Abstract
Metabotropic glutamate receptors (mGluRs), particularly mGluR2/3, mGluR5 and mGluR7, have received much attention in medication development for the treatment of drug addiction and other neuropsychiatric diseases. However, little is known as to whether mGluR ligands also alter natural sexual behavior, a possible unwanted effect when used in humans. In the present study, we used classical copulatory behaviors to evaluate the effects of LY379268 (a selective mGluR2/3 agonist), MPEP (a selective mGluR5 antagonist) and AMN082 (a selective mGluR7 agonist), on male sexual performance in rats. We found that systemic administration of LY379268 (1, 3 mg/kg, i.p.) had no effect, while MPEP (20 mg/kg, but not 10 mg/kg, i.p.) and AMN082 (10, 20 mg/kg, but not 3 mg/kg) produced a significant and dose-dependent reduction in both sex-seeking and sex-performance behaviors, manifested as an increase in mount or intromission latency and time required for ejaculation, and a reduction in mount or intromission frequency. This inhibition lasted for about 30–60 min. These findings suggest that compounds that target mGluR5 or mGluR7, but not mGluR2/3, may have short-term inhibitory effects on male sexual performance.
Keywords: mGluR2/3, mGluR5, mGluR7, LY379268, MPEP, AMN082, sexual behavior
Introduction
Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS) and is extensively involved in many aspects of CNS function (Albrecht et al. 2007; Fonnum 1984). Abnormal glutamate neurotransmission, especially excessive glutamate release, can disrupt neuronal function and result in a variety of neurological and psychiatric disorders, such as schizophrenia, epilepsy, anxiety, stroke, pain, and drug addiction (Albrecht et al. 2007; Foster and Kemp 2006; Kalivas 2009). Glutamate action is mediated by activation of ionotropic and metabotropic glutamate receptors (mGluRs). mGluRs are divided into three groups based on their sequence homology, intracellular signal transduction mechanisms and pharmacological properties (Ferraguti and Shigemoto 2006). Group Ι mGluRs include mGluR1 and mGluR5, which are positively coupled to phospholipase C via Gq proteins. Group Π mGluRs include mGluR2 and mGluR3. Group Щ mGluRs include mGluR4, mGluR6, mluR7 and mGluR8, which are negatively coupled to adenylyl cyclase via Gi/o proteins (Cartmell and Schoepp 2000; Ferraguti and Shigemoto 2006). Since group II and III mGluRs functionally modulate presynaptic glutamate release (Bellone et al. 2008; Cartmell and Schoepp 2000; Spooren et al. 2003), substantial efforts have been made towards development of selective agonists or antagonists as potential medications for the treatment of neuropsychiatric diseases and drug addiction (Foster and Kemp 2006; Kalivas 2009; Parsons et al. 1998).
Among the eight mGluRs, the mGluR2/3s are the most well characterized. mGluR2/3s are predominantly expressed on presynaptic glutamatergic terminals and negatively regulate glutamate release (Bellone et al. 2008; Schoepp 2001). LY379268 is a highly potent and systemically effective mGluR2/3 receptor agonist (Monn et al. 1999). Extensive research during the past decade suggests that LY379268 and several other mGluR2/3 agonists may have significant therapeutic potential in animal models of stroke, epilepsy, schizophrenia, pain and drug addition (Foster and Kemp 2006; Imre 2007; Olive 2009). For example, systemic administration of LY379268 significantly inhibits phencyclidine- or d-amphetamine-induced locomotor behavior (Cartmell et al. 1999), cocaine self-administration (Adewale et al. 2006; Baptista et al. 2004) and reinstatement of drug-seeking behavior (Bossert et al. 2006a; Peters and Kalivas 2006). This action has been thought to be related to attenuation of cocaine-induced increase in extracellular DA and glutamate in the NAc (Xi et al. 2010a; Xi et al. 2010b).
In contrast to mGluR2/3s, mGluR5s are located predominantly on postsynaptic cells (Lujan et al. 1996), and activation of mGluR5s potentiates excitatory glutamate transmission (Cartmell and Schoepp 2000; Spooren et al. 2003). Recent studies suggest that mGluR5 blockade by MPEP or MTEP produces significant analgesic effects (Sevostianova and Danysz 2006; Varty et al. 2005; Zhu et al. 2004), anxiolytic activity (Varty et al. 2005), and anti-addictive effects (Backstrom and Hyytia 2006; 2007; Chiamulera et al. 2001; Herzig et al. 2005; Kenny et al. 2003). Fenobam, an orally active mGluR5 antagonist (Porter et al., 2005), has been approved by the U.S. Food and Drug Administration (FDA) for Phase II/III clinical trials for the treatment of Fragile X syndrome and L-DOPA-induced dyskinesia (Berry-Kravis et al, 2009).
Compared with group Ι and Π mGluRs, group III mGluRs are the least investigated due to the lack of selective group III mGluR pharmacological agents. With the recent development of AMN082, a systemically active mGluR7 agonist (Mitsukawa et al. 2005), we and others have recently reported that AMN082 produces significant anti-depressive (Palucha et al. 2007), anxiolytic (Cryan et al. 2003), and anti-addictive effects (to cocaine and alcohol) (Li et al. 2010; Li et al. 2009; Salling et al. 2008).
