Abstract
The phosphatase and tensin homolog delete on chromosome 10 (PTEN) regulates innate immune responses inversely with phosphoinositide 3-kinase (PI3K) and its direct downstream target gene, Akt. The Forkhead box O (Foxo) transcription factors are essential in the regulation of tissue development, immune homeostasis and cell survival. This study was designed to investigate the role of PTEN-mediated Akt/β-catenin/Foxo1 signaling in the regulation of in vivo and in vitro innate immune responses in a mouse model of hepatic inflammatory injury induced by 90 min of liver partial warm ischemia followed by 6 h of reperfusion (IRI). We found that knockdown of PTEN with small interfering RNA (siRNA) promoted Akt/β-catenin/Foxo1 signaling, leading to the resistance against liver IR-damage, local enhancement of antiapoptotic function, and down-regulation of innate TLR4 expression. A specific PI3K blockade inhibited Akt/β-catenin signaling, increased Foxo1-mediated TLR4-driven local inflammation and recreated cardinal features of liver IRI. Moreover, knockdown of PTEN in LPS-stimulated mouse bone marrow derived-macrophages, enhanced β-catenin activity, which in turn provided a negative regulatory feedback to the Foxo1 function, leading to the inhibition of TLR4 and NF-κB, with ultimate depression of proinflammatory cytokine programs in vitro. Conclusion: our novel findings identify PTEN-mediated Akt/β-catenin/Foxo1 axis as a key regulator of innate inflammatory response in the mouse liver. By identifying molecular mechanisms of PTEN-mediated Akt/β-catenin/Foxo1 signaling in TLR4 innate immune regulation, our study provides the rationale for novel therapeutic approaches to manage inflammation injury in IR-stressed liver.
Keywords: TLR4, Inflammation, Innate Immunity, Liver Injury
Ischemia/reperfusion injury (IRI) in the liver remains a clinical problem and a major complication of liver resection and transplantation (1). Liver IRI represents a continuum of exogenous antigen-independent inflammatory processes that include endothelial activation, increased expression of adhesion molecules, Kupffer cell/neutrophil activation, and cytokine release, followed by ultimate endothelial cell and hepatocyte death (1,2). We and others have characterized TLR4-dependent innate immune mechanisms that initiate liver IRI cascade, translocation of NF-κB and the transcription of cytokine, chemokine, and cell adhesion genes, ultimately resulting in the development of local inflammation and apoptosis (3,4).
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN), a lipid phosphatase, which dephosphorylates PI(3,4,5)P3 to PI(4,5)P2, is known to antagonize phosphoinositide 3-kinase (PI3K) (5,6). The balance between PTEN and PI3K determines PI(3,4,5)P3 levels and opposing effects on growth and cell survival (7). PI(3,4,5)P3 is thought to mediate these effects by inducing phosphorylation/activation of the Akt kinase (8), which enhances PIP3-PI3K/Akt survival pathway. PTEN inhibition heightens PI3K/Akt activity, increases cardioprotection (9) and reduces brain damage (10), whereas Akt increases β-catenin translocation to the nucleus via phosphorylation of GSK-3β (11).
Forkhead box O (Foxo) proteins of the Forkhead family of transcription factors play important roles in the control of cell differentiation, proliferation, and survival (12). Indeed, Foxos were found essential in resistance to oxidative stress through regulation of cell survival (13). The phosphorylation of Foxo by Akt blocks its function, inhibits DNA binding, nuclear exclusion, and subsequent sequestration into the cytoplasm (14). Dephosphorylation of Foxo, on the other hand, increases its nuclear accumulation and activity, leading to enhanced target gene expression and apoptosis (15). Interestingly, increased Foxo1 phosphorylation was shown to promote cardiomyocyte survival and inhibit cell apoptosis in response to oxidative stress (16). Increased nuclear Foxo1 activity results in the activation of innate antimicrobial peptide (AMP), which modulates the defense inflammatory responses without harming the host tissues (17). Although these findings imply an essential regulatory role of Foxo1 in cell survival, apoptosis, oxidative stress, and macrophage function, little is known about putative function and molecular mechanisms of PTEN-mediated Foxo1 signaling in the regulation of immune homeostasis in the liver damage.
Here, we identify PTEN mediated Akt/β-catenin/Foxo1 axis as a novel regulatory mechanism in IR-triggered liver inflammation and injury. It’s signaling promoted cytoprotection and resistance to IRI by increasing β-catenin activity, as well as providing a negative feedback mechanism to Foxo1 signaling. This study reveals a novel regulatory linkage between PTEN mediated Akt/β-catenin/Foxo1 and TLR4 pathway, and provides a novel mechanism for β-catenin-mediated immune modulation in IR-stressed livers.
