Abstract
The majority of vulvar intraepithelial neoplasia (VIN) is high-grade and is related to high-risk human papillomavirus (HRHPV) (most commonly HPV16). It is considered to be the precursor of HRHPV-related vulvar squamous cell carcinoma. Vulvar condyloma acuminatum is low-risk HPV (LRHPV)-related (most commonly types 6 and 11) and has virtually no risk of neoplastic progression. While infection with multiple LR- and HRHPV types has been reported for cervical squamous intraepithelial lesions, coexisting vulvar condyloma and adjacent high-grade VIN have not been well characterized.
Eleven cases of concurrent condyloma acuminatum and adjacent flat high-grade VIN and three cases of high-grade VIN with prominent condylomatous architecture were analyzed using immunohistochemical (IHC) analysis of p16 expression, in situ hybridization (ISH) for HPV detection (HPV6/11, HPV16, HPV 18, and HPV WS [types 6,11,16,18,31,33,35,45,51,52] probes), and HPV typing by PCR-based method (in select cases).
All patients had underlying immunosuppressive conditions (human immunodeficiency virus infection or post-transplant therapy). Among the 11 cases of concurrent high-grade VIN and condyloma, the lesions were directly adjacent to one another in 5 cases (with 2 of these demonstrating an intimate admixture of lesions), and in 6 cases were found in separate tissue sections from the same specimen. Diffuse/strong p16 expression was seen in all high-grade VIN lesions, whereas patchy/weak staining was found in all condylomata. All condylomata contained HPV 6 or 11 as detected by ISH. All of the accompanying high-grade VIN lesions had HRHPV detected. Ten contained HPV 16 (9 by ISH, 1 by PCR), with the remaining case containing multiple HPV types by PCR. All condylomatous high-grade VIN lesions demonstrated diffuse/strong p16 expression and had evidence of HRHPV (one with HPV 16 by ISH, one with HPV 18 by ISH, and one with multiple HPV types by PCR), with no detection of HPV 6 or 11 by ISH.
The restriction of LRHPV to condylomatous components and HRHPV to high-grade VIN components of adjacent lesions suggests these are independent lesions caused by different HPV types. Diffuse p16 expression can highlight small foci of high-grade VIN which may be overlooked in more abundant condylomatous tissue from immunosuppressed patients. The presence of only HRHPV in those VIN lesions with high-grade cytologic features but prominent condylomatous architecture supports their classification as forms of pure high-grade VIN and distinguishes them from condyloma acuminatum.
Keywords: Human papillomavirus, vulvar cancer, vulvar intraepithelial neoplasia, condyloma acuminatum
Introduction
High-risk human papillomavirus (HRHPV) is an established cause of a significant proportion of vulvar intraepithelial neoplasia (VIN).1–3 The incidence of VIN has been increasing, along with a decrease in age at diagnosis.4–8 High-grade VIN, VIN 3 in particular, is considered the immediate precursor of HRHPV-related squamous cell carcinoma of the vulva. Similar to cervical high-grade squamous intraepithelial lesions (HSIL), the majority of VIN 3 lesions contain HPV 16.9–16 While it has been recognized that, in the cervix, HRHPV infection is responsible for a spectrum of squamous intraepithelial lesion grades (low-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia 1 [LSIL/CIN 1], high-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia 2 [HSIL/CIN 2], and high-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia 3 [HSIL/CIN 3]), the majority of vulvar HRHPV- related lesions are high-grade (VIN 2 and VIN 3) and the existence of VIN 1 has been questioned.17 The reported cases of flat VIN 1 harbor high-risk HPV in a significant number of cases; however, they mostly contain high-risk HPV types other than HPV 16.18
Low-risk human papillomavirus (LRHPV) infection is extremely common in the vulva14,19 and is responsible for the development of vulvar condyloma acuminatum.14,19 These lesions most commonly contain HPV6 and HPV11 and have virtually no risk of neoplastic progression.11,20,21 Nevertheless, with the increase in the incidence of condyloma acuminatum, it poses a significant health care problem that is associated with growing costs.22
Infections with multiple high- and low-risk HPV types have been reported in cervical and vulvar specimens containing intraepithelial lesions.16,18,21,23–28 Some studies have demonstrated that, in the cervix, LSIL/CIN 1 and HSIL/CIN 3 that are present in the same specimen harbor different HPV subtypes in over 30% of cases, indicating that some of these cases may contain independent lesions related to infection with multiple HPV types.24,25 It is reasonable to expect that concurrent infection by high- and low-risk HPV types in the vulva can also lead to the simultaneous development of independent, morphologically distinct lesions. However, cases of coexisting vulvar condyloma acuminatum and adjacent high-grade VIN have not been well characterized with respect to HPV detection within the morphologically distinct lesional components. There are occasional reports of the presence of HRHPV in vulvar specimens diagnosed as condyloma acuminatum, but the morphology of these lesions has not been described in detail.11,20,21 Therefore, the current study was undertaken to investigate the correlation of morphology and HPV type along with immunohistochemical analysis of p16 expression in a series of vulvar lesions comprised of coexisting high-grade VIN and condyloma acuminatum.