Such data suggest that mGluR ligands may have potential utility in the treatments of a number of CNS disorders, including drug addiction. Given that a number of dopaminergic drugs with significant anti-psychotic and anti-addiction profiles have unwanted effects on natural (food, sex) reward (Barrett et al. 2004; Lile et al. 2004; Melis and Argiolas 1995), the question arises as to whether any mGluR compounds have similar unwanted effects on natural reward. In this regard, animal models of food/sucrose/milk-taking or -seeking behaviors have been used to evaluate the effects of various compounds on food reward (Baptista et al. 2004; Bossert et al. 2006b; Lu et al. 2007; Peters and Kalivas 2006), but little is known as to whether any of the above-noted mGluR compounds alter male sexual behavior. Therefore, in the present study, we used classical copulatory behavior (Agmo 1997) to investigate the effects of the mGluR agents LY379268, MPEP and AMN082 on male sexual behavior in rats.
Materials and Methods
Animals
Both male and ovariectomized female Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA) weighing 275 to 300 g upon arrival were used. Male and female rats were housed separately in a climate-controlled animal colony room on a reversed light-dark cycle (lights on at 7:00 PM, lights off at 7:00 AM) with free access to food and water. The animals were maintained in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the U.S. National Academy of Sciences, and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health.
Experiment 1: Male sexual behavior
Mating test cages
Powder coated white cages (24 × 18 × 12 inch) were purchased from Quality Cage Company (Portland, Oregon, USA). The cage is made of 16 gauge wire. There is a 5 inch wide ramp and 10 × 12 inch balcony in the cage, made with easy-on-tiny feet wire. The front 10 inch of the top opens the full-width for animal access, and secures with a spring and hook latch. The body of the cage attaches securely to a metal tray with the same white color.
Screening and selecting experimental subjects
The estrus in the ovariectomized females was induced by subcutaneous (s.c.) administration of β-estradiol-3 benzoate (20 μg/rat) 48 hours before the test and progesterone (1mg/rat, s.c.) 4 hours before the test. All the experiments were conducted within 4~8 hours after the injection of progesterone. During the sexual behavior test, a male rat was placed in the mating cage 5 min prior to introduction of a sexually receptive female rat. The male rat was allowed to copulate with the female rat until the first post-ejaculatory intromission. Only males ejaculating within 15 minutes after the introduction of the female and with the interval between ejaculation and the next intromission less than 15 minutes were included in behavior studies.
Mating test procedure
On the behavior test day, a sexually receptive female was first put into the mating cage for 20 min in order to provide female olfactory stimuli for the male before the start of behavioral testing. Then, the male rat was placed into the mating cage, 5 min prior to a female rat re-introduction. During the 5 min before the female was introduced, the sex-seeking behavior as assessed by the number of level changes (LC) - the move from the base tray to the upper ramp or balcony, and the move from the ramp or balcony to the base tray, was counted. After the female was introduced, the following copulatory behavior parameters were recorded: mount latency (ML) - the time from the introduction of female to the first mount; intromission latency (IL) - the time from the introduction of female to the first intromission; mount frequency MF) - the number of mounts prior to ejaculation; intromission frequency (IF) - the number of intromissions prior to ejaculation; time for ejaculation (TE) - the time from the introduction of female to the first ejaculation; post-ejaculatory interval (PEI) - the time from ejaculatory behavior to the first intromission of the second copulatory series. Each testing lasted for 30 min beginning from the introduction of a sexually receptive female rat.
Drug effect testing
Three groups (n=8–10 per group) of rats were used to evaluate the effects of LY379268, MPEP and AMN082, respectively, on male sexual behavior. Each selected male rat randomly received one dose of the following drugs: LY379268 (vehicle, 1, 3 mg/kg, i.p.), MPEP (vehicle, 10, 20 mg/kg, i.p.) or AMN082 (vehicle, 3, 10, 20 mg/kg, i.p), and tested repeatedly for 3–5 times with 43–7 days of between test intervals. The drug sequence was counter-balanced. To determine the time course of drug action (if any), the highest effective dose of each drug was tested again in the same group of animals with two different pretreatment times (60 or 120 min prior to testing).
Experimental 2: Locomotor activity
Before receiving any drug, rats were placed in a locomotor detection chamber (Accuscan, Columbus, OH, USA) to record baseline locomotor activity for 1 h. Rats were then randomly received either the vehicle (1 ml/kg 0.5% Tween-80) or one dose (10, 20 mg/kg, i.p.) of MPEP to determine whether MPEP alone alters basal levels of locomotion. The order of testing for various doses of MPEP was counterbalanced. Following the injection, locomotor activity was recorded for 3h in 10 min bins, and distance counts were used to evaluate the effects of MPEP on basal levels of locomotion.
Experiment 3: Rotarod performance
To further determine whether MPEP alters locomotor performance or locomotor coordination ability, we observed the effects of MPEP on rat rotarod performance on a fast-running rotarod device. Performance on an accelerating rotarod was assessed using a four-station rat rotarod (AccuScan Instruments Inc., Columbus, Ohio, USA). The speed of rotation of the rotarod was increased from 2.5 to 40 rpm over 2 min and the time (Sec) the animal remained on the rod was determined as the mean of three trials. After 53–7 days of habituation and training on the rotarod device, three groups of rats randomly received either the vehicle or one dose (10, 20 mg/kg, i.p.) of MPEP before the rotarod test began. After the drug injection, animals were placed on the rotarod device to observe their locomotor performance over 30 min intervals for a total duration of 3 hrs.