Experimental Procedures
Animals
Male C57BL/6 wild-type (WT) mice at 8–10 weeks of age were used (The Jackson Laboratory, Bar Harbor, ME). Animals were housed in UCLA animal facility under pathogen-free conditions, and received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86–23 revised 1985). The animal study was approved by IACUC.
Preparation of siRNA
The siRNA against PTEN was designed using the siRNA selection program (18). The sense and antisense strands of murine PTEN siRNA were 5′-AGAGAUCGUUAGCAGAAACTT-3′ (sense) and 5′-GUUUCUGCUAACGAUCUCUT T-3′ (antisense). The scramble (control) siRNA were 5′-GCGCGCUUUGUAGGAUUCGTT-3′ (sense) and 5′-CGAAUCCUACAAAGCG CGCTT-3′ (antisense). All siRNA were synthesized in 2′-deprotected, duplexed, desalted and purified siRNA form (Qiagen Inc., Chatsworth, CA).
Liver IRI model
We used a well-established model of partial warm hepatic IRI (19), in which mice anesthetized by isoflurane and treated with carprofen (5mg/kg) were injected with heparin (100U/kg). An atraumatic clip was used to interrupt the artery/portal venous blood supply to the left/middle liver lobes under aseptic conditions. After 90min of ischemia, the clip was removed. At the conclusion of experiment at 6h of reperfusion, animals were sacrificed with isoflurane overdose, and blood and tissue samples were collected.
PTEN siRNA or scramble siRNA (2mg/kg) was given i.v. 4h prior to the ischemia (18,20). We have previously documented the efficacy of this siRNA approach in the liver (18), with >40% of i.v. infused siRNA accumulating in the ischemic mouse livers, data confirmed by others (21). In some experiments, PI3K inhibitor (LY294002; Calbiochem; 0.5mg/kg) or vehicle [10% dimethyl sulfoxide (DMSO) and 90% PBS] was given i.p. 30 min prior to the ischemic insult. Sham-operated mice underwent the same procedures without vascular occlusion.
Hepatocellular damage assay
Serum alanine aminotransferase (sALT) levels, an indicator of hepatocellular injury, were measured in blood samples with an autoanalyzer (ANTECH Diagnostics, Los Angeles, CA).
Liver histology
Liver paraffin sections (5-μm) were stained with hematoxylin and eosin (H&E). The severity of liver IRI was graded blindly using Suzuki’s criteria on a scale from 0 to 4 (22). No necrosis, congestion/centrilobular ballooning is given a score of 0, while severe congestion and >60% lobular necrosis is given a value of 4.
Immunohistochemistry
Liver macrophages were detected using primary mAb against CD11b (Mac-1, M1/70; BD Biosciences, San Jose, CA). The secondary Ab, biotinylated goat anti-rat IgG (Vector, Burlingame, CA) was incubated with immunoperoxidase (ABC Kit, Vector). Positive cells were counted blindly in 10 HPF/section (×400).
Myeloperoxidase assay
The presence of myeloperoxidase (MPO) was used as an index of neutrophil accumulation in the liver (23). One unit of MPO activity (U/g) was defined as the quantity of enzyme degrading 1μmol peroxide per minute at 25°C per gram of tissue.
Caspase-3 assay
Caspase-3 activity was determined by an assay kit (Calbiochem, La Jolla, CA) as described (20).
Quantitative RT-PCR
Total RNA was extracted from frozen livers and cells using RNAse Mini Kit (Qiagen, Valencia, CA); A total of 5.0μg of RNA was reverse-transcribed into cDNA. Quantitative PCR was performed as described (23). Primer sequences for the amplification of TNF-α, IL-1β, MCP-1, CXCL-10, PTEN, and HPRT are shown (Supplementary Table I). Target gene expressions were calculated by their ratios to the housekeeping gene HPRT.
Apoptosis assay
Apoptosis in formalin-fixed paraffin-embedded liver sections was detected by TUNEL staining (Calbiochem, Gibbstown, NJ) (23). Negative controls were prepared through omission of terminal transferase. Positive controls were generated by treatment with DNase. TUNEL-positive cells were counted in 10 HPF/section (×400).
Western blots
Proteins (30μg/sample) from cell cultures or liver samples were subjected to 12% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Polyclonal rabbit anti-mouse TLR4 (IMGENEX, San Diego, CA), NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA), cleaved caspase-3, Bcl-2, Bcl-xL, Akt, Phospho-Akt, PTEN, Phospho-PTEN, Foxo1, Phospho-Foxo1, β-catenin, Phospho-β-catenin (Ser552), and β-actin (Cell Signaling Technology, Danvers, MA) were used. Relative quantities of protein were determined by densitometer and expressed in absorbance units (AU).
