (1.38–1.45) | 1.38 (1.34–1.41) | 0.46 | |
| Anti-hypertensive medications (%) | Baseline | 15.5 | 16.1 | 17.5 | 0.48 |
| Last DPP annual | 27.7 | 28.8 | 25.7 | 0.26 | |
| DPPOS year 1 | 36.9 | 39.1 | 35.3 | 0.43 | |
| DPPOS year 5 | 50.9 | 52 | 46.7 | 0.09 | |
| Lipid lowering medications (%) | Baseline | 5.4 | 5.6 | 4.4 | 0.53 |
| Last DPP annual | 15.5 | 15 | 11.8 | 0.04 | |
| DPPOS year 1 | 21.1 | 20.8 | 17.8 | 0.12 | |
| DPPOS year 5 | 37.4 | 39.2 | 32.5 | 0.01‡ |
Data are presented as mean (95%) and percentage as appropriate.
P-value from treatment group comparison using ANOVA is not adjusted for multiple comparisons. P-value from treatment group comparison using ANOVA is not adjusted for multiple comparisons, with pairwise significance indicated by:
(P < 0.01 for placebo vs. metformin);
(P < 0.01 for placebo vs. lifestyle);
(P < 0.01 for metformin vs. placebo).
The visits represent baseline when randomization occurred; last DPP annual with average follow-up of 2.9 years (end of masked phase of the study); DPPOS year 1 with average follow-up of 5 years (start of follow-up study after bridge when all groups were offered group-implemented lifestyle curriculum); and DPPOS year 5 with average follow-up of 9 years (end of first phase of DPPOS after 4 years of group-implemented quarterly lifestyle sessions).
DPP, Diabetes Prevention Program; DPPOS, Diabetes Prevention Program Outcomes Study.
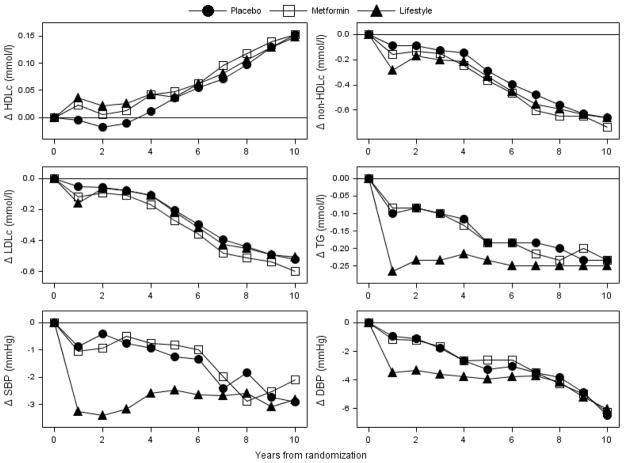
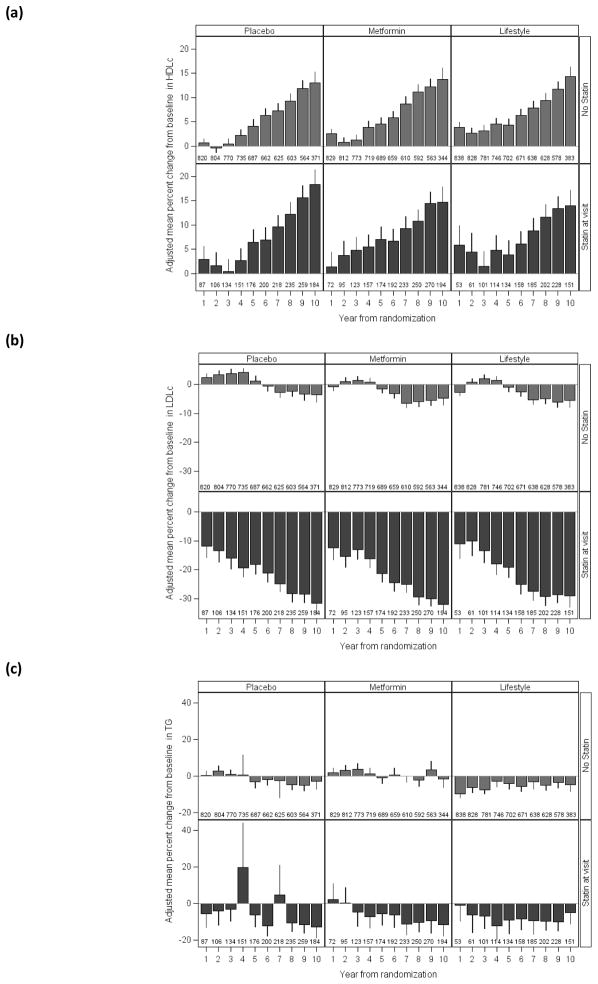
Figure 1 depicts annual changes from baseline by treatment group. This analysis adjusts for medication use at each time point; however, without adjustment, little difference is seen from the patterns depicted. Significant falls in systolic blood pressure were seen in all three treatment groups among those on anti-hypertensive medications, although these occurred earlier (during the DPP) in the intensive lifestyle intervention group (data not shown). For LDL cholesterol (and non-HDL cholesterol), similar declines in all groups were seen from year 4 (during the bridge period) onwards, with