Methods
The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. Vulvar specimens containing both high-grade VIN and condyloma acuminatum were retrieved from the files of the Department of Pathology of The Johns Hopkins Hospital. The hematoxylin and eosin (H&E)-stained slides of all cases were reviewed by two authors (KM and AY) and selected cases and images were reviewed by the co-authors. Immunohistochemical analysis of p16 and Ki-67 expression and HPV detection were performed as described below. While performing slide review and case selection for this study, we also encountered three cases in which vulvar lesions had a strikingly condylomatous architecture, beyond that which is usually seen in warty VIN, coupled with cytologic features typical of high-grade squamous intraepithelial lesions; these cases were also included.
In situ hybridization for HPV was performed using HPV 16, HPV 6/11, and HPV wide spectrum (HPVWS) probes (see below). For the cases in which a specific HRHPV type (HPV 16) was not detected by in situ hybridization, HPV typing was performed using PCR-based INNO-LiPA assay (see below). For two cases of condylomatous VIN in which multiple HPV types, including HPV 18, were detected by PCR-based method, in situ hybridization for HPV 18 was also subsequently performed.
Immunohistochemical analysis of p16 and Ki-67 expression
Immunohistochemical analysis for p16 was performed using prediluted anti-p16 clone E6H4 (CINtec, mtm laboratories AG, Germany) with primary antibody incubation time of 28 minutes at room temperature. Antigen retrieval was performed under standard optimized conditions using CC1 Mild reagent. Immunoperoxidase labeling was detected with the iView DAB Detection kit (Ventana, Tucson, AZ) on the automated BenchMark XT IHC Staining Module (Ventana Medical Systems, Tucson, AZ) as previously described.29 Based on the extent of immunolabeling, p16 immunoexpression (both nuclear and cytoplasmic staining) was recorded as diffuse positive (defined as diffuse band-like staining within at least the lower one-half to two-thirds of the epithelium), patchy positive, or negative.
Immunohistochemical analysis for Ki-67 was carried out using anti-Ki-67 mouse monoclonal antibodies (clone 30-9, Ventana, Tucson, AZ), prediluted 1:1 with an incubation time of 16 minutes at 37°C. Antigen retrieval was performed under standard optimized conditions using CC1 reagent. Immunoperoxidase labeling was detected with iView DAB Detection kit (Ventana, Tucson, AZ) on the automated BenchMark XT IHC Staining Module (Ventana Medical Systems, Tucson, AZ). For Ki-67, nuclear staining was evaluated. Ki-67 proliferative activity was scored as low, moderately elevated, or markedly elevated based on the presence and number of positive nuclei in the epithelial layers above the parabasal area.
HPV DNA detection
In situ hybridization for HPV
In situ hybridization for HPV was performed in all cases. After deparaffinization and rehydration, biotin-labeled HPV probe solutions (Dako Corp, Carpinteria, CA) were applied to individual sections. These included a wide spectrum probe (HPV WS [cocktail of HPV 6, 11, 16, 18, 31, 33, 35, 45, 51, and 52]) and separate type-specific probes for HPV 16, HPV 6/11, and HPV 18 (in selected cases). Detection of hybridized probe was performed by tyramide-catalyzed signal amplification using the Dako Genpoint Kit (Dako Corp, Carpinteria, CA). Chromogenic detection was performed with Diaminobenzidine/H2O2. Controls included tissue sections positive for HPV wide spectrum and the SiHa cell line for HPV 16. Biotin-labeled plasmid probes served as a negative control in each case. Cases with a discrete reaction product (either dot-like or diffuse) located in the lesional cell nuclei were interpreted as positive. Positive reaction with HPV WS probe in the absence of signal with HPV 6/11 probe was considered evidence of high-risk HPV presence.
INNO-LiPA HPV typing method
For the cases in which a specific HRHPV type (HPV 16) was not detected by in situ hybridization, HPV typing was performed using PCR-based INNO-LiPA assay. To extract the DNA, paraffin was solubilized with octane. The tissue was pelleted by centrifugation and washed with 100% ethanol. After removing the ethanol after centrifugation, the tissue was dried with the addition of 10 µL acetone in a 55°C heating block. Dried tissues were resuspended in a 1X digestion buffer of 200 µg/mL proteinase K and 0.1% laureth-12 and incubated at 55°C until the tissue was fully digested. Protease was heat-inactivated by incubation at 95°C for 10 minutes. Polymerase chain reactions were run on a Gene Amp PCR System 9700 engine thermocycler (Applied Biosystems, Foster City, CA). HPV type-specific detection was conducted using the INNO-LiPA HPV Genotyping Kit according to the manufacturer's instructions (Innogenetics, Gent, Belgium). This system is capable of detecting 28 specific HPV types.30–32 The LiPA strips were manually interpreted using the reference guide provided.
Results
Eleven cases of concurrent condyloma acuminatum and adjacent flat high-grade VIN were studied. In 5 of these cases, high-grade VIN and condyloma were immediately adjacent to each other, and in 6 cases, they were present in separate tissue sections within the same specimen. In two of the former cases, foci of cytologically high grade epithelium were present within the condyloma.