Cell isolations and in vitro cultures
Murine bone marrow derived-macrophages (BMMs) were isolated, as described (20). Cells (1×106/well) were cultured for 7 days, and then transfected with PTEN siRNA/scramble siRNA. After 24h, cells were supplemented with LPS (100ng/ml)for additional 6h. In some experiments, cells were pretreated for 1h with PI3K inhibitor (LY294002, 10μM, Calbiochem) or DMSO (6.5μl/ml).
Statistical analysis
All data are expressed as Mean±SD. Differences between experimental groups were analyzed by Student-t test. All differences were considered statistically significant at the p-value of <0.05.
Results
Knockdown of PTEN improves hepatocellular function and ameliorates liver IRI
We analyzed the hepatocellular function in mouse livers subjected to 90min of warm ischemia followed by 6h reperfusion. As shown in Fig. 1A, sALT levels (IU/L) in mice pretreated with PTEN siRNA were decreased (p<0.0005), as compared with untreated or scramble siRNA-treated WT controls (6650±3277 vs. 33075±5059 and 36225±5051, respectively). These data correlated with Suzuki’s histological grading of liver IRI (Fig. 1B and 1C). Indeed, knockdown of PTEN resulted in minimal liver sinusoidal congestion, vacuolization without edema or necrosis (Fig. 1B(c); score=1.2±0.4). In contrast, livers in untreated or scramble siRNA-treated WT mice revealed moderate to severe edema and extensive hepatocellular necrosis (Fig. 1B(b) and (d); score=3.6±0.51 and 3.5±0.53, respectively, p<0.0001). The MPO assay, reflecting liver neutrophil activity (U/g), was depressed in PTEN siRNA-treated group, compared with untreated or scramble siRNA-treated WT groups (Fig. 1D: 0.25±0.15 vs. 3.34±0.33 and 3.13±0.21, respectively, p<0.01 and p<0.005).
Figure 1.
Pretreatment of mice with PTEN siRNA (2mg/kg i.v. at −4h) ameliorates liver IRI, compared with untreated or scramble siRNA-treated WT controls (6h of reperfusion after 90min warm ischemia).
(A) The sALT (IU/L) levels. *p<0.0005, n=4–6/group. Mean±SD are shown.
(B) Representative liver histology of ischemic liver lobes: (a) Sham; (b) untreated WT; (c) PTEN siRNA-treatment (d) scramble siRNA-treatment. (H&E staining; magnification ×100, n=4–6/group)
(C) Suzuki’s histological grading of liver IR-damage *p<0.0001, n=4–6/group. Mean±SD are shown.
(D) Liver neutrophil accumulation, assessed by MPO activity (U/gm). *p<0.01, **p<0.005. n=4–6/group. Mean±SD are shown.
Knockdown of PTEN inhibits Foxo1 signaling and regulates innate immune response in liver IRI
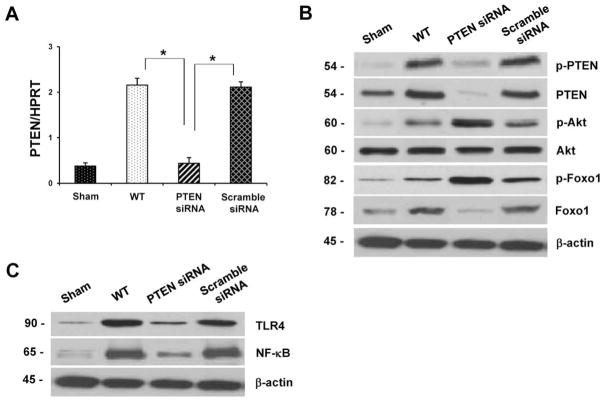
We investigated the role of Foxo1 signaling after PTEN knockdown in IR-induced liver inflammation. As shown in Figure 2A, treatment with PTEN siRNA decreased hepatic expression of mRNA levels coding for PTEN, compared with controls (p<0.01). Moreover, unlike in sham controls, hepatic IR increased Western blot-assisted expression (AU) of total PTEN and phosphorylated PTEN in untreated (Fig. 2B, 2.7–2.9 and 1.9–2.1, respectively) or scramble siRNA (2.1–2.3 and 2.0–2.2, respectively) treated mice. In contrast, knockdown of PTEN diminished total PTEN (0.1–0.2) and phosphorylated PTEN (0.1–0.2). Interestingly, PTEN knockdown increased phosphorylation of Akt (2.4–2.6) and Foxo1 (2.6–2.8), but diminished nuclear total Foxo1 (0.2–0.4), compared with untreated or scramble siRNA-treated WT controls (0.3–0.5 and 0.5–0.9, 1.9–2.0, respectively). Unlike enhanced IR-induced TLR4 (Fig. 2C, 2.4–2.6) and NF-κB (2.2–2.5) expression seen in untreated or scramble siRNA treated WT livers, PTEN knockdown resulted in augmented Akt and Foxo1 phosphorylation, along with decreased Foxo1 nuclear accumulation/transcriptional activity, leading to the inhibition of TLR4 (0.5–0.7) and NF-κB (0.4–0.6) expression.
