Abstract
OBJECTIVE
Obesity in adult males is associated with hypogonadotropic hypogonadism. We evaluated the effect of obesity on plasma testosterone concentrations in pubertal and post pubertal young males.
DESIGN AND METHODS
Morning fasting blood samples were obtained from 25 obese (BMI≥95th percentile) and 25 lean(BMI<85th percentile) males between the ages 14–20 years with Tanner staging ≥4. Total and free testosterone and estradiol concentrations were measured by liquid chromatography tandem mass spectrometry and equilibrium dialysis. Free testosterone was also calculated using SHBG and albumin. C-reactive protein (CRP), insulin and glucose concentrations were measured and homeostasis model of insulin resistance (HOMA-IR) was calculated.
RESULTS
After controlling for age and Tanner staging, obese males had a significantly lower total testosterone(10.5 vs 21.44nmol/l), free testosterone(0.22 vs 0.39nmol/l) and calculated free testosterone(0.26 vs 0.44nmol/l) concentrations as compared to lean males(p<0.001 for all). Obese males had higher CRP concentrations (2.8 vs 0.8mg/l; p<0.001), and HOMA-IR (3.8 vs 1.1; p<0.001) than lean males. Free testosterone concentrations were positively related to age and negatively to BMI, HOMA-IR and CRP concentrations. Total and free estradiol concentrations were significantly lower in males with subnormal testosterone in concentrations.
CONCLUSION
Testosterone concentrations of young obese pubertal and post pubertal males are 40–50% lower than those with normal BMI. Obesity in young males is associated with low testosterone concentrations which are not secondary to an increase in estradiol concentrations. Our results need to be confirmed in a larger number of subjects.
Keywords: Testosterone, Estradiol, Hypogonadism, Obese, Boys, young males, insulin resistance
INTRODUCTION
Prevalence of obesity in the pediatric population has tripled from 1971–1974 to 2003–2004 and there has been a rise in the cases of type 2 diabetes[1, 2]. We have previously shown that type 2 diabetes and obesity are associated with a high prevalence(25–33%) of hypogonadotropic hypogonadism in middle-aged and elderly men[3–5]. The rate of hypogonadotropic hypogonadism in young type 2 diabetic males between the ages of 18 and 35 years is >50%[6]. In all these studies, free testosterone concentrations are negatively related to BMI. This raises the question whether obesity is associated with lower testosterone concentrations even in younger males.
On the basis of the above, we hypothesized that obese boys and young obese males (age 14–20 years) have significantly lower total and free testosterone(TT and FT) and sex hormone binding globulin(SHBG) concentrations as compared to lean boys and young lean males. In addition, since low T concentrations have been related to elevated CRP concentrations and indices of insulin resistance, we also measured plasma concentrations of CRP, glucose and insulin [7–9]. HOMA-IR was calculated as an index of insulin resistance.
MATERIALS AND METHODS
This is a cross-sectional observational study. Twenty-five obese(defined as BMI≥95th percentile for age) and 25 lean(defined as BMI<85th percentile for age as per CDC guidelines) males between the ages of 14–20 years and Tanner stage≥4 were recruited at the Endocrine and Diabetes Center of Women and Children's Hospital of Buffalo. Tanner staging was assessed by one of the investigators(MM) trained by a board certified pediatric endocrinologist(TQ) using an orchidometer. Males with testicular volume between 12–15 ml were classified as Tanner stage 4 and those with testicular volume >15 ml were classified as Tanner stage 5. Height was measured to the nearest 0.1 cm using a wall mounted stadiometer by trained personnel and weight were measured to the nearest 0.1 kg using a digital scale. Blood pressure and heart rate were recorded. Subjects were healthy and without significant co-morbidities. Specifically, subjects with history of hypogonadism, panhypopituitarism, severe depression or psychiatric illness, diabetes, head trauma, renal failure, hemochromatosis, cirrhosis, hepatitis C, HIV, treatment with testosterone or oral steroids were excluded. Additionally active infection or recent surgery or hospitalization in the 6 weeks prior to the study was exclusion criteria as well. All subjects gave their informed consent to participate in the study which was approved by Institutional Review Board of the Children’s Hospital and University at Buffalo. Parental consent was taken in addition to children’s consent for subjects less than 18 years of age.