The other three vulvar specimens displayed strikingly condylomatous architecture at low magnification (beyond what is usually observed in cases of warty high-grade VIN) and marked cytologic atypia as well as increased mitotic activity in the upper epithelial layers, similar to that seen in flat high-grade VIN and not typical of condyloma. These cases were classified as condylomatous high-grade VIN.
The patients’ ages ranged from 27–57 years (median/mean age 39/39.8 years). Prior or concurrent abnormal cervical cytology and/or other evidence of HRHPV infection was noted in 13 (of 14) patients. All patients had underlying conditions with evidence of immunosuppression: 13 had human immunodeficiency virus infection and one was undergoing post-transplant immunosuppressive therapy.
In cases with concurrent high-grade VIN and condyloma, all high-grade VIN lesions had HRHPV DNA detected: 10/11 contained HPV 16 (9 by in situ hybridization [ISH] and 1 by PCR) and one case had positive HPV WS in situ probe and no detectable signal by HPV 16 or HPV 6 and 11 probes. In this latter case, HPV 33, 44, 52, and 54 were detected by PCR. All condylomata acuminata (11/11) had HPV 6 or HPV 11 types detected by ISH (Figures 1 and 2). These data are summarized in Table 1.
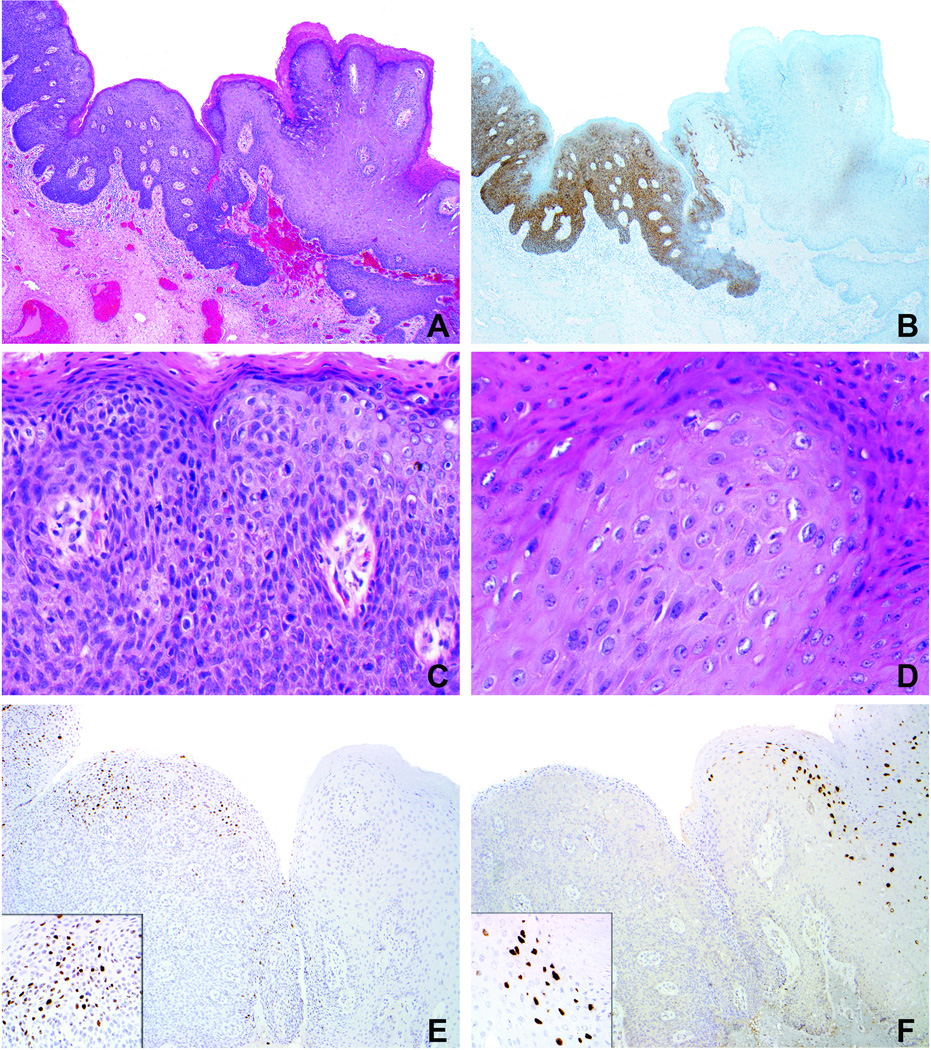
Figure 1.
Case 3 (Table 1). A. Condyloma acuminatum (right) with adjacent flat high-grade VIN (left). B. Diffuse p16 expression in high-grade VIN and extremely focal to negative staining in condyloma. C. High-grade VIN exhibits loss of maturation, marked nuclear atypia, and frequent mitotic figures. D. Condyloma is composed of more mature keratinocytes with enlarged nuclei and some koilocytotic change. E. In situ hybridization preparation demonstrates nuclear signals with the HPV 16 probe in the area of high-grade VIN (no signal in condyloma). F. In situ hybridization preparation demonstrates nuclear signals with the HPV 6/11 probe in the area of condyloma (no signal in high-grade VIN).