Drugs
β-estradiol-3 benzoate and progesterone were purchased from Sigma (St. Louis, MO, USA). LY379268 [(−)-2-oxa-4-aminobicylco hexane-4,6-dicarboxylic acid] and MPEP [2-methyl-6-(phenylethynyl)-pyridine] were purchased from Tocris Bioscience (Ellisville, MO, USA). AMN082 [N,N'-dibenzyhydryl-ethane-1,2-diamine dihydrochloride] was purchased from Ascent Scientific (Bristol, UK). β-estradiol-3 benzoate and progesterone were dissolved in corn oil. LY379268 was dissolved in 0.9% saline. MPEP and AMN082 were dissolved in 0.5% Tween-80 (Sigma, St. Louis, MO, USA). The drug doses used in this study are the same as those commonly used in animal models of CNS disorders described above.
Statistical analysis
All data are presented as means (± S.E.M.). One-way analysis of variance (ANOVA) was used to analyze the effects of LY379268, MPEP or AMN082 on each parameter of copulatory behavior. Individual group comparisons were carried out using the Student-Newman-Keuls method.
Results
MPEP and AMN082, but not LY379268, inhibit sex-seeking behavior in male rats
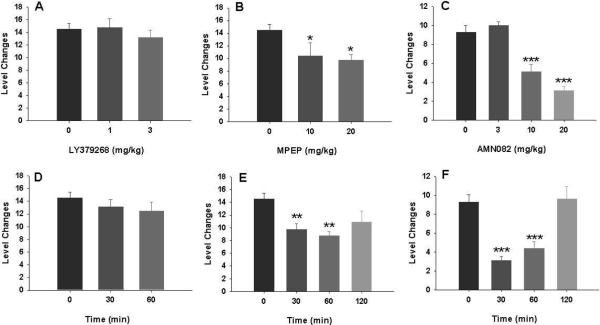
Figure 1 illustrates the effects of each representative mGluR ligand on sex-seeking behavior 3– operationally defined as changes in level within the mating cage after the cage was occupied by a sexually receptive female, demonstrating that LY379268 (1, 3 mg/kg, i.p., 30 min prior to testing) had no effect (F2,23=0.63, P>0.05), while MPEP (10, 20 mg/kg) (F2,22=4.09, P<0.05) or AMN082 (3, 10, 20 mg/kg) (F3,27=26.39, P<0.001) produced a significant dose-dependent reduction in sex-seeking behavior in the cage before the female partner was re-introduced. This effect lasted for about 303–60 min and recovered after 2 hrs.
Figure 1.
Effects of mGluR ligands on sex-seeking behavior in the appetitive phase of male sexual behavior. Pretreatment with LY379268 has no effect (A, D), while pretreatment with MPEP (B, E) or AMN082 (C, F) dose-dependently inhibited sex-seeking behavior (i.e., lever changes). This inhibitory effect produced by 20 mg/kg MPEP or AMN082 lasted for about 30–60 min (E, F). *p<0.05, **p<0.01, ***p<0.001, when compared to vehicle treatment group.
LY379268 has no effect on male sexual performance
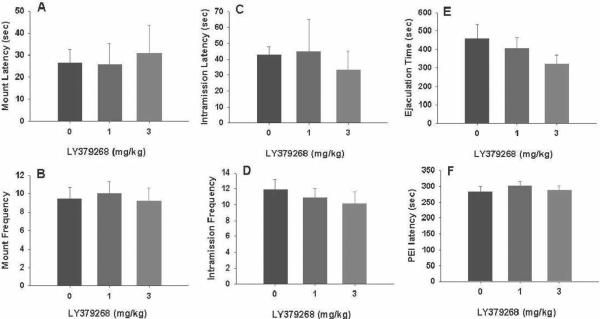
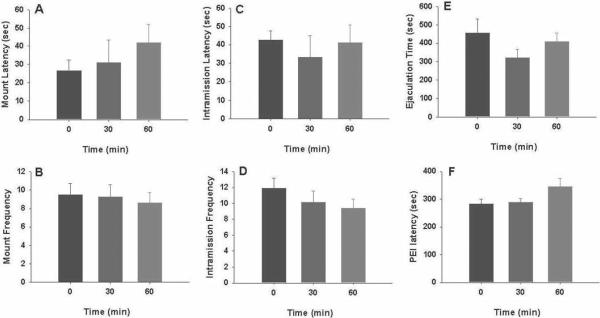
Figure 2 illustrates that pretreatment with LY379268 (1 or 3 mg/kg, i.p., 30 min prior to testing) failed to alter any measurement of male sexual behavior. Figure 3 illustrates the time-course of action of 3 mg/kg LY379268, showing the failure to alter male sexual behavior as compared to the vehicle group.
Figure 2.
Effects of LY379268 (1, 3 mg/kg, i.p., 30 min prior to testing) on consummatory male sexual behavior. Systemic administration of LY379268 has no effect on male sexual performance as assessed by mount latency (A) and frequency (B), intromission latency (C) and frequency (D), ejaculation latency (E) and postejaculatory interval (F).
Figure 3.
The time course of the effects produced by LY379268 on male sexual behavior. Systemic administration of LY379268 (3 mg/kg, i.p., 30, 60, 120 min prior to testing) has no effect on consummatory phase of sexual behavior.