One fasting blood sample was drawn between 8 and 10 am to measure total and free T and estradiol, SHBG, LH and FSH. Total T and estradiol were measured by liquid chromatography and tandem mass spectrometry(LC-MS/MS). A detailed description of the methodology has previously been published[10]. The sensitivity of the testosterone assay(LOQ), set at a coefficient of variation(CV) of ≤20%, was 0.01nmol/l. The intra-assay CV ranged from 7.6–10.8% and inter-assay CV ranged from 9.8–13.4% at total T concentrations between 0.35–41.64nmol/l. The CV of the estradiol assay is 15% at an estradiol concentration of 0.05nmol/l and 13% at 0.69nmol/l. The LOQ for estradiol in this assay is 7.34pmol/l. Three subjects(two lean and one obese) had estradiol concentrations below the LOQ of the assay. Concentrations below LOQ are often encountered in studies and discarding these observations would create a bias making the remaining lower concentrations as selectively “too high”. Conversely, assigning them a value of zero would also introduce a bias, making the concentrations on the lower side as falsely low. A commonly recommended method is to assign a value of LOQ/2 for the purpose of statistical analysis[11]. Therefore, men with total estradiol concentrations below LOQ were assigned a value of 3.67pmol/l for analysis.
Tracer equilibrium dialysis is considered the gold standard for measuring free steroid hormone concentrations and this methodology was used to determine the FT and free estradiol concentrations in our subjects. However, due to inadequacy of blood sample in some subjects, the FT concentrations could not be measured directly in 9 lean and 5 obese subjects. Free estradiol concentrations could not be measured in 13 lean and 4 obese subjects.
FT concentrations were calculated in all subjects from the concentrations of TT, SHBG and albumin by the formula of Sodergard and Vermuelen et al[12–14]. The formula to calculate FT was [FT]={[T] − (N[FT])}/Kt {[SHBG] − [T] + N[FT]} where Kt is the association constant of T for SHBG(1×109 L/mol), T=total testosterone, FT=free testosterone and N=1+Ka[A] (where Ka=association constant of T for albumin[3.6×104 L/mol] and A=albumin); T and protein concentrations are in mol/L[10].
SHBG, LH and FSH concentrations were measured by a solid-phase, chemiluminescent immunometric assay (Siemens, IMMULITE 2500). All these assays were done by Quest Laboratories. The LOQ and total CV for SHBG, LH and FSH are 0.02 nmol/L and 4.0–6.6%, 0.07 IU/L and 2.7–3.8%, and 0.3 IU/L and 3.5–4.0% respectively.
Plasma CRP concentrations (LOQ: 0.35ng/ml, intra-assay CV 3.5%, inter-assay CV 5%) were measured using a high-sensitivity enzyme-linked immunosorbent assay(ELISA) kit from Alpha Diagnostics International(San Antonio,TX). Insulin concentrations (LOQ: 0.3µU/mL, intra-assay CV 2%, inter-assay CV 5%) were determined using an ELISA kit from Diagnostic Systems Laboratories Inc.(Webster,TX). Glucose concentrations (intra-assay CV 2%, inter-assay CV 2%) were measured in plasma by YSI 2300 STAT Plus glucose analyzer(Yellow Springs, Ohio). These assays were done at the research laboratories of the division of Endocrinology and Metabolism, University at Buffalo. HOMA-IR was calculated from fasting insulin and glucose level using the formula [fasting insulin(mU/L) × fasting glucose(mmol/L)]/22.5.
Statistical analysis
Group comparisons were performed by one-way ANOVA, two-tailed t tests, Mann-Whitney rank sum tests, and χ2 tests as appropriate. Adjustment for variables such as age, BMI, SHBG and Tanner stage in group comparisons was done with ANCOVA and generalized linear model analysis. CRP concentrations were not normally distributed(determined by visual estimation of data as well as Kolmogorov-Smirnov and Shapiro-Wilk tests) and were log-transformed to perform the parametric statistical tests. Pearson correlation between variables was done using SPSS software(SPSS Inc, Chicago, Illinois). Data are presented as means±SD for normally distributed data and median[25th, 75th percentile] for non-normal data. p<0.05 was considered significant.