Figure 2.
Knockdown of PTEN inhibits Foxo1 signaling and regulates innate immune response in liver IRI. For details see Figure 1 legend.
(A) Quantitative RT-PCR-assisted detection of PTEN expression in liver IRI. Data were normalized to β-actin gene expression. *p<0.01, n=4–6/group. Mean±SD are shown.
(B and C) Western blot-assisted analysis of phospho-PTEN (B), PTEN (B), phospho-Akt (B), Akt (B), phospho-Foxo1 (B), Foxo1 (B), TLR4 (C), and NF-κB (C) in IR livers. β-actin served as an internal control. Representative of three experiments.
Inhibition of PTEN-mediated Foxo1 signaling reduces macrophage trafficking and inflammatory program in liver IRI
As enhanced PTEN-mediated Foxo1 signaling following PTEN knockdown contributed to the inhibition of TLR4/NF-κB, we then asked whether PTEN-mediated Foxo1 might affect macrophage function in vivo. As shown in Fig. 3A and 3B, treatment with PTEN siRNA reduced the frequency of CD11b+ macrophages sequestered in the ischemic livers, as compared with controls (17.2±5.98 vs. 37.7±13.17 and 37.5±11.58, respectively, p<0.0005). Consistent with the immunostaining data, decreased mRNA levels coding for TNF-α (p<0.05), IL-1β (p<0.01), MCP-1 (p<0.005), and CXCL-10 (p<0.05) were consistently found in PTEN siRNA-treated livers, compared with untreated or siRNA-treated WT control groups (Fig. 3C).
Figure 3.
Inhibition of PTEN-mediated Foxo1 reduces macrophage trafficking and proinflammatory gene programs in liver IRI. For details see Figure 1 legend.
(A) Representative liver sections stained for CD11b expression (magnification ×400).
(B) Cell quantification/HPF. *p<0.0005, n=4–6/group.
(C) Quantitative RT-PCR-assisted detection of TNF-α, IL-1β, MCP-1, CXCL-10 in IR livers. Data were normalized to β-actin gene expression. *p<0.05, **p<0.01, ***p<0.005, n=4–6/group. Mean±SD are shown.
Inhibition of PTEN-mediated Foxo1 signaling promotes anti-apoptotic function and reduces hepatocellular apoptosis in liver IRI
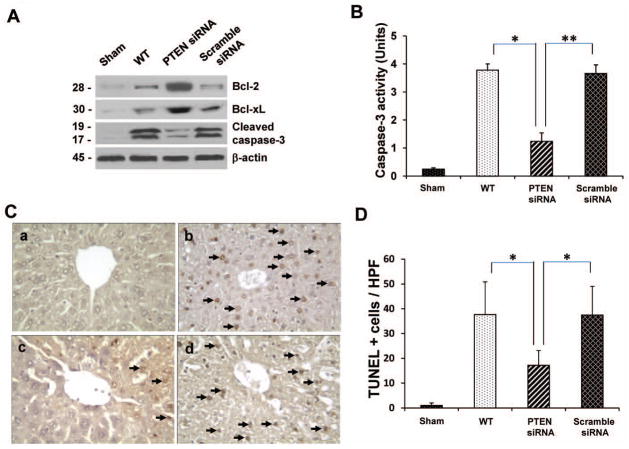
To determine whether PTEN-mediated Foxo1 signaling affects hepatic IR-induced apoptosis, we next analyzed the expression of anti-apoptotic (Bcl-2/Bcl-xL) and pro-apoptotic (cleaved caspase-3) gene products. Indeed, siRNA-facilitated PTEN knockdown markedly increased Western blot-assisted expression (AU) of Bcl-2, and Bcl-xL in the ischemic livers (Fig. 4A, 2.2–2.4 and 2.0–2.2, respectively), compared with untreated or scramble siRNA-treated WT groups (0.2–0.4). Moreover, PTEN knockdown inhibited the expression of cleaved caspase-3 (0.2–0.3 vs. 2.3–2.5 in controls). This data was confirmed by decreased caspase-3 activity in PTEN siRNA-treated but not in untreated or scramble siRNA-treated WT livers (Fig. 4B: 1.24±0.302 vs. 3.78±0.22 and 3.66±0.31, p<0.0005 or p<0.001, respectively). We further analyzed the hepatocellular apoptosis by TUNEL staining (Fig. 4C and 4D). Indeed, knockdown of PTEN decreased the frequency of TUNEL+ cells/HPF in the ischemic liver lobes (Fig. 4C(c); 18.1±6.45, p<0.0005), compared with untreated (Fig. 4C(b); 36.4±10.62) or scramble siRNA-treated WT group (Fig. 4C(d); 35.6±8.82).