concurrent rises in mean HDL cholesterol in all groups of 0.06–0.08 mmol/l. The triglyceride pattern was, however, a little different and, like systolic blood pressure, occurred earlier (during the DPP). We also examined the possibility that the marked improvements in lipids during the DPPOS were primarily driven by an increase in statin drug use, which increased from 4.4% at DPP baseline to 18% at DPPOS year 1 and 31% at DPPOS year 5. However, the same pattern was seen irrespective of statin use, although the absolute differences from baseline were, as expected, much greater for triglycerides and LDL cholesterol in the statin users (Fig. 2). The HDL cholesterol rise after 4 years’ follow-up (bridge period) was of a similar magnitude in all groups. At the end of DPPOS follow-up, there were no lipid/lipoprotein differences by treatment group after adjustment for statin use. Developing diabetes and thus receiving an intensification of therapy did not explain these lipid patterns, as similar lipid (and blood pressure) changes were seen in those who never developed diabetes. Also, in further analyses, diabetic status was not a significant predictor of the HDL cholesterol rise.
Figure 1.
Changes in cardiovascular disease risk factors from baseline by treatment group. Mean changes from baseline are adjusted for baseline level. For triglyceride changes, the means are calculated in the log scale and changes are calculated as (per cent change − 1) × mean at baseline. Data presented are based on number of participants with annual visits, which include: year 1 = 2711, year 2 = 2717, year 3 = 2698, year 4 = 2635, year 5 = 2584, year 6 = 2552, year 7 = 2519, year 8 = 2518, year 9 = 2473, year 10 = 1636.
Figure 2.
Per cent change in (a) HDL cholesterol, (b) LDL cholesterol and (c) triglycerides by treatment group and statin use at visit. Per cent changes in mean (95% CI) are adjusted for baseline levels. The numbers represent participants included for the year and statin status. Statin status is based on reported concomitant medications at current year.