RESULTS
Twenty-five obese and 25 lean pubertal and post-pubertal males participated in the study. Eight lean and 7 obese males were Tanner stage 4 with the remaining subjects being Tanner stage 5. Table 1 illustrates the anthropometric characteristics and laboratory results of the population studied. The groups were comparable with regard to age. The mean T concentration in obese males was 50% lower than the lean males. Mean FT concentration(measured by equilibrium dialysis) was lower by 46% while mean calculated free testosterone concentration(cFT) was lower by 42% as compared to lean males. The results were similar after adjusting for age and Tanner stage. Males in Tanner stage 5 had higher cFT(0.39±0.19 vs 0.27±0.10nmol/l, p=0.04) than males in Tanner stage 4. Among males with Tanner stage 5, 18 were obese and 17 were obese. The cFT concentrations of these obese males were lower than those of the lean males(0.28±0.12 vs 0.50±0.18 nmol/l, p<0.001).
Table 1.
Comparison of sex hormone concentrations of lean and obese pubertal and post-pubertal males. T, estradiol and SHBG concentrations were adjusted for age and tanner stage. To convert T into ng/dl, multiply by 28.8. To convert estradiol into pg/ml, multiply by 0.27. Data are expressed as Means ± S.D. CRP concentrations were not normally distributed; therefore median [25th, 75th percentile] is mentioned.
Total Testosterone (TT)
Free Testosterone (FT)
Calculated Free Testosterone (cFT)
C-reactive protein (CRP)
homeostasis model of insulin resistance (HOMA-IR)
sex hormone binding globulin(SHBG)
| Lean | Obese | p | |
|---|---|---|---|
| Number of subjects | 25 | 25 | |
| Age | 16.5±1.4 | 16.0±1.5 | 0.17 |
| BMI | 20.9±2.2 | 36.0±5.3 | <0.001 |
| BMI Z Score | −0.1±0.9 | 2.4± 0.4 | <0.001 |
| BMI percentile | 49±25 | 99±1 | <0.001 |
| Race: Caucasians African Americans Others |
17 (68%) 7(28%) 1(4%) |
15(60%) 5(20%) 5(20%) |
0.21 |
| Sys BP | 120±11 | 130±10 | 0.001 |
| Dia BP | 68±9 | 74±11 | 0.06 |
| Heart Rate | 67±13 | 75±15 | 0.05 |
| Tanner stage | 4.7±0.5 | 4.7±0.5 | 0.9 |
| TT (nmol/l) | 21.4±8.3 | 10.5±5.2 | <0.001 |
| cFT (nmol/l) | 0.44±0.18 | 0.26±0.11 | <0.001 |
| FT by equilibrium dialysis(nmol/l) | 0.39±0.17 (n=16) | 0.22±0.11 (n=20) | 0.001 |
| SHBG (nmol/l) | 37.6±17.2 | 21.4±11.6 | 0.001 |
| Total estradiol (pmol/l) | 66.1±40.7 | 75.6±36.3 | 0.33 |
| Free estradiol (pmol/l) by equilibrium dialysis | 1.39±0.95 (n=12) | 1.95±0.99 (n=21) | 0.03 |
| CRP (mg/l) | 0.15 [0.07, 0.70] | 1.47 [0.91, 4.41] | <0.001 |
| Glucose (mmol/l) | 4.38 ±0.39 | 4.44±0.56 | 0.65 |
| Insulin (µU/mL) | 5.3±2.4 | 18.1±15.7 | <0.001 |
| HOMA-IR | 1.1±0.5 | 3.8±4.1 | <0.001 |
| LH | 3.5±1.7 | 3.2±1.4 | 0.49 |
| FSH | 4.0±3.9 | 3.2±2.1 | 0.46 |
No large studies have been published on reference range for FT in children. In our study, the 5th percentile of cFT in lean subjects was 0.23nmol/l. 40% of the obese subjects had cFT concentrations <0.23nmol/l(p=0.006 as compared to lean group). Males with cFT concentrations below or above 0.23nmol/l are compared in table 2. None of the subjects in the study had high LH or FSH concentrations. Males with cFT concentrations <0.23nmol/l were younger and had a higher BMI. After adjustment for age, BMI and Tanner stage, these males had higher glucose, insulin and HOMA-IR as compared to males with cFT concentrations ≥0.23nmol/l. Obese males with cFT concentrations <0.23nmol/l (n=10) also had higher HOMA-IR concentrations(7.4±6.0) than obese males with normal cFT concentrations(1.4±1.0, n=15, p=0.002), after adjustment for age, BMI and Tanner stage.