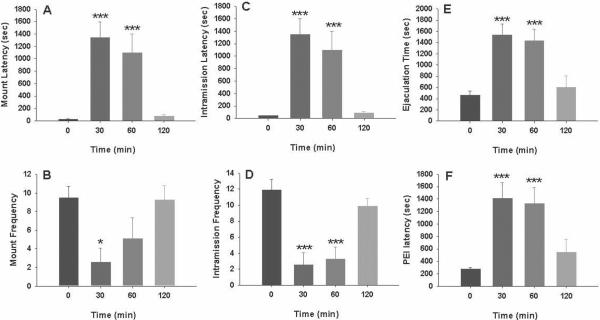
MPEP inhibits male sexual behavior at high doses
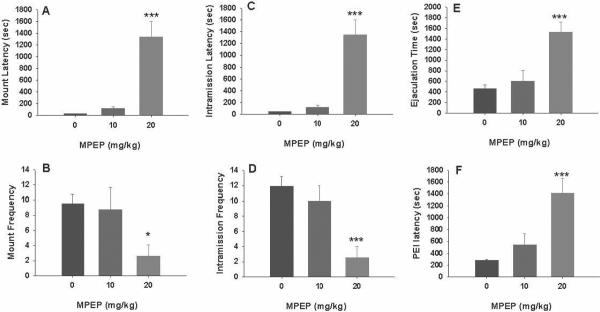
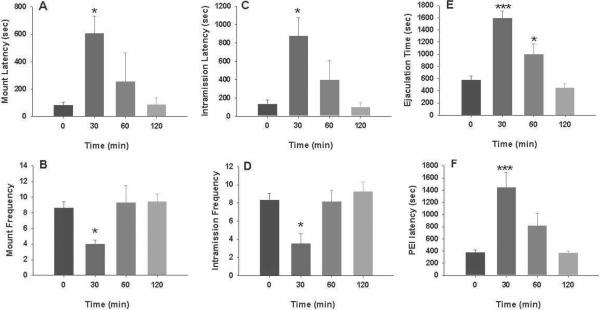
Figure 4 illustrates that MPEP (10, 20 mg/kg, i.p., 30 min prior to testing) significantly inhibited male sexual behavior, as assessed by a statistically significant increase in mount latency (Fig. 4A: F2,22=32.44, P<0.001), intromission latency (Fig. 4C: F2,22=32.45, P<0.001), time to ejaculation (Fig. 4E: F2,22=14.05, P<0.001) and post-ejaculation latency (Fig. 4F: F2,22=13.30, P<0.001), and a significant reduction in mount frequency (Fig. 4B: F2,22=3.94, P<0.05) and intromission frequency (Fig. 4D: F2,22=10.25, P<0.001). Individual group comparisons revealed statistically significant increases in latencies or decreases in frequencies of mounting, intromission and ejaculation after 20 mg/kg, but not 10 mg/kg, of MPEP (the statistical analysis results are labeled on each figure panel). Figure 5 shows the time courses of drug action measured at 30, 60 or 120 min after 20 mg/kg MPEP administration, demonstrating that the inhibitory effects produced by MPEP on male sexual behavior lasted for about 303–60 min, and recovered about 2 hr after drug administration.
Figure 4.
Effects of MPEP on consummatory male sexual behavior. Systemic administration of MPEP (10, 20 mg/kg, i.p., 30 min prior to testing) dose-dependently inhibited sexual behavior as evaluated by mount latency (A) and frequency (B), intromission latency (C) and frequency (D), ejaculation latency (E) and postejaculatory interval (F). *p<0.05, ***p<0.001, when compared to vehicle treatment group.
Figure 5.
Time course of the effects produced by MPEP on male sexual behavior. MPEP (20 mg/kg, i.p.), when administered 30 min or 60 min, but not 120 min, prior to testing, significantly inhibited sexual behavior as evaluated by mount latency (A) and frequency (B), intromission latency (C) and frequency (D), ejaculation latency (E) and postejaculatory interval (F). *p<0.05, **p<0.01, ***p<0.001, when compared with vehicle treatment group.
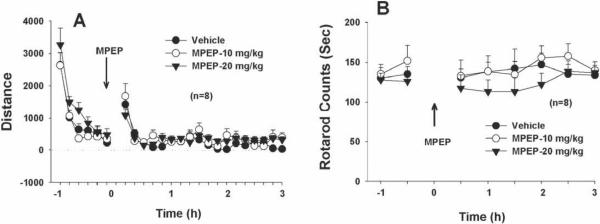
MPEP has no effect on locomotion or rotarod locomotor performance
To determine whether the reduction in sexual performance produced by MPEP was due to locomotor impairment, animals were tested for locomotor and rotarod performance. Figure 6A illustrates that systemic administration of MPEP, at the same doses noted above, did not alter basal levels of locomotion (F2,21=0.62, p=NS). Figure 6B illustrates that systemic administration of MPEP also failed to alter rotarod locomotor performance or locomotor coordination ability on a fast-turning rotarod device (main treatment effect, F5,10=0.77, p=NS)
Figure 6.
Effects of MPEP on locomotion and rotarod performance in rats. Panel A shows that MPEP (10–20 mg/kg, i.p.) had no effect on basal levels of locomotor activity. Panel B shows that the same doses of MPEP had no significant effect on rotarod locomotor performance.