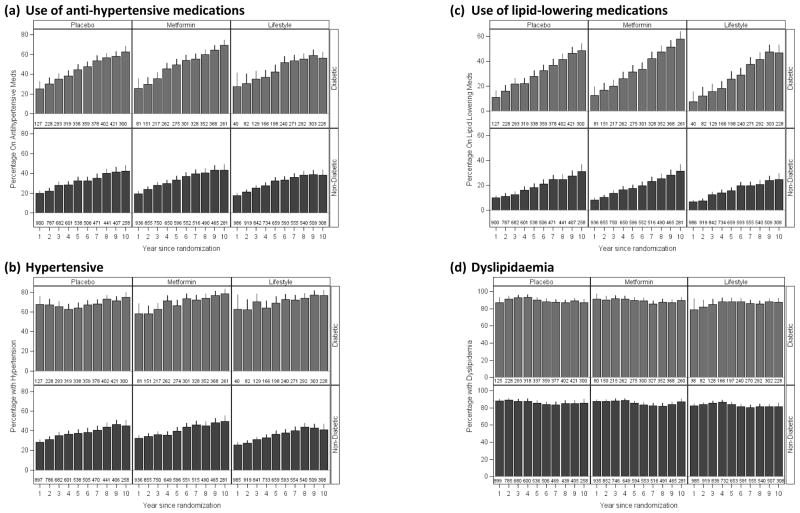
We also examined the effect that current smoking status had on the changes in risk factors. Overall, smoking rates decreased to 3.6% (lifestyle) to 6.5% (placebo) by DPPOS year 5 when rates were higher in the metformin group compared with the placebo group. Generally, systolic blood pressure and HDL cholesterol levels were worse, and improvements somewhat smaller, in current smokers, although only one significant difference by smoking status was observed after 1 year in the lifestyle group, when a 3.5-mmHg drop was seen in non-smokers and a 1.4-mmHg rise in current smokers. Categorical outcomes are shown in Fig. 3 where, for either hypertension or lipid medication use, a tendency for lower use during the DPPOS in the lifestyle group was seen, which became significant after 9 years for blood pressure medication use and 7 years for lipid medication. The comparisons of lifestyle vs. either metformin or placebo were significant. Figure 3 also shows the proportion of each group who are either on drug treatment for hypertension (or dyslipidaemia) or who met Amercian Diabetes Association blood pressure (or original 2001 National Cholesterol Education Program Adult Treatment Panel III [27]) guidelines for lipid treatment. Prevalance of hypertension was consistently lower in the lifestyle group throughout the DPPOS (overall P < 0.001), while for dyslipidaemia significant differences among groups were seen after 9 or 10 years’ follow-up. For example, after 10 years, lifestyle had a lower prevalence (47%) compared with metformin and placebo (both 55%). Because diabetes status will affect both treatment guidelines and goals, as well as general clinical practice, the impact of developing diabetes was further examined by using both diabetes status in mixed models at each time point and by stratification according to diabetes status, i.e. censoring participants at the time of diabetes diagnosis for those without diabetes and starting follow-up at diabetes diagnosis for those with diabetes (Fig. 3). As expected, those with diabetes consistently use more lipid-lowering and anti-hypertensive medications. It should also be noted that, despite the lower incidence of diabetes in the lifestyle group, which translated to a smaller proportion of participants subject to the more stringent criteria for hypertension, participants without diabetes in the lifestyle group had lower prevalence of hypertension compared with those in the metformin group (Fig. 3). After 10 years, 41% of the participants in the lifestyle group without diabetes had hypertension compared with 50% and 45% in the metformin and placebo groups, respectively.
Figure 3.
Proportion of each treatment group meeting guideline criteria by diabetes status at each visit. Data presented are percentage meeting guideline with 95% CI. The numbers represent participants included for the year and diabetes status. Diabetes status is current status for the year. Hypertension was defined as use of anti-hypertensive medications or systolic/diastolic blood pressure ≥ 140/90 for participants without diabetes or systolic/diastolic blood pressure ≥ 130/80 with diabetes. Dyslipidaemia is meeting any of the three criteria: triglyceride ≥ 1.7 mmol/l, LDL cholesterol ≥ 2.6 mmol/l or use of lipid-lowering medications.
Little impact of participation in the bridge lifestyle intervention on these risk factors was seen. Similarly, no major differences were seen in the results reported here by racial group, sex or age, and all interaction terms between treatment group and these demographic variables were non-significant except for systolic and diastolic blood pressure changes in the placebo group and diastolic blood pressure in the metformin group. Men in the placebo group experienced less reduction in systolic/diastolic blood pressure compared with women [systolic/diastolic blood pressure changes (SE) in mmHg were −0.12 (0.47)/−2.06 (0.31) vs. −1.89 (0.32)/−3.51 (0.21)]. Similarly, in the metformin group men showed a lesser reduction in diastolic blood pressure [−2.16 (0.29)] than women [−3.53 (0.21)].