Figure 4.
Inhibition of PTEN-mediated Foxo1 promotes anti-apoptotic function and reduces cell apoptosis in liver IRI. For details see Figure 1 legend.
(A) Western-assisted analysis of Bcl-2, Bcl-xl, and cleaved caspase-3 in IR livers. β-actin served as an internal control. Representative of three experiments.
(B) Caspase 3 activity (U). Mean±SD; n=4–6/group. *p<0.0005, **p<0.001.
(C) TUNEL-assisted detection of apoptosis in IR livers. Representative staining of apoptotic positive cells (magnification ×400).
(D) Quantification of apoptotic cells/HPF in PTEN siRNA-treated vs. WT/scramble siRNA-treated group. *p<0.0005, n=4–6/group.
Inhibition of PI3K blocks Akt/β-catenin/Foxo1 signaling and recreates liver IRI
As PI3K/Akt/β-catenin pathway was shown to regulate cell survival in vitro (24), we then investigated cross talk between PI3K and Foxo1 after PTEN knockdown in our model. WT mice underwent adjunctive treatment with a specific PI3K inhibitor (LY294002) and PTEN siRNA prior to the ischemic insult. By 6h of reperfusion, sALT levels (IU/L) were increased in mice given PI3K inhibitor plus PTEN siRNA (Fig. 5A, 29700±3780), as compared with PTEN siRNA monotherapy group (6190±3592; p<0.0005). These data correlated with Suzuki’s grading of histological liver damage (Fig. 5B and 5C). Unlike PTEN siRNA-treated mice with minimal liver sinusoidal congestion without edema, vacuolization or necrosis (Fig. 5B(d); score=1.2±0.42, p<0.0001), those after adjunctive PI3K inhibitor and PTEN siRNA revealed liver edema, severe sinusoidal congestion, cytoplasmic vacuolization, and extensive hepatocellular necrosis (30–50%; Fig. 5B(c); score=3.7±0.48). Furthermore, inhibition of PI3K in PTEN siRNA-treated livers decreased Western blot-assisted (AU) phosphorylation of Akt (Fig. 5D, 0.6–0.8), β-catenin (0.2–0.4), and Foxo1 (0.3–0.5), while increasing the expression of total Foxo1 (1.9–2.1) and decreasing nuclear β-catenin (0.4–0.6), compared with PTEN siRNA monotherapy (Fig. 5D, 2.4–2.6, 2.3–2.5, and 1.4–1.7, 1.0–1.2 and 2.4–2.6, respectively).
Figure 5.
Inhibition of PI3K (LY294002; 0.5mg/kg i.p. at −30 min) blocks Akt/β-catenin/Foxo1 signaling and recreates liver IRI. For details see Figure 1 legend.
(A) The sALT levels (IU/L). *p<0.0005, n=4/group. Mean ± SD are shown.
(B and C) Suzuki’s histological score of liver IRI: (a) Sham; (b) DMSO control (3.4±0.52); (c) PI3K inhibitor LY294002 (3.7±0.48); (d) PTEN siRNA treatment (1.2±0.42). Representative liver histology (H&E staining; magnification ×100, n=4/group): *p<0.0001, Mean±SD are shown.
(D) Western blot-assisted expression of phospho-Akt, Akt, phospho-Foxo1, Foxo1, phospho-β-catenin, and β-catenin in IR livers. β-actin served as an internal control. Representative of three experiments.