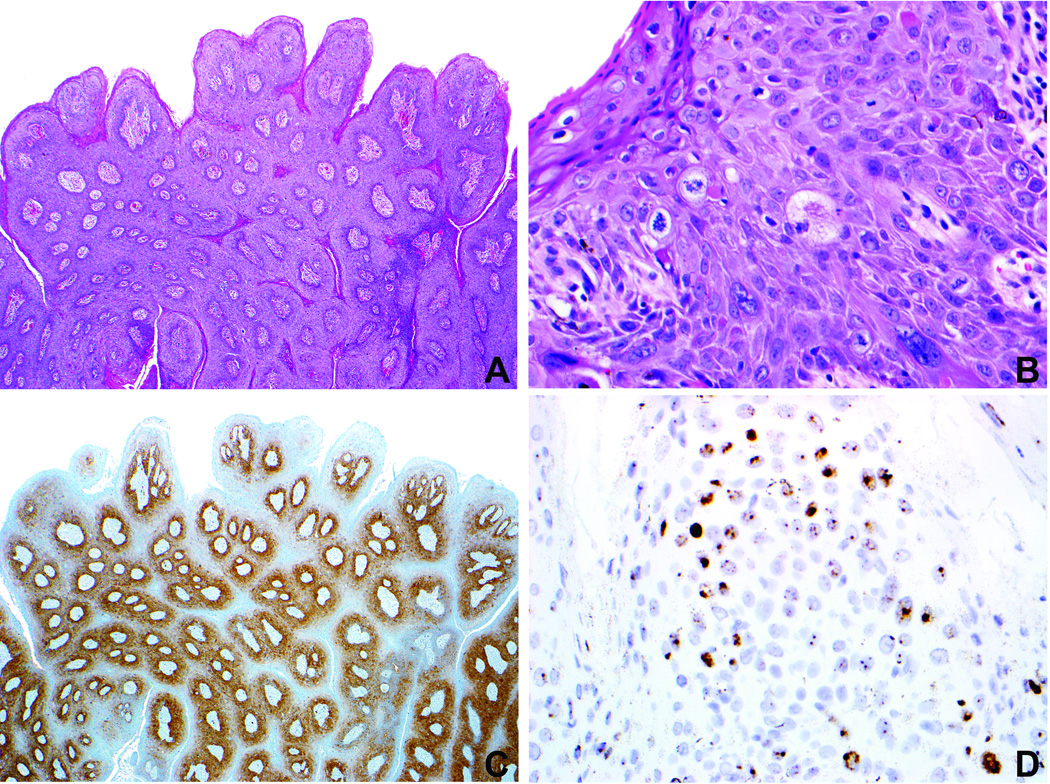
Figure 2.
Case 11 (Table 1). A. Focus of high-grade VIN (arrow, inset) within a condyloma acuminatum. B. High-grade VIN displays diffuse/strong p16 expression (arrow) whereas the surrounding condyloma displays patchy/weak p16 expression. C. In situ hybridization preparation demonstrates nuclear signals with the HPV 6/11 probe in the condyloma (no signal in focus of high-grade VIN). D. In situ hybridization preparation demonstrates nuclear signals with the HPV 16 probe in the high-grade VIN component (arrow; no signals in the surrounding condyloma).
Table 1.
HPV detection and immunohistochemical analysis of p16 expression in cases with coexisting high-grade VIN and condyloma acuminatum.
| Case number |
High-grade VIN | Condyloma acuminatum | ||
|---|---|---|---|---|
| HPV type | P16 expression | HPV type | P16 expression | |
| 1 | HPV 16 | diffuse | HPV 6 or HPV 11** | patchy |
| 2 | HPV 16* | diffuse | HPV 6 or HPV 11 | patchy |
| 3 | HPV 16 | diffuse | HPV 6 or HPV 11 | patchy |
| 4 | HPV 16 | diffuse | HPV 6 or HPV 11 | patchy |
| 5 | HPV 16 | diffuse | HPV 6 or HPV 11 | patchy |
| 6 | HPV 16 | diffuse | HPV 6 or HPV 11 | patchy |
| 7 | HPV 16 | diffuse | HPV 6 or HPV 11 | patchy |
| 8 | HPV 16 | diffuse | HPV 6 or HPV 11 | patchy |
| 9 | HPV 33,44,52,54* | diffuse | HPV 6 or HPV 11 | patchy |
| 10 | HPV 16 | diffuse | HPV 6 or HPV 11 | patchy |
| 11 | HPV 16 | diffuse | HPV 6 or HPV 11 | patchy |
HPV types detected by PCR; for the remainder of the cases HPV was detected by in situ hybridization.
HPV in all condylomata was detected by in situ hybridization using combined probe for HPV 6 and HPV 11, therefore detection of individual types (HPV 6 or 11) is not possible.
Among cases of high-grade VIN with prominent condylomatous architecture and high-grade cytologic features, one had HPV 16 detected by ISH; the remaining two were positive by HPV WS ISH with no detectable HPV 6 or 11 by ISH. These two cases had HPV 18, 39, 51, 66, 74 and HPV 18, 35, 39 detected by PCR. HPV 18 was detected by in situ hybridization in the former but not in the latter (Figure 3). These data are summarized in Table 2.