Discussion
These results add to the evidence for the long-term benefits of the DPP interventions on blood pressure and lipoprotein control and support the hypothesis that intensive lifestyle intervention, in particular, has long-lasting benefits on blood pressure and triglycerides. Particularly encouraging was the finding that, despite greater overall improvement in the cardiovascular disease risk factor profile in all groups, both lipid and blood pressure medication use was least for lifestyle participants. Finally, consistent with the modest impact on weight loss and diabetes (compared with the effect in the DPP itself) of introducing lifestyle intervention during the bridge period [16], little effect on cardiovascular disease risk factors was noted. While it is difficult to separate out the long-term effects of the DPP interventions per se from the impact of follow-up in the DPPOS, it would seem likely that the original DPP lifestyle intervention has a sustainable benefit.
Clearly, these results do not prove that the DPP interventions will definitely reduce cardiovascular disease events in the long term, a secondary outcome in the DPPOS. However, when taken together with our previous reports on risk factors in the DPP [7] and on the incidence and regression of the metabolic syndrome [8], they would be consistent with such an outcome. However, it could be argued that, as absolute risk factor levels at the end of the current DPPOS follow-up do not differ by DPP intervention group (even after adjustment for medication use), similar outcomes might be achieved with more intensive blood pressure and lipid medication use alone. However, this is to ignore the considerable cost savings of less medication use in the lifestyle group and the lower exposure to potential medication side effects. These benefits are, of course, in addition to the reduced diabetes incidence, which we have shown still to still be apparent after a further 5 years’ follow-up in the DPPOS [14] and general health benefits. Thus although there is a strong coming together of risk factors and adiposity by the end of the DPPOS follow-up reported here, meaningful benefits of the interventions are apparent. A full cost-effectiveness analysis is in press.
A number of further findings merit discussion. Particularly striking is the greatly improved lipoprotein profile seen in all participants. Mean LDL cholesterol fell approximately 0.52 mmol/l from DPP baseline, with most (c. 0.39 mmol/l) occurring during the DPPOS itself. Similarly, HDL cholesterol rose approximately 0.16 mmol/l since DPP baseline and, again, most occurring during the DPPOS for, as we previously reported, the DPP had little effect on HDL cholesterol itself. Also remarkable is that (Fig. 2) the major breakpoints in the LDL and HDL cholesterol curves seem to be c. around year 4 (3.4–5.0 years) from DPP baseline, which on average corresponds to the beginning of the DPPOS, although the range of duration of follow-up at first DPPOS visit was 3.4–7.1 years.
We considered a number of factors to explain these findings. Firstly, was this a laboratory artifact? The quality control data showed little change and, at most, could only explain a 0.1-mmol/l fall in LDL cholesterol and rise of 0.05 mmol/l in HDL cholesterol. Furthermore, the marked change at year 4 follow-up would have occurred in terms of calendar time over a 3- to 4-year period (because of staggered recruitment). Thus a laboratory/assay artifact or a seasonal effect is unlikely.
Secondly, could these changes merely reflect improvements in the general population? There appears to be some support for this, as a recent report [28], using National Health and Nutrition Examination Survey (NHANES) data for 2003–2004 (approximately years 5 and 6 of our post-DPP baseline follow-up) showed a major (10%) rise from 1999 to 2000 in the proportion of individuals at goal HDL cholesterol levels, while a 30-year analysis of trends in serum lipids from the NHANES data sets from 1976 up to 2006 report a 0.08-mmol/l rise in HDL cholesterol [29]. Similar rises are apparent in the Pittsburgh Epidemiology of Diabetes Complications study (T. J. Orchard, personal observation) and in the placebo group of the LIPID arm of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial [13], both studies where, unlike in NHANES, assay methodology has not changed. Thus, a general rise in HDL cholesterol may indeed be occurring. In addition, it should be noted that, unlike in the NHANES data where a rise in triglycerides has been seen in recent years, presumably reflecting increasing weight, in the DPPOS we did not see any rise in triglycerides.