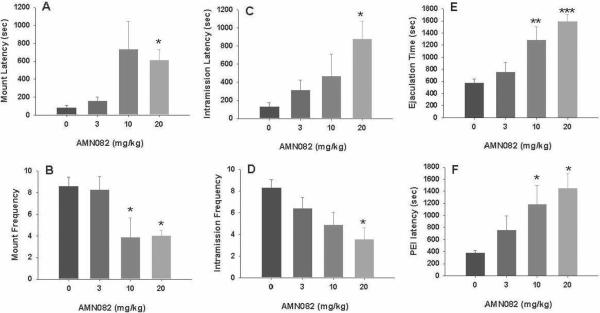
AMN082 inhibits male sexual behavior in a dose-dependent manner
Figure 7 illustrates that systemic administration of AMN082 (3, 10, 20 mg/kg i.p., 30 min prior to testing) significantly inhibited consummatory sexual behavior as assessed by a significant increase in mount latency (Fig. 7A: F3,26=3.30, P<0.05), intromission latency (Fig. 7C: F3,26=3.34, P<0.05), time to ejaculation (Fig. 7E: F3,26=10.40, P<0.001) and post-ejaculation latency (Fig. 7F: F3,26=4.31, P<0.05), and by a significant reduction in mount frequency (Fig. 7B: F3,26=4.93, P<0.01) and intromission frequency (Fig. 7D: F3,26=3.77, P<0.05). Individual group comparisons revealed a significant increase in time to ejaculation and ejaculation interval after 10 mg/kg and 20 mg/kg, but not 3 mg/kg, AMN082. Figure 8 shows the time courses of drug action measured at 30, 60 or 120 min after AMN082 administration, demonstrating that the inhibitory effects of 20 mg/kg AMN082 lasted for about 303–60 min, and recovered by 2 hrs after drug administration.
Figure 7.
Effects of AMN082 on consummatory male sexual behavior. Activation of mGluR7 by AMN082 (3, 10, 20 mg/kg, i.p., 30 min prior to testing) dose-dependently inhibited sexual behavior as evaluated by mount latency (A) and frequency (B), intromission latency (C) and frequency (D), ejaculation latency (E) and postejaculatory interval (F). *p<0.05, **p<0.01, ***p<0.001, when compared with vehicle treatment group.
Figure 8.
Time course of the effects of AMN082 on male sexual behavior. AMN082 (20 mg/kg, i.p.), when administered 30 min or 60 min, but not 120 min, prior to testing, significantly inhibited sexual behavior as evaluated by mount latency (A) and frequency (B), intromission latency (C) and frequency (D), ejaculation latency (E) and postejaculatory interval (F). *p<0.05, ***p<0.001, when compared with vehicle treatment group.
Discussion
Sexual activity consists of appetitive and consummatory phases. The appetitive phase is characterized by behaviors that denote sex-seeking, sexual excitement or arousal. Since these reflect the willingness or desire of an animal to initiate and engage in sexual interaction, the changes in behavior and motivation observed in this phase are used to evaluate sexual craving and sex-seeking in experimental animals (Balthazart et al. 1997; Mendelson and Pfaus 1989; Pfaus et al. 2007). The number of level changes made by the male in a bi-level chamber that a sexually receptive female had previously occupied, is the most commonly used parameter to measure the appetitive phase (Mendelson and Pfaus 1989; Van Furth and Van Ree 1996). The consummatory phase is characterized by copulatory sexual responses such as mount, intromission and ejaculation. Thus, frequency and latency of mount, intromission and ejaculation are the commonly used parameters of measurements (Agmo 1997).
The present findings show that systemic administration of LY379268 (1, 3 mg/kg, i.p.), a selective mGluR2/3 agonist, has no effect on male sexual behavior. This is consistent with previous reports that LY379268, at the same doses, failed to alter sweetened milk or sucrose intake (Baptista et al. 2004; Bossert et al. 2006b), suggesting that mGluR2/3s are not involved in natural (food, sex) reward. Previous studies have shown that LY379268, at the same dose range, produces significant anti-epileptic (Moldrich et al. 2001), anti-stroke (Bond et al. 1999), anti-nociceptic (Simmons et al. 2002), anti-addictive effects (Adewale et al. 2006; Baptista et al. 2004; Bossert et al. 2006b; Peters and Kalivas 2006). These data suggest that LY379268 or other mGluR2/3 agonists may have no significant effects on natural (food, sex) reward when used in humans. The lack of effect of LY379268 on natural reward could be related to the lack of mGluR2/3s in the medial preoptic area (mPOA), a critical brain region involved in sexual behaviors (Chiamulera et al. 2001; Paterson et al. 2003).
In contrast to LY379268, the mGluR5 antagonist MPEP dose-dependently inhibited male sexual behavior in both appetitive and consummatory phases. This inhibition is transient and lasts for about 303–60 min. Previous studies have shown that MPEP, within the dose range of 03–50 mg/kg, altered neither food self-administration (Tessari et al., 2004), nor food-conditioned place preference (Herzig et al. 2005). Similarly, MTEP (0.33–10 mg/kg), another selective mGluR5 antagonist, also failed to alter sweetened and condensed milk self-administration (Martin-Fardon et al. 2009). Thus, mGluR5 blockade may selectively inhibit sexual, but not food-taking, behavior. In vivo receptor binding assays suggest that MPEP produces >75% receptor occupancy when administered intraperitoneally (10 mg/kg) (Anderson et al., 2003) and almost complete receptor blockade when given intravenously (10 mg/kg) (Patel et al., 2005). These findings suggest that the presently observed effects produced by 103–20 mg/kg (i.p.) MPEP are most likely mediated by mGluR5 blockade.