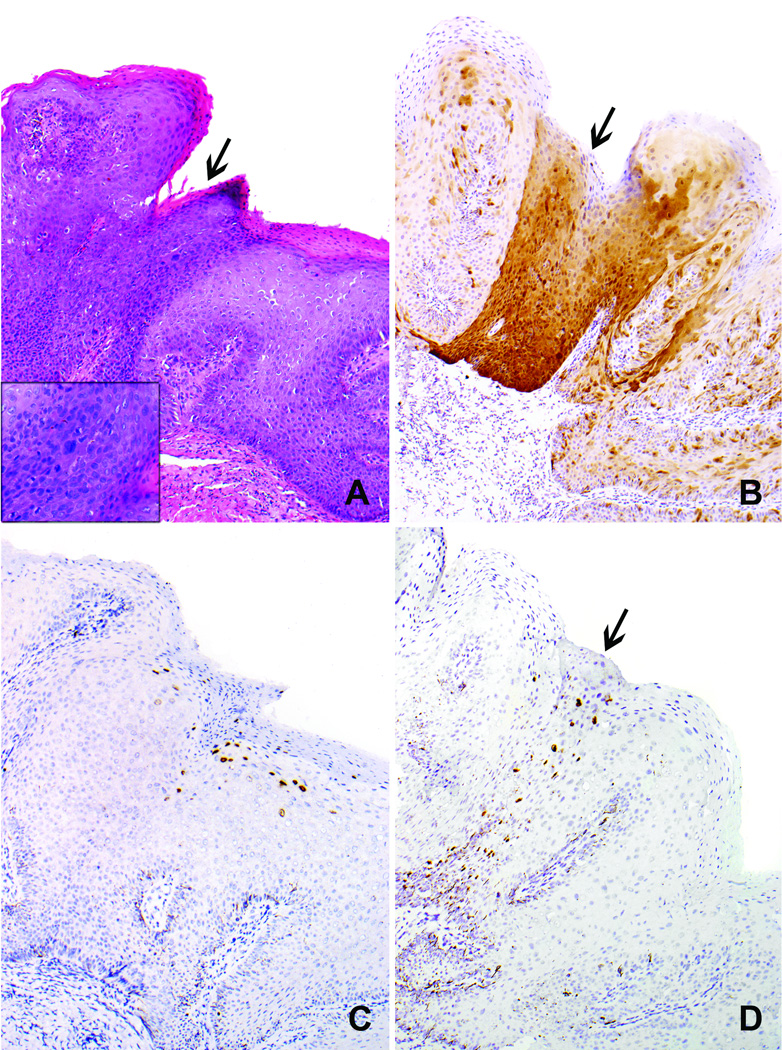
Figure 3.
Case 1 (Table 2). A. Condylomatous high-grade VIN demonstrates striking papillary architecture at low magnification, simulating a condyloma acuminatum. B. Immaturity and notable nuclear atypia consistent with high-grade VIN are evident at higher magnification. C. The lesion demonstrates diffuse p16 expression. D. In situ hybridization preparation demonstrates positive signals with the HPV wide spectrum probe [HPV types 6,11,16,18,31,33,35,45,51,52] and HPV 18 (not shown). There was no detectable HPV with the HPV 6/11 probe (data not shown). These findings support a diagnosis of a high-risk HPV-related high-grade VIN.
Table 2.
HPV detection and immunohistochemical analysis of p16 expression in cases of condylomatous high grade VIN.
| Case number |
In situ hybridization for HPV | HPV DNA detection PCR analysis |
P16 expression |
||
|---|---|---|---|---|---|
| HPV WS | HPV 6/11 | HPV 6/11 | |||
| 1 | Positive | Not detected | Not detected | 18*,39,51,66,74 | diffuse |
| 2 | Positive | Not detected | Not detected | 18**,35,39 | diffuse |
| 3 | Positive | Positive | Not detected | Not performed | diffuse |
HPV 18 was also detected by in situ hybridization.
No detectable HPV 18 by in situ hybridization.
All high-grade VIN areas had diffuse expression of p16, as did the 3 cases of condylomatous high-grade VIN. In contrast, weak and patchy to absent p16 expression was seen in all condylomata acuminata. Ki-67 proliferative activity was variably elevated in all the lesions without notable differences between high-grade VIN and condyloma (data not shown).