Table 2.
Comparison of sex hormone concentrations of subjects with cFT less than or more than 0.23 nmol/l.
| cFT <0.23 nmol/l | cFT ≥0.23 nmol/l | p | |
|---|---|---|---|
| Number of subjects | 11 | 39 | |
| Age | 15.3±1.4 | 16.5±1.3 | 0.02 |
| BMI | 35.5±7.8 | 26.3±7.7 | 0.003 |
| TT (nmol/l) | 6.73±2.85 | 18.56±8.08 | <0.001 |
| cFT (nmol/l) | 0.16±0.04 | 0.41±0.02 | <0.001 |
| FT (nmol/l) by equilibrium dialysis | 0.14±0.08 (n=9) | 0.34±0.15 (n=27) | 0.001 |
| SHBG (nmol/l) | 20.6±11.9 | 32.0±17.1 | 0.02 |
| Total estradiol (pmol/l) After adjustment* |
43.7±33.0 45.9±33.0 |
77.8±37.0 77.1±37.0 |
0.02 0.02 |
| Free estradiol (pmol/l) by equilibrium dialysis After adjustment$ |
1.14±0.84 (n=10) 1.25±0.84 |
2.06±0.92(n=23) 2.02±0.92 |
0.01 0.01 |
| CRP (mg/l) After adjustment# |
1.47 [1.06, 4.27] 0.67±0.60 |
0.51 [0.13, 2.09] 0.66±0.30 |
0.03 0.97 |
| Glucose (mmol/l) After adjustment$ |
4.7±0.7 4.6±0.7 |
4.4±0.3 4.4±0.3 |
0.18 0.05 |
| Insulin (µU/mL) After adjustment$ |
24.1±22.0 22.5±22.0 |
8.3±5.4 8.8±5.4 |
0.04 0.001 |
| HOMA-IR After adjustment$ |
5.5±5.9 5.3±5.9 |
1.6±1.0 1.7±1.0 |
0.05 0.002 |
| Sys BP After adjustment$ |
131±9 126±9 |
123±12 124±12 |
0.06 0.64 |
| Dia BP After adjustment$ |
75±11 74±11 |
70±10 70±10 |
0.23 0.35 |
| Pulse | 74±14 | 70±15 | 0.36 |
| Tanner stage | 4.5±0.5 | 4.7±0.4 | 0.30 |
| LH | 2.9±1.6 | 3.5±1.5 | 0.33 |
| FSH | 4.2±3.2 | 3.4±3.1 | 0.51 |
Total estradiol concentrations were adjusted for age, BMI, SHBG and tanner stage.
Free estradiol, glucose, insulin, HOMA-IR and blood pressure values were adjusted for age, BMI and tanner stage.
CRP concentrations were not normally distributed; therefore median [25th, 75th percentile] is mentioned. The geometric mean after adjustment for age, BMI and tanner stage is also shown.