The presently observed reduction in male sexual performance is unlikely due to sedation or locomotor impairment after MPEP administration, since the same doses of MPEP have no effect on either basal levels of locomotion or rotarod locomotor performance. This is consistent with previous reports that MPEP significantly inhibits locomotion or rotarod performance only at higher doses (30, 50, 100 mg/kg) (Herzig et al., 2005; Spooren et al., 2000; Varty et al., 2005).
An additional important finding is that the selective mGluR7 agonist AMN082 produced a significant dose-dependent inhibition of sexual behavior. This is consistent with previous reports that AMN082 significantly inhibits locomotion and sucrose self-administration in mice (Palucha et al. 2007; Salling et al. 2008), but conflicts with our previous report that AMN082 failed to alter sucrose self-administration or locomotor activity in rats (Li et al. 2009). We note that AMN082, at both 10 mg/kg and 20 mg/kg, significantly inhibits male sexual behavior, while AMN082 only at 20 mg/kg (but not 10 mg/kg) inhibits rotarod locomotor performance as reported previously (Li et al. 2009). These data suggest that low-dose AMN082-induced inhibition of sexual performance is unlikely to be mediated by non-specific locomotor deficit. However, with the increased dose, AMN082 may also produce significant locomotor impairment, which may contribute to reduction in sexual performance.
The mechanisms underlying the action produced by these compounds are unclear. A large amount of evidence suggests that the mesolimbic and hypothalamic dopamine (DA) systems play an important role in the central regulation of male sexual performance (Hull et al. 1995; Melis and Argiolas 1995). It has been reported that DA receptor agonists stimulate sexual excitement and arousal in both humans and rats, while DA receptor antagonists inhibit appetitive and consummatory sexual behaviors (Balthazart et al. 1997; Clement et al. 2009). However, we have previously reported that neither MPEP nor AMN082 altered extracellular DA levels in the nucleus accumbens (NAc) (Li et al. 2008; Xi et al. 2004), a critical brain region involved in brain reward function and drug addiction (Wise 2004), suggesting that a DA-dependent mechanism in the NAc may not be involved in MPEP- or AMN082-induced reduction in male sexual performance.
In addition to DA, recent studies suggest that glutamate-related mechanisms are also involved in the regulation of male sexual behavior (Dominguez 2009). In male rats, both the medial preoptic area (mPOA) in the mediobasal hypothalamus and the ventral tegmental area (VTA) in the midbrain have been shown to be critical brain regions involved in appetitive and consummatory sexual behaviors (Dominguez 2009; Georgescu and Pfaus 2006a; 2006b). It was reported that during mating, extracellular glutamate and phosphorylated NMDA receptors are up-regulated in the mPOA (Dominguez et al. 2007; Dominguez et al. 2006; Melis et al. 2004). The concentration of glutamate in the paraventricular nucleus of the hypothalamus is also increased in male rats during sexual activity (Melis et al. 2004). Further, microinjections of glutamate directly into the mPOA increase erections (Giuliano et al. 1996) and the urethrogenital reflex (Marson and McKenna 1994), while microinjection of dizocilpine, a noncompetitive NMDA antagonist, into the paraventricular nucleus impaired copulatory activity (Melis et al. 2004). Systemic injections of MK-801, an NMDA receptor antagonist, impaired male sexual behavior in sexually naive and sexually experienced male rats (Powell et al. 2003). These findings suggest that augmented glutamate transmission in mPOA plays an important role in male sexual performance. Accordingly, attenuation of glutamate transmission in this brain region would inhibit sexual behavior. Since high densities of mGluR5s and mGluR7s, while a low density of mGluR2/3s, are expressed in hypothalamus, including mPOA (Ferraguti and Shigemoto 2006), this may explain why mGluR5 and mGluR7, but not mGluR2/3, ligands altered male sexual behavior in the present study. As stated above, mGluR5s have been shown to be located primarily on postsynaptic cells, and mGluR5 activation potentiates glutamate transmission (Olive 2009). Thus, blockade of mGluR5 in the mPOA by MPEP would attenuate glutamate transmission, and therefore, inhibit glutamate-dependent male sexual performance. In addition, we have recently reported that activation of mGluR7s by AMN082 inhibits glutamate release in the NAc by a presynaptic mechanism (Li et al., 2008), suggesting that a similar glutamate-dependent mechanism may underlie the antagonism of male sexual behavior by AMN082. More studies are required to determine whether MPEP or AMN082 alters glutamate transmission in the mPOA.
In conclusion, the present study demonstrates that the mGluR2/3 agonist LY379268 fails to, while both the mGluR5 antagonist MPEP and the mGluR7 agonist AMN082 significantly inhibit male sexual behavior in a dose-dependent manner. This inhibition is reversible and lasts for about 30–60 min. These findings suggest that MPEP and AMN082 as well as other mGluR5/mGluR7 compounds may have transient, unwanted side-effects on male sexual behavior when used for treatment of neuropsychiatric diseases such as schizophrenia, anxiety, depression and mood disorder, and drug addiction.