Discussion
Infections with multiple HPV types, including co-infection with high- and low-risk HPV types, are commonly observed in lower genital tract squamous intraepithelial lesions.16,18,21,23–28 Distinct lesion morphology associated with HRHPV infection (i.e. high-grade VIN) vs. LRHPV (i.e. condyloma acuminatum) has been well established. The cases in this study emphasize the possibility of high-grade VIN occurring adjacent to or even admixed within a condyloma acuminatum, particularly in immunosuppressed patients. Recognition of this possibility is important both clinically and pathologically. An extensive condylomatous growth can overwhelm or obscure an adjacent or admixed flat high-grade VIN, leading to failure to sample the more clinically significant lesion. Similarly, small foci of high-grade VIN may be overlooked when evaluating more abundant condylomatous lesional tissue microscopically, leading to failure to diagnose the more significant component of the pathologic process. When diagnosing these cases, it is important to emphasize the presence of high-grade VIN, as this component is clinically more relevant than the often abundant background of condyloma acuminatum. High-grade VIN encompasses both VIN 2 and VIN 3.17 It has been demonstrated that combining the two VIN grades (VIN 2 and VIN3) as high-grade VIN leads to good intra-observer agreement.33 Since the distinction of VIN 2 and VIN 3 does not alter clinical management, high-grade VIN represents a clinically relevant diagnostic category. In our practice, we use the term high-grade VIN with parenthetical notation of the sub-grade, i.e. high-grade VIN (VIN 2) or (VIN 3), but commonly use high-grade VIN (VIN 2–3) because lesions with both grades are common in our experience.
A rare subset of HRHPV-related vulvar lesions with prominent condylomatous architecture, as evidenced by the 3 such cases identified in the current study, is probably more closely related to typical high-grade VIN rather than condyloma acuminatum, based on morphology (high-grade cytology), HPV typing data (detection of high-risk rather than low-risk types), and p16 expression (diffuse). These cases may represent what has been previously reported as condyloma acuminatum containing HRHPV.11,20,21 The use of the term “condyloma” should be avoided in such cases as it implies a LRHPV-related lesion with virtually no risk of progression to carcinoma, which can be misleading for clinical management. Analysis of additional examples of this uncommon variant would be useful to confirm our findings.
The prevalence of HPV-related lesions containing both low- and high-risk HPV in patients with immunosuppression, particularly human immunodeficiency virus (HIV) infection, is higher than in the general population.34–36 These conditions represent known predisposing factors for persistent HPV infections, with both frequent lesion persistence/recurrence and increased potential for development of invasive carcinoma. All patients in this study had immunosuppressive conditions, including HIV infection in the majority of patients and post-transplant therapy in one, suggesting that immunosuppressed patients may be at increased risk for developing the variants of high-grade VIN described herein. Thus, awareness of the potential for high-grade VIN to develop in intimate association with condyloma acuminatum or display architectural features simulating a low-risk HPV-related lesion (condyloma acuminatum) is especially important in this patent population.
Although HPV DNA detection with PCR-based methodology using the entire tissue section is considered a “gold standard” and is most commonly used in research, it often generates a list of several HPV types and does not provide information regarding the HPV type that is implicated in the development of a lesion. The attribution of an individual HPV type as likely causal of a particular lesional focus is possible with in situ hybridization techniques (ISH). In this report, we describe evidence of high- and low-risk HPV in adjacent vulvar lesions as detected by ISH. All but one (10 of 11) of the high-grade VIN lesions contained HPV16, which is the HPV type most commonly detected in high-grade VIN in other studies.9–16 All condylomata acuminata contained HPV 6/11, as detected by in situ hybridization.
While the in situ hybridization technique was successful in detecting specific HPV types in the majority of cases, its imperfect sensitivity is recognized. Immunohistochemical analysis of p16 expression is frequently used as an adjunct to morphologic diagnosis of high-risk HPV-related lesions. Multiple studies have shown that p16 is a sensitive marker of HRHPV-related VIN.37–40 This data is concordant with our findings of diffuse p16 expression in all VINs that were associated with the detection of HRHPV. All vulvar condylomata were associated with focal/patchy to absent expression of p16, which is consistent with what was previously described for anal condylomata.41 Therefore, p16 is useful in the detection of vulvar lesions related to HRHPV and in their discrimination from LRHPV-related condyloma acuminatum and other non-HPV-related vulvar lesions.
Immunohistochemical expression of Ki-67 was variably elevated in all lesions in this study, including both high-grade VIN and condyloma acuminatum (data not shown). Although this marker does not discriminate between the two HPV-related entities, it has been reported to be useful in differentiating these from non-HPV-related vulvar lesions.21,23,41
In summary, mixed infections with low- and high-risk HPV types can occur in the vulva and lead to the development of adjacent morphologically distinct lesions. The possibility of finding small foci of high-grade VIN adjacent to condyloma acuminatum is important to recognize when evaluating vulvar specimens, especially from patients with immunosuppressive conditions. Vulvar lesions with strikingly condylomatous architecture and high-grade cytologic features are related to high-risk HPV and, therefore, likely represent variants of high-grade VIN.
Acknowledgments
This study was supported in part by NIH/NCI/ R21CA150033. Dr. Yemelyanova is supported by Cervical SPORE career development award NIH/ NCI/ P50 CA098252. Dr. Ronnett has been compensated by Roche mtm laboratories AG for educational lectures (webinars).
The authors thank Aleksandra Ogurtsova for technical assistance with HPV detection by PCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: For the remaining authors none were declared.