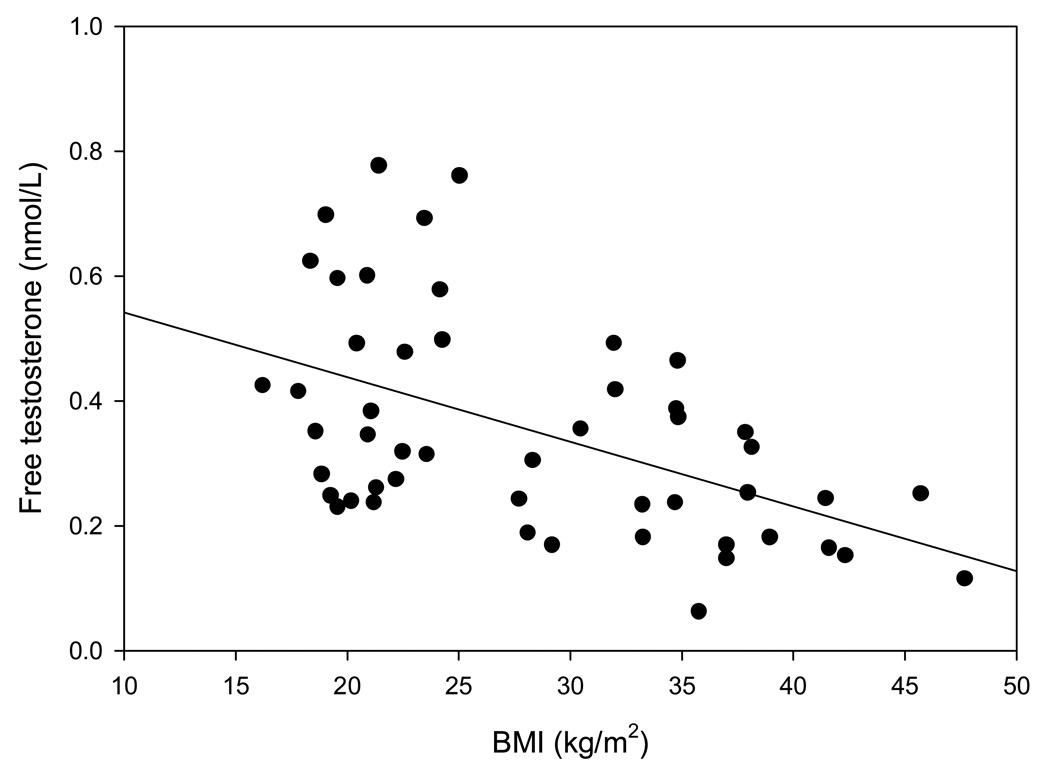
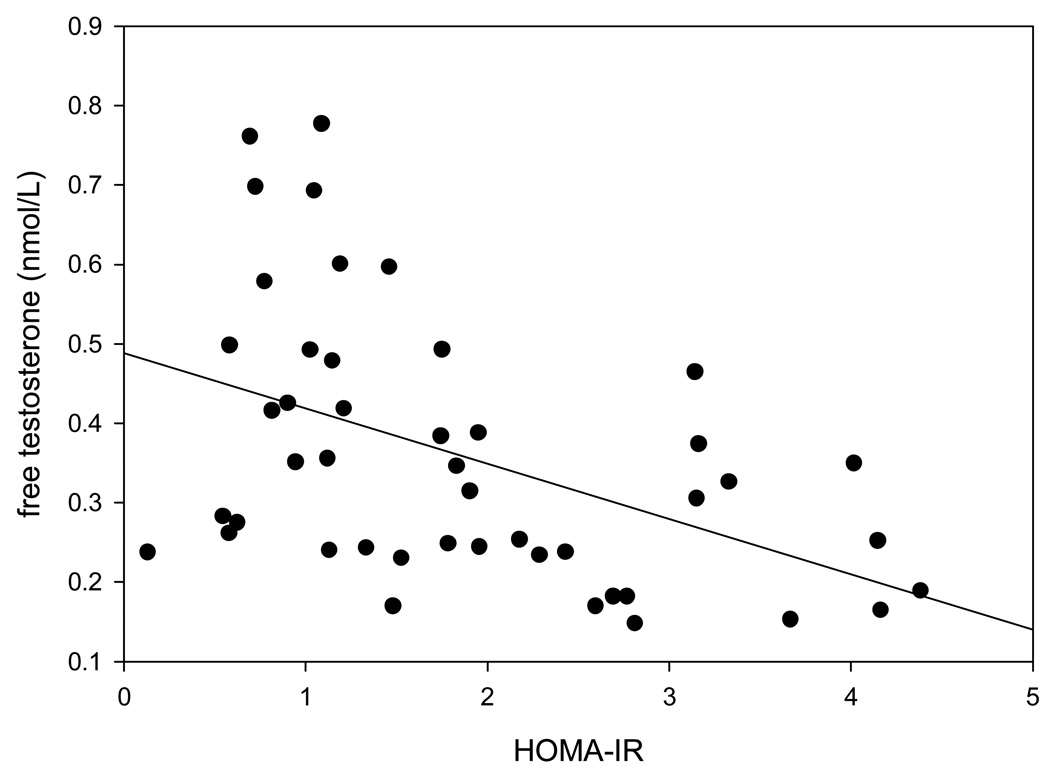
cFT concentrations were positively related to age(r=0.39, p=0.005) and negatively to BMI(figure 1), CRP concentrations(r= −0.32, p=0.03) and to HOMA-IR(figure 2). The results were similar with TT and with directly measured FT (data not shown). As expected, cFT concentrations were strongly related to TT (r= 0.90, p<0.001) and directly measured FT concentrations(r= 0.69, p<0.001). TT and total estradiol concentrations were directly related (r=0.45, p=0.003), as were FT and free estradiol concentrations (r=0.37, p=0.05).