We further explored the potential role of statin use in this HDL cholesterol rise and could not find any clear evidence for this explanation for, as shown in Fig. 3, the HDL cholesterol rise was similar in users and non-users of statins. Statin use in our placebo group at year 4 (approximately 2003/2004) population was 17%, which is comparable with the 23% reported from NHANES for dyslipidaemic subjects with the metabolic syndrome in 2003–2004 [28]. We also could not explain this HDL cholesterol increase on the basis of the minimal use of either fibrates or niacin. Other than oestrogen therapy and alcohol use, there are few factors known to increase HDL cholesterol. Although we cannot explain the HDL cholesterol rise, it is unlikely to be unique to the DPPOS.
Our findings highlight the relatively greater benefit of lifestyle for blood pressure control than for lipids and provide a further stimulus to the incorporation of lifestyle intervention into the basic therapy of all those with, or at risk of, hypertension. It is possible that the nature of the lifestyle intervention (a behaviourally delivered combination of low-fat diet and modest exercise) may have a greater impact on blood pressure than on lipids, particularly HDL cholesterol, which rose only modestly during the DPP. The relatively high polyunsaturated/saturated fatty acid ratio of the diet may have muted any HDL cholesterol rise [8], while the modest level of exercise may be adequate for a blood pressure effect but not a lipid (e.g. HDL raising) effect. Metformin therapy continued to show benefits during DPPOS follow-up and, although not as effective in many regards as lifestyle, was still associated with benefit on blood pressure and showed overall a somewhat greater reduction in LDL cholesterol than seen in the lifestyle group (data not shown). These results thus further support the early adoption of metformin therapy in the glucose intolerant for cardiovascular protection as well as diabetes risk reduction. We did not observe any significant major race or gender differences, consistent with the overall results for diabetes prevention.
A number of study limitations merit discussion. Firstly, we do not present cardiovascular disease event data as there are insufficient numbers for meaningful analyses. Secondly, as these data are from a clinical trial, they may not be generalizable to all those with impaired glucose tolerance and/or early diabetes. However, follow-up participation in the DPPOS was high (> 85%) and the original DPP population was enriched with at-risk minorities, which adds to its relevance. Care and treatment decisions in the DPPOS were determined by the participants’ personal healthcare providers, rather than the trial investigators, so medical management was not standardized. While this has the advantage of providing an element of real-world translation, the closer monitoring of glucose tolerance and cardiovascular disease risk factors in the trial remains a potential bias. We have only examined the data using an intention-to-treat approach and it is possible that results would have been different if we limited the analysis to those who closely followed the protocol. Finally, during the DPP trial, approximately 25% of participants developed diabetes, a figure which had risen to over 50% after 10 years of follow-up. This may have profound effects on cardiovascular disease risk factor management, although we did account for any diabetes effect in multivariate analyses. It should also be noted that the full effect of lifestyle and metformin therapy on cardiovascular disease risk will also be partially mediated by diabetes prevention/delay per se, given the effect diabetes has on cardiovascular disease risk beyond the standard risk factors.
In conclusion, extended follow-up of the DPP cohort has shown that, 10 years after randomization, all groups have excellent and comparable lipid and blood pressure control and thus any future difference in cardiovascular disease events by treatment group would likely reflect a legacy effect of the DPP intervention period. Nonetheless, the current equivalency of cardiovascular disease risk status by DPP treatment group with less medication use in the lifestyle group is an advantage of lifestyle intervention.
Supplementary Material
Acknowledgments
The Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS. During the DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centres and the Coordinating Center for the design and conduct of the study, and collection, management, analysis and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, supported data collection at many of the clinical centres. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the Office of Research on Women’s Health, the National Center for Minority Health and Human Disease, the Centers for Disease Control and Prevention and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP, Lipha (Merck-Sante) provided medication and LifeScan Inc. donated materials during the DPP and DPPOS. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. A complete list of centres, investigators and staff can be found in the Supporting Information (Appendix S1).