References
- 1.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 2.van der Avoort IA, Shirango H, Hoevenaars BM, et al. Vulvar squamous cell carcinoma is a multifactorial disease following two separate and independent pathways. Int J Gynecol Pathol. 2006;25:22–29. doi: 10.1097/01.pgp.0000177646.38266.6a. [DOI] [PubMed] [Google Scholar]
- 3.Skapa P, Zamecnik J, Hamsikova E, et al. Human papillomavirus (HPV) profiles of vulvar lesions: possible implications for the classification of vulvar squamous cell carcinoma precursors and for the efficacy of prophylactic HPV vaccination. Am J Surg Pathol. 2007;31:1834–1843. doi: 10.1097/PAS.0b013e3180686d10. [DOI] [PubMed] [Google Scholar]
- 4.Joura EA, Losch A, Haider-Angeler MG, et al. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J Reprod Med. 2000;45:613–615. [PubMed] [Google Scholar]
- 5.Jones RW, Baranyai J, Stables S. Trends in squamous cell carcinoma of the vulva: the influence of vulvar intraepithelial neoplasia. Obstet Gynecol. 1997;90:448–452. doi: 10.1016/s0029-7844(97)00298-6. [DOI] [PubMed] [Google Scholar]
- 6.Judson PL, Habermann EB, Baxter NN, et al. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107:1018–1022. doi: 10.1097/01.AOG.0000210268.57527.a1. [DOI] [PubMed] [Google Scholar]
- 7.Saraiya M, Watson M, Wu X, et al. Incidence of in situ and invasive vulvar cancer in the US, 1998–2003. Cancer. 2008;113:2865–2872. doi: 10.1002/cncr.23759. [DOI] [PubMed] [Google Scholar]
- 8.Bodelon C, Madeleine MM, Voigt LF, et al. Is the incidence of invasive vulvar cancer increasing in the United States? Cancer Causes Control. 2009;20:1779–1782. doi: 10.1007/s10552-009-9418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoevenaars BM, van der Avoort IA, de Wilde PC, et al. A panel of p16(INK4A), MIB1 and p53 proteins can distinguish between the 2 pathways leading to vulvar squamous cell carcinoma. Int J Cancer. 2008;123:2767–2773. doi: 10.1002/ijc.23857. [DOI] [PubMed] [Google Scholar]
- 10.Hording U, Daugaard S, Junge J, et al. Human papillomaviruses and multifocal genital neoplasia. Int J Gynecol Pathol. 1996;15:230–234. doi: 10.1097/00004347-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Buscema J, Naghashfar Z, Sawada E, et al. The predominance of human papillomavirus type 16 in vulvar neoplasia. Obstet Gynecol. 1988;71:601–606. [PubMed] [Google Scholar]
- 12.Gasco M, Sullivan A, Repellin C, et al. Coincident inactivation of 14-3-3sigma and p16INK4a is an early event in vulval squamous neoplasia. Oncogene. 2002;21:1876–1881. doi: 10.1038/sj.onc.1205256. [DOI] [PubMed] [Google Scholar]
- 13.van Beurden M, ten Kate FJ, Smits HL, et al. Multifocal vulvar intraepithelial neoplasia grade III and multicentric lower genital tract neoplasia is associated with transcriptionally active human papillomavirus. Cancer. 1995;75:2879–2884. doi: 10.1002/1097-0142(19950615)75:12<2879::aid-cncr2820751214>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Barzon L, Militello V, Pagni S, et al. Distribution of human papillomavirus types in the anogenital tract of females and males. J Med Virol. 2010;82:1424–1430. doi: 10.1002/jmv.21733. [DOI] [PubMed] [Google Scholar]
- 15.Garland SM, Insinga RP, Sings HL, et al. Human papillomavirus infections and vulvar disease development. Cancer Epidemiol Biomarkers Prev. 2009;18:1777–1784. doi: 10.1158/1055-9965.EPI-09-0067. [DOI] [PubMed] [Google Scholar]
- 16.Insinga RP, Liaw KL, Johnson LG, et al. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:1611–1622. doi: 10.1158/1055-9965.EPI-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sideri M, Jones RW, Wilkinson EJ, et al. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med. 2005;50:807–810. [PubMed] [Google Scholar]
- 18.Srodon M, Stoler MH, Baber GB, et al. The distribution of low and high-risk HPV types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN) Am J Surg Pathol. 2006;30:1513–1518. doi: 10.1097/01.pas.0000213291.96401.48. [DOI] [PubMed] [Google Scholar]
- 19.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 20.McLachlin CM, Kozakewich H, Craighill M, et al. Histologic correlates of vulvar human papillomavirus infection in children and young adults. Am J Surg Pathol. 1994;18:728–735. doi: 10.1097/00000478-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Pirog EC, Chen YT, Isacson C. MIB-1 immunostaining is a beneficial adjunct test for accurate diagnosis of vulvar condyloma acuminatum. Am J Surg Pathol. 2000;24:1393–1399. doi: 10.1097/00000478-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Hoy T, Singhal PK, Willey VJ, et al. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin. 2009;25:2343–2351. doi: 10.1185/03007990903136378. [DOI] [PubMed] [Google Scholar]