PTEN-mediated Akt activates β-catenin and negatively regulates Foxo1 signaling in vitro
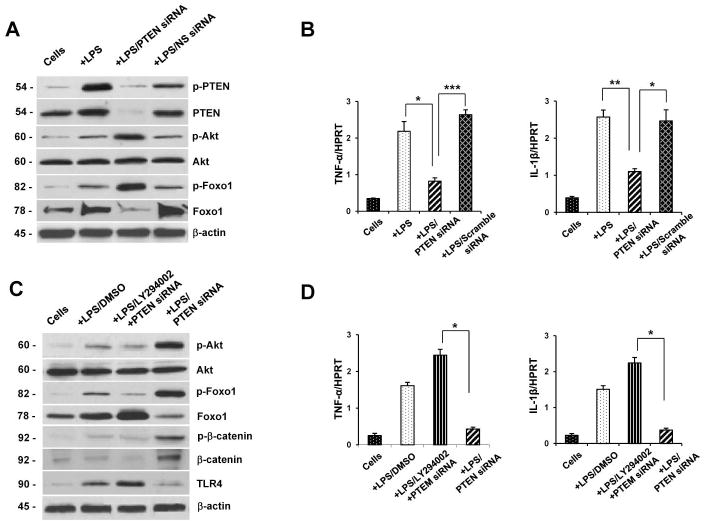
We elucidated the mechanism of Foxo1-mediated innate regulation in LPS-stimulated BMM cultures. Knockdown of PTEN diminished Western blot-assisted expression (AU) of total PTEN (Fig. 6A, 0.05–0.1) and phosphorylated PTEN (0.1–0.3), compared with nonspecific (NS) siRNA-transfected (2.2–2.4 and 1.2–1.4, respectively) or LPS-stimulated (2.4–2.6 and 2.3–2.5, respectively) cells. However, PTEN knockdown upregulated phosphorylation of Akt (2.2–2.4) and Foxo1 (2.4–2.6), while downregulating total Foxo1 (0.3–0.5) expression, compared with NS siRNA-treated BMMs (0.7–0.8, 0.8–0.9, and 2.6–2.8) or cells alone (0.3–0.4, 0.2–0.3 and 1.1–1.2). Moreover, PTEN knockdown inhibited mRNA levels coding for TNF-α and IL-1β in LPS-stimulated BMMs (Fig. 6B).
Figure 6.
PTEN-mediated Akt activates β-catenin and negatively regulates Foxo1 signaling in vitro.
(A) Western-assisted expression of phospho-PTEN, PTEN, phospho-Akt, Akt, phospho-Foxo1, Foxo1, phospho-Erk, and phospho-p38 in LPS-stimulated BMMs after treatment with PTEN siRNA. β-actin was used as an internal control. Representative of three experiments.
(B) Quantitative RT-PCR-assisted detection of TNF-α and IL-1β in LPS-stimulated BMMs after treatment with PTEN siRNA.*p<0.05, **p<0.01, ***p<0.005, n=3–4/group. Mean±SD are shown.
(C) Western-assisted expression of phospho-Akt, Akt, phospho-Foxo1, Foxo1, phospho-β-catenin, β-catenin and TLR4 in LPS-stimulated BMMs after treatment with PI3K inhibitor LY294002. β-actin was used as an internal control. Representative of three experiments.
(D) Quantitative RT-PCR-assisted detection of TNF-α and IL-1β in LPS-stimulated BMMs after treatment with PI3K inhibitor LY294002. *p<0.005, n=3–4/group. Mean±SD are shown.
Our in vivo data has implicated β-catenin as an important cytoprotective molecule in hepatic IRI. To investigate whether PI3K and PTEN-mediated Akt signaling activates β-catenin to regulate innate immunity networks in vitro, we next used PI3K inhibitor (LY 294002) in LPS-stimulated BMM cultures. Indeed, siRNA-facilitated knockdown of PTEN increased Western blot-assisted (AU) phosphorylation of Akt (Fig. 6C, 2.3–2.5) and Foxo1 (2.5–2.7) but diminished nuclear total Foxo1 (0.7–0.9). Interestingly, PTEN knockdown upregulated phosphorylated β-catenin (1.2–1.4) and nuclear total β-catenin (1.4–1.6) but downregulated TLR4 (0.2–0.3) expression in the culture. Conversely, inhibition of PI3K in PTEN siRNA-treated cells readily inhibited phosphorylation of Akt (Fig. 6C, 0.3–0.5), Foxo1 (0.4–0.6) and β-catenin (0.1–0.3), yet promoted the expression of total Foxo1 (2.6–2.8), TLR4 (1.5–1.7), while depressing nuclear β-catenin (0.2–0.3). Consistent with these findings, PI3K inhibition has led to increased mRNA levels coding for TNF-α and IL-1β in PTEN siRNA-treated LPS-stimulated cells (Fig. 6D).