The mGluR2/3 agonist LY379268 has no effect on male sexual behavior in rats.
The mGluR5 antagonist MPEP inhibits male sexual behavior in rats.
The mGluR7 agonist AMN082 inhibits male sexual behavior in rats.
These findings suggest potential side-effects of some mGluR ligands in humans
Acknowledgement
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–31. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- Agmo A. Male rat sexual behavior. Brain Res Brain Res Protoc. 1997;1:203–9. doi: 10.1016/s1385-299x(96)00036-0. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci. 2007;12:332–43. doi: 10.2741/2067. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford ND, Varney MA. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–86. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997;62:571–80. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–7. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–73. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65:2913–23. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. Journal of medical genetics. 2009;46(4):266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A, Ragumoorthy N, Monn JA, Hicks CA, Ward MA, Lodge D, O'Neill MJ. LY379268, a potent and selective Group II metabotropic glutamate receptor agonist, is neuroprotective in gerbil global, but not focal, cerebral ischaemia. Neurosci Lett. 1999;273:191–4. doi: 10.1016/s0304-3940(99)00663-1. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006a;31:2197–209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006b;173:148–52. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–70. [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30:1324–33. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–4. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Clement P, Pozzato C, Heidbreder C, Alexandre L, Giuliano F, Melotto S. Delay of ejaculation induced by SB-277011, a selective dopamine D3 receptor antagonist, in the rat. J Sex Med. 2009;6:980–8. doi: 10.1111/j.1743-6109.2008.01173.x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci. 2003;17:2409–17. doi: 10.1046/j.1460-9568.2003.02667.x. [DOI] [PubMed] [Google Scholar]
- Dominguez JM. A role for preoptic glutamate in the regulation of male reproductive behavior. Neuroscientist. 2009;15:11–9. doi: 10.1177/1073858408322679. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Balfour ME, Lee HS, Brown JL, Davis BA, Coolen LM. Mating activates NMDA receptors in the medial preoptic area of male rats. Behav Neurosci. 2007;121:1023–31. doi: 10.1037/0735-7044.121.5.1023. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Gil M, Hull EM. Preoptic glutamate facilitates male sexual behavior. J Neurosci. 2006;26:1699–703. doi: 10.1523/JNEUROSCI.4176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Foster AC, Kemp JA. Glutamate- and GABA-based CNS therapeutics. Curr Opin Pharmacol. 2006;6:7–17. doi: 10.1016/j.coph.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothalamus in the regulation of female rat sexual behaviors I. Behavioral effects of glutamate and its selective receptor agonists AMPA, NMDA and kainate. Pharmacol Biochem Behav. 2006a;83:322–32. doi: 10.1016/j.pbb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothalamus in the regulation of female rat sexual behaviors. II. Behavioral effects of selective glutamate receptor antagonists AP-5, CNQX, and DNQX. Pharmacol Biochem Behav. 2006b;83:333–41. doi: 10.1016/j.pbb.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Rampin O, Brown K, Courtois F, Benoit G, Jardin A. Stimulation of the medial preoptic area of the hypothalamus in the rat elicits increases in intracavernous pressure. Neurosci Lett. 1996;209:1–4. doi: 10.1016/0304-3940(96)12594-5. [DOI] [PubMed] [Google Scholar]
- Herzig V, Capuani EM, Kovar KA, Schmidt WJ. Effects of MPEP on expression of food-, MDMA- or amphetamine-conditioned place preference in rats. Addict Biol. 2005;10:243–9. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–71. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13:444–64. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–8. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Li X, Gardner EL, Xi ZX. The metabotropic glutamate receptor 7 (mGluR7) allosteric agonist AMN082 modulates nucleus accumbens GABA and glutamate, but not dopamine, in rats. Neuropharmacology. 2008;54:542–51. doi: 10.1016/j.neuropharm.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li J, Gardner EL, Xi ZX. Activation of mGluR7s Inhibits Cocaine-Induced Reinstatement of Drug-Seeking Behavior by a Nucleus Accumbens Glutamate-mGluR2/3 Mechanism in Rats. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li J, Peng XQ, Spiller K, Gardner EL, Xi ZX. Metabotropic glutamate receptor 7 modulates the rewarding effects of cocaine in rats: involvement of a ventral pallidal GABAergic mechanism. Neuropsychopharmacology. 2009;34:1783–96. doi: 10.1038/npp.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Morgan D, Birmingham AM, Davies HM, Nader MA. Effects of the dopamine reuptake inhibitor PTT on reinstatement and on food- and cocaine-maintained responding in rhesus monkeys. Psychopharmacology (Berl) 2004;174:246–53. doi: 10.1007/s00213-003-1738-x. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–8. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Marson L, McKenna KE. Stimulation of the hypothalamus initiates the urethrogenital reflex in male rats. Brain Res. 1994;638:103–8. doi: 10.1016/0006-8993(94)90638-6. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–90. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Dopamine and sexual behavior. Neurosci Biobehav Rev. 1995;19:19–38. doi: 10.1016/0149-7634(94)00020-2. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Mascia MS, Cortis L, Argiolas A. Extracellular excitatory amino acids increase in the paraventricular nucleus of male rats during sexual activity: main role of N-methyl-d-aspartic acid receptors in erectile function. Eur J Neurosci. 2004;19:2569–75. doi: 10.1111/j.0953-816X.2004.03362.x. [DOI] [PubMed] [Google Scholar]