- 23.Logani S, Lu D, Quint WG, et al. Low-grade vulvar and vaginal intraepithelial neoplasia: correlation of histologic features with human papillomavirus DNA detection and MIB-1 immunostaining. Mod Pathol. 2003;16:735–741. doi: 10.1097/01.MP.0000081051.55284.2A. [DOI] [PubMed] [Google Scholar]
- 24.Park J, Sun D, Genest DR, et al. Coexistence of low and high grade squamous intraepithelial lesions of the cervix: morphologic progression or multiple papillomaviruses? Gynecol Oncol. 1998;70:386–391. doi: 10.1006/gyno.1998.5100. [DOI] [PubMed] [Google Scholar]
- 25.Agorastos T, Miliaras D, Lambropoulos AF, et al. Detection and typing of human papillomavirus DNA in uterine cervices with coexistent grade I and grade III intraepithelial neoplasia: biologic progression or independent lesions? Eur J Obstet Gynecol Reprod Biol. 2005;121:99–103. doi: 10.1016/j.ejogrb.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Mejlhede N, Pedersen BV, Frisch M, et al. Multiple human papilloma virus types in cervical infections: competition or synergy? APMIS. 2010;118:346–352. doi: 10.1111/j.1600-0463.2010.2602.x. [DOI] [PubMed] [Google Scholar]
- 27.Rousseau MC, Pereira JS, Prado JC, et al. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508–1517. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 28.Hampl M, Sarajuuri H, Wentzensen N, et al. Effect of human papillomavirus vaccines on vulvar, vaginal, and anal intraepithelial lesions and vulvar cancer. Obstet Gynecol. 2006;108:1361–1368. doi: 10.1097/01.AOG.0000245786.86267.80. [DOI] [PubMed] [Google Scholar]
- 29.Yemelyanova A, Ji H, Shih I, et al. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: immunohistochemical analysis of 201 cases. Am J Surg Pathol. 2009;33:1504–1514. doi: 10.1097/PAS.0b013e3181ac35f5. [DOI] [PubMed] [Google Scholar]
- 30.Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37:2508–2517. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153:1731–1739. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hamont D, van Ham MA, Bakkers JM, et al. Evaluation of the SPF10-INNO LiPA human papillomavirus (HPV) genotyping test and the roche linear array HPV genotyping test. J Clin Microbiol. 2006;44:3122–3129. doi: 10.1128/JCM.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preti M, Mezzetti M, Robertson C, et al. Inter-observer variation in histopathological diagnosis and grading of vulvar intraepithelial neoplasia: results of an European collaborative study. BJOG. 2000;107:594–599. doi: 10.1111/j.1471-0528.2000.tb13298.x. [DOI] [PubMed] [Google Scholar]
- 34.Conley LJ, Ellerbrock TV, Bush TJ, et al. HIV-1 infection and risk of vulvovaginal and perianal condylomata acuminata and intraepithelial neoplasia: a prospective cohort study. Lancet. 2002;359:108–113. doi: 10.1016/S0140-6736(02)07368-3. [DOI] [PubMed] [Google Scholar]
- 35.Jamieson DJ, Paramsothy P, Cu-Uvin S, et al. Vulvar, vaginal, and perianal intraepithelial neoplasia in women with or at risk for human immunodeficiency virus. Obstet Gynecol. 2006;107:1023–1028. doi: 10.1097/01.AOG.0000210237.80211.ff. [DOI] [PubMed] [Google Scholar]
- 36.Chiasson MA, Ellerbrock TV, Bush TJ, et al. Increased prevalence of vulvovaginal condyloma and vulvar intraepithelial neoplasia in women infected with the human immunodeficiency virus. Obstet Gynecol. 1997;89:690–694. doi: 10.1016/s0029-7844(97)00069-0. [DOI] [PubMed] [Google Scholar]
- 37.Rufforny I, Wilkinson EJ, Liu C, et al. Human papillomavirus infection and p16(INK4a) protein expression in vulvar intraepithelial neoplasia and invasive squamous cell carcinoma. J Low Genit Tract Dis. 2005;9:108–113. doi: 10.1097/00128360-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Santos M, Landolfi S, Olivella A, et al. p16 overexpression identifies HPV-positive vulvar squamous cell carcinomas. Am J Surg Pathol. 2006;30:1347–1356. doi: 10.1097/01.pas.0000213251.82940.bf. [DOI] [PubMed] [Google Scholar]
- 39.Santos M, Montagut C, Mellado B, et al. Immunohistochemical staining for p16 and p53 in premalignant and malignant epithelial lesions of the vulva. Int J Gynecol Pathol. 2004;23:206–214. doi: 10.1097/01.pgp.0000130108.03231.89. [DOI] [PubMed] [Google Scholar]
- 40.Riethdorf S, Neffen EF, Cviko A, et al. p16INK4A expression as biomarker for HPV 16-related vulvar neoplasias. Hum Pathol. 2004;35:1477–1483. doi: 10.1016/j.humpath.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Pirog EC, Quint KD, Yantiss RK. P16/CDKN2A and Ki-67 enhance the detection of anal intraepithelial neoplasia and condyloma and correlate with human papillomavirus detection by polymerase chain reaction. Am J Surg Pathol. 2010;34:1449–1455. doi: 10.1097/PAS.0b013e3181f0f52a. [DOI] [PubMed] [Google Scholar]