Discussion
The molecular mechanism of IR-stressed liver damage involves activation of multiple and interlocked signaling pathways (1). PTEN, a key downregulator of PI3K/Akt pro-survival signaling, was shown essential in heart (25) and brain (26) ischemic injury. PTEN regulates PI3K/Akt pathway by decreasing translocation of Akt to the cellular membrane (5). Activation of Akt, on the other hand, inhibits Foxo1, a process leading to increased cell survival (14), control of glucose homeostasis (27), and maintenance of stem cell differentiation (28). Consistent with the role of PTEN - PI3K/Akt axis in the inflammation cascade, our current in vivo study has shown that knockdown of PTEN ameliorated liver IRI and improved hepatocellular function, evidenced by decreased sALT levels, histological liver damage, local macrophage activation, neutrophil accumulation, and pro-inflammatory cytokine/chemokine gene expression. Notably, knockdown of PTEN activated Akt-mediated β-catenin and inhibited Foxo1, which implies a specific role for Akt/β-catenin/Foxo1 signaling in the regulation of IR-triggered inflammation in the liver. Several factors may contribute to Akt/β-catenin/Foxo1-mediated cytoprotection. First, we found that IR insult promoted PTEN phosphorylation and downregulated Akt activation, whereas knockdown of PTEN activated Akt. The latter phosphorylated Foxo1, resulting in the reduction of its DNA-binding capacity and exporting Foxo1 from the nucleus to the cytoplasm, thereby inhibiting Foxo1 transcriptional activity. Thus, PTEN-mediated Akt might directly inhibit Foxo1 signaling via Akt-mediated phosphorylation of Foxo1 in IR-triggered liver inflammation. Second, activation of Akt after PTEN knockdown triggered β-catenin (Ser552) phosphorylation, leading to increased translocation of β-catenin into the nucleus, and enhancing its activity, which in turn negatively regulated Foxo1 signaling. Third, knockdown of PTEN decreased IR-induced TLR4/NF-κB expression in the ischemic liver. Since TLRs participate in the response to pathogen-associated molecular patterns (PAMPs) and endogenous ligands (DAMPs) released from damaged hepatocytes, TLR4 signaling is critical for the induction of innate immunity (4). NF-κB is a ubiquitous inducible transcription factor, which stimulates gene expression and promotes inflammatory response (29). We have previously demonstrated that hepatic IR increases IκBα phosphorylation, resulting in NF-κB activation (20). Moreover, our current data shows that enhanced TLR4/NF-κB expression leads to increased macrophage trafficking/activation, as demonstrated by liver sequestration of CD11b+ cells, and local expression of proinflammatory cytokines (TNF-α/IL-1β) and chemokines (MCP-1/CXCL-10). In contrast, activation of Akt/β-catenin/Foxo1 after PTEN knockdown inhibited TLR4/NF-κB, along with decreased macrophage-mediated proinflammatory response. These findings imply the regulatory role of PTEN-mediated Akt/β-catenin/Foxo1 signaling in IR-stressed liver.
We found that PTEN knockdown-facilitated activation of Akt/β-catenin/Foxo1 increased the expression of antiapoptotic Bcl-2/Bcl-xL yet decreased caspase-3 activity. Indeed, Foxo transcription factors are at the interface of crucial cellular processes, orchestrating programs of gene expression that regulate apoptosis (13). Phosphorylation of Foxo1 by Akt resulted in decreased Foxo1 activity and increased cell survival (16). Our TUNEL assay confirmed increased frequency of apoptotic cells, whereas PTEN knockdown markedly decreased apoptotic cell death in ischemic liver samples. Thus, PTEN-mediated Akt/β-catenin/Foxo1 axis is essential in promoting cytoprotection in IR-liver inflammation.
To further elucidate the mechanism by which PTEN-mediated Akt/β-catenin/Foxo1 axis may regulate IR-liver inflammation, we disrupted PI3K/Akt signaling by using a specific PI3K inhibitor (LY294002). Indeed, Akt is a direct downstream target positively regulated by PI3K, whereas activation of PI3K/Akt has been implicated in cytoprotection in liver (20) and heart (30) injury models. Our current data shows that PTEN knockdown improved hepatic function, promoted phosphorylation of Akt and inhibited nuclear Foxo1 in IR-stressed liver. However, PI3K inhibition in PTEN siRNA-treated mice decreased Akt phosphorylation and increased nuclear Foxo1, resulting in augmented IR-hepatocellular damage. These results are consistent with our previous in vitro and in vivo findings when PI3K blockade recreated liver IRI and increased TLR4-driven inflammation (20). Iinterestingly, PTEN knockdown upregulated β-catenin (Ser552) phosphorylation, whereas inhibition of PI3K in PTEN siRNA-treated mice downregulated phosphorylated β-catenin in ischemic liver. These are consistent with the report of activated PI3K/Akt to mediate β-catenin signaling in intestinal cells (31). The β-catenin has been implicated as an integral component in the Wnt signaling pathway and in cell development/tissue homeostasis (32). Indeed, Akt-induced phosphorylation of β-catenin via a binding motif containing Ser552 increases its nuclei accumulation and transcriptional activity (33). Activation of β-catenin inhibits NF-κB by impairing its DNA binding/transcription coding activity, and resulting in depressed expression of NF-κB target genes, including Fas and TRAF1 (34). These results were further supported by our in vitro studies in which inhibition of PI3K in PTEN siRNA-transfected BMMs decreased phosphorylation of Akt, Foxo1 and β-catenin but increased nuclear Foxo1, leading to enhanced TLR4-mediated proinflammatory response after LPS stimulation. Therefore, our results provide direct evidence that PI3K is required for PTEN-mediated Akt/β-catenin/Foxo1 signaling. Activated β-catenin can negatively regulate Foxo1, leading to inhibition of TLR4-mediated inflammation in the mechanism of liver IRI.