Abbreviations
- DPP
Diabetes Prevention Program
- DPPOS
Diabetes Prevention Program Outcomes Study
Footnotes
Competing interests
TJO has been a consultant for Astra Zeneca. RG has speaker honoraria from Merck, GSK and Daiichi-Sankoyo; research grants from Abbott, GSK and Roche; and is on the Consultant Board of GSK, Daiichi-Sankoyo and Pfizer. MT, EB-C, SF, KM, SM, MM, RR, CS, HS and KW have nothing to declare.
Additional Supporting Information may be found in the online version of this article:
Appendix S1. DPPOS Research Group Investigators.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than for missing material) should be directed to the corresponding author for the article.
References
- 1.Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Coronary heart disease risk and impaired glucose tolerance: the Whitehall Study. Lancet. 1980;8183:1373–1376. doi: 10.1016/s0140-6736(80)92651-3. [DOI] [PubMed] [Google Scholar]
- 2.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701–710. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan X-R, Li G-W, Hu Y-H, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindström J, Eriksson JG, et al. for the Finnish Diabetes Prevention Study Group. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orchard TJ, Temprosa M, Goldberg R, et al. for the Diabetes Prevention Program Research Group. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: The Diabetes Prevention Program Randomized Trial. Ann Intern Med. 2005;142:611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 11.Duckworth W, Abraira C, Moritz T, et al. for the VADT Investigators. Intensive glucose control and complications in American veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 12.ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care. 2003;26:977–980. doi: 10.2337/diacare.26.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venditti EM, Bray GA, Carrion-Petersen ML, et al. First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes (Lond) 2008;32:1537–1544. doi: 10.1038/ijo.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y, Temprosa M, Fowler S, et al. Evaluating the accuracy of an aneroid sphygmomanometer in a clinical trial setting. Am J Hypertens. 2009;22:263–266. doi: 10.1038/ajh.2008.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- 19.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantification of high-density lipoprotein cholesterol. Clin Chem. 1982;28:1279–1288. [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Hainline A Jr, Karon J, Lippel K, editors. 2. Bethesda, MD: Lipid Research Clinics Program, Lipid and Lipoprotein Analysis, US Department of Health and Human Services, National Institutes of Health; 1983. Manual of Laboratory Operations. [Google Scholar]
- 22.Auwerx JH, Marzetta CA, Hokanson JE, Brunzell JD. Large buoyant LDL-like particles in hepatic lipase deficiency. Arterioscler Thromb Vasc Biol. 1989;9:319–325. doi: 10.1161/01.atv.9.3.319. [DOI] [PubMed] [Google Scholar]
- 23.Purnell JQ, Marcovina SM, Hokanson JE, et al. Levels of lipoprotein(a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM. Results from follow-up in the Diabetes Control and Complications Trial. Diabetes. 1995;44:1218–1226. doi: 10.2337/diab.44.10.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown H, Prescott R. Applied Mixed Models in Medicine. New York: John Wiley and Sons Inc; 1999. [Google Scholar]
- 25.Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O. An R2 statistic for fixed effects in the linear mixed model. Stat Med. 2008;27:6137–6157. doi: 10.1002/sim.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford: Clarendon Press; 1994. [Google Scholar]
- 27.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) J Am Med Assoc. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 28.Ghandehari H, Kamal-Bahl S, Wong ND. Prevalence and extent of dyslipidemia and recommended lipid levels in US adults with and without cardiovascular comorbidities: The National Health and Nutrition Examination Survey 2003–2004. Am Heart J. 2008;156:112–119. doi: 10.1016/j.ahj.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, et al. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III and 1999–2006. Am J Cardiol. 2010;106:969–975. doi: 10.1016/j.amjcard.2010.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.