- Mendelson SD, Pfaus JG. Level searching: a new assay of sexual motivation in the male rat. Physiol Behav. 1989;45:337–41. doi: 10.1016/0031-9384(89)90136-4. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Yamamoto R, Ofner S, Nozulak J, Pescott O, Lukic S, Stoehr N, Mombereau C, Kuhn R, McAllister KH, van der Putten H, Cryan JF, Flor PJ. A selective metabotropic glutamate receptor 7 agonist: activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc Natl Acad Sci U S A. 2005;102:18712–7. doi: 10.1073/pnas.0508063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich RX, Jeffrey M, Talebi A, Beart PM, Chapman AG, Meldrum BS. Anti-epileptic activity of group II metabotropic glutamate receptor agonists (--)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268) and (--)-2-thia-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY389795) Neuropharmacology. 2001;41:8–18. doi: 10.1016/s0028-3908(01)00044-2. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Jr., Wright RA, Johnson BG, Andis SL, Kingston A, Tomlinson R, Lewis R, Griffey KR, Tizzano JP, Schoepp DD. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–40. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–989. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha A, Klak K, Branski P, van der Putten H, Flor PJ, Pilc A. Activation of the mGlu7 receptor elicits antidepressant-like effects in mice. Psychopharmacology (Berl) 2007;194:555–62. doi: 10.1007/s00213-007-0856-2. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect. 1998;11:523–69. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–64. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–9. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J Sex Med. 2007;4(Suppl 4):269–79. doi: 10.1111/j.1743-6109.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6:1506–33. doi: 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, et al. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–21. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Powell WS, Dominguez JM, Hull EM. An NMDA antagonist impairs copulation and the experience-induced enhancement of male sexual behavior in the rat. Behav Neurosci. 2003;117:69–75. doi: 10.1037//0735-7044.117.1.69. [DOI] [PubMed] [Google Scholar]
- Salling MC, Faccidomo S, Hodge CW. Nonselective suppression of operant ethanol and sucrose self-administration by the mGluR7 positive allosteric modulator AMN082. Pharmacol Biochem Behav. 2008;91:14–20. doi: 10.1016/j.pbb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006;51:623–30. doi: 10.1016/j.neuropharm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Shimura T, Yamamoto T, Shimokochi M. The medial preoptic area is involved in both sexual arousal and performance in male rats: re-evaluation of neuron activity in freely moving animals. Brain Res. 1994;640:215–22. doi: 10.1016/0006-8993(94)91875-9. [DOI] [PubMed] [Google Scholar]
- Simmons RM, Webster AA, Kalra AB, Iyengar S. Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol Biochem Behav. 2002;73:419–27. doi: 10.1016/s0091-3057(02)00849-3. [DOI] [PubMed] [Google Scholar]
- Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol. 2003;14:257–77. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, Bergmann R, Kuhn R. Effects of the prototypical mGlu(5) receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine on rotarod, locomotor activity and rotational responses in unilateral 6-OHDA-lesioned rats. Eur J Pharmacol. 2000;406:403–10. doi: 10.1016/s0014-2999(00)00697-x. [DOI] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate receptor 5 receptors inhibits nicotine- and cocaine-taking behavior and prevents nicotine-triggered relapse to nicotine-seeking. Eur J. Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Patel S, Ndubizu O, Hamill T, Chaudhary A, Burns HD, Hargreaves R, Gibson RE. Screening cascade and development of potential Positron Emission Tomography radiotracers for mGluR5: in vitro and in vivo characterization. Mol Imaging Biol. 2005;7:314–23. doi: 10.1007/s11307-005-0005-4. [DOI] [PubMed] [Google Scholar]
- Van Furth WR, Van Ree JM. Appetitive sexual behavior in male rats: 2. sexual reward and level-changing behavior. Physiol Behav. 1996;60:1007–12. doi: 10.1016/0031-9384(96)00009-1. [DOI] [PubMed] [Google Scholar]
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, Hodgson RA, Lu SX, Nicolussi E, Pond AJ, Parker EM, Hunter JC, Higgins GA, Reggiani A, Bertorelli R. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology (Berl) 2005;179:207–17. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos A, Ashby C, Gardner E. The metabotropic glutamate receptor 5 antagonist MPEP blocks reinstatement of drug-seeking triggered by cocaine, but not by stress or cues. Abstract at the 66th Annual Meeting of the College on Problems of Drug Dependence; San Juan, Puerto Rico. 2004. [Google Scholar]
- Xi ZX, Kiyatkin M, Li X, Peng XQ, Wiggins A, Spiller K, Li J, Gardner EL. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacology. 2010a;58:304–13. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr. Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 2010b;112:564–76. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CZ, Wilson SG, Mikusa JP, Wismer CT, Gauvin DM, Lynch JJ, 3rd, Wade CL, Decker MW, Honore P. Assessing the role of metabotropic glutamate receptor 5 in multiple nociceptive modalities. Eur J Pharmacol. 2004;506:107–18. doi: 10.1016/j.ejphar.2004.11.005. [DOI] [PubMed] [Google Scholar]