Figure 7 depicts putative molecular mechanism by which Akt/β-catenin/Foxo1 signaling may regulate IR-triggered inflammation response in the liver. PTEN knockdown increases Akt activity, which may then directly phosphorylate Foxo1 and inhibit its activity to modulate innate TLR4 function. Moreover, Akt-mediated β-catenin activation provides a negative feedback to the nuclear Foxo1. The β-catenin directly inhibits NF-κB activation, resulting in suppression of pro-inflammatory gene programs that otherwise drive the hepatocellular damage in IR-stressed liver forward.
Figure 7.
Schematic illustration of interlocked signaling pathways by which Akt/Foxo1 axis may affect innate immune responses in IR-stressed liver. See text for details.
In conclusion, PTEN-mediated Akt/β-catenin/Foxo1 axis regulates IR-triggered inflammation injury in the mouse liver. Knockdown of PTEN activates β-catenin, which then inhibits Foxo1, leading to local cytoprotection against TLR4-driven inflammation. By identifying molecular mechanisms of PTEN-mediated Akt/β-catenin/Foxo1 signaling in TLR4 innate immune regulation, our study provides the rationale for novel therapeutic approaches to manage panoply of local inflammatory responses in IR-stressed liver.
Supplementary Material
Primers used in qRT-PCR studies.
Acknowledgments
Financial Support: NIH Grants RO1 DK062357; DK 062357-06S1 (JWKW); The Diann Kim Foundation; The Dumont Research Foundation.
List of Abbreviations
- BMMs
bone marrow derived-macrophages
- Foxo
Forkhead box O
- IRI
ischemia/reperfusion injury
- MPO
myeloperoxidase
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homolog delete on chromosome 10
- sALT
serum alanine aminotransferase
- siRNA
small interfering RNA
- TLR4
Toll-like receptor 4
- TUNEL
terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling
- WT
wild-type
References
- 1.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: New insights into mechanisms of innate adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 3.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 4.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 6.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 7.Gunzl P, Schabbauer G. Recent advances in the genetic analysis of PTEN and PI3K innate immune properties. Immunobiology. 2008;213:759–765. doi: 10.1016/j.imbio.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann MP, Hemmings BA, Hafen E. Living with lethal PIP3 levels: viability of files lacking PTEN restored by a PH domain mutation in Akt/PKB. Science. 2002;295:2088–2091. doi: 10.1126/science.1068094. [DOI] [PubMed] [Google Scholar]
- 9.Cai Z, Semenza GL. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res. 2005;97:1351–1359. doi: 10.1161/01.RES.0000195656.52760.30. [DOI] [PubMed] [Google Scholar]
- 10.Omori N, Jin G, Li F, Zhang WR, Wang SJ, Hamakawa Y, Nagano I, et al. Enhanced phosphorylation of PTEN in rat brain after transient middle cerebral artery occlusion. Brain Res. 2002;954:317–322. doi: 10.1016/s0006-8993(02)03366-8. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 12.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 13.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 14.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 15.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, Inhester T, et al. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463:369–373. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- 18.Ke B, Shen XD, Gao F, Qiao B, Ji H, Busuttil RW, Volk HD, et al. Small interfering RNA targeting heme oxygenase-1 (HO-1) reinforces liver apoptosis induced by ischemia-reperfusion injury in mice: HO-1 is necessary for cytoprotection. Hum Gene Ther. 2009;20:1133–1142. doi: 10.1089/hum.2009.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, Busuttil RW, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, Busuttil RW, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: Regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 2012;56:359–366. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Kamo N, Shen XD, Ke B, Busuttil RW, Kupiec-Weglinski JW. Sotrastaurin, a protein kinase C inhibitor, ameliorates ischemia and reperfusion injury in rat orthotopic liver transplantation. Am J Transplant. 2011;11:2499–2507. doi: 10.1111/j.1600-6143.2011.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Havasi A, Gall JM, Mao H, Schwartz JH, Borkan SC. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol. 2009;20:1919–1928. doi: 10.1681/ASN.2009030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behl Y, Krothapalli P, Desta T, Roy S, Graves DT. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes. 2009;58:917–925. doi: 10.2337/db08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, Chen J. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem. 2010;112:1500–1512. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest. 2011;121:3456–3466. doi: 10.1172/JCI57984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 30.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, et al. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 31.Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, et al. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–881. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 33.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, et al. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used in qRT-PCR studies.