Figure 1.
Relation of cFT concentrations with BMI (r= −0.51, p<0.001).
Figure 2.
Relation of cFT concentrations with HOMA-IR (r= −0.45, p=0.002). Two HOMA-IR values of 15 and 19 were not included in the graph to depict the relationship clearly. These two patients were obese and had cFT concentrations of 0.114 and 0.063 nmol/L respectively. Eliminating those two values changed the correlation coefficient (r) to −0.47 and p to 0.001.
DISCUSSION
Our data clearly show that obese young pubertal and post pubertal males have significantly lower total and free T concentrations compared to their lean counterparts. Consistent with the studies in adults, the concentrations of LH and FSH were not elevated and hence were inappropriately low indicating central suppression of the hypothalamo-hypophyseal-gonadal axis[3, 6, 14]. There is no universally accepted reference range for T concentrations in pubertal and post-pubertal children. Furthermore studies on T measurement are often hampered by the use of inaccurate assays. One study has published reference ranges for TT by LC/MS-MS in a large number of children[15]. The reference range for males with Tanner stages 4 and 5 was 5.6–29.4 nmol/l. However, obese children were not excluded from that study. Hence, based upon our study results, it is possible that this reference range is spuriously low. None of lean boys in our study had TT concentration <5.6nmol/L(the lowest TT concentration in this group was 10.4nmol/l). 12% of the obese boys had TT concentration <5.6nmol/L(p=0.23 as compared to lean group). No studies on reference ranges for free T are available. Our study had too few patients to provide reference ranges for TT or FT concentrations.
There are 4 previous studies on T concentrations in obese boys (table 3). Apart from producing variable results, none of them have reported results based on appropriately measured FT concentrations. Nor are the results based on T concentrations measured by the current standard method of LC-MS/MS. This method is more sensitive and specific and is now considered the method of choice for measuring T, especially in subjects with low T concentrations, such as children and women[16]. In one study, obese prepubertal boys(Tanner stage 1) had higher TT concentrations than lean prepubertal boys whereas obese pubertal boys(Tanner stage 2) had TT concentrations similar to those in lean boys[17]. Another study found lower TT concentrations in obese boys aged 8–18 years[18]. A small study of 20 males between the ages of 12–19 years(tanner stage≥2) found lower TT concentrations in obese and type 2 diabetic males as compared to lean males[9]. Similarly, Taneli et al found that TT concentrations at Tanner stages 2 and 4 were lower in obese boys as compared to lean boys[19]. These studies also found lower SHBG concentrations in obese boys. Since approximately half of the TT is bound to SHBG, it is likely that lower SHBG concentrations can, at least partly, account for the lower TT concentrations in obese boys. Taneli et al found lower FT concentrations in the obese boys at Tanner stage 2 but not at Tanner stage 4[19]. The FT concentrations were, however, measured by direct radioimmunoassay, an inaccurate method that underestimates FT concentrations by manifold and is dependent upon SHBG concentrations[20, 21]. For accurate estimation, FT should be measured by equilibrium dialysis [22].
Table 3.
Summary of published studies that have compared T and estradiol concentrations in obese and lean boys. However the different methodologies used in various studies limit direct comparisons.
| population | controls | age | total T (nmol/L) | free T(nmol/L) | Total estradiol (pmol/L) |
|
|---|---|---|---|---|---|---|
| Moriarty-Kelsey[9] | 6 obese non-DM and 7 obese DM boys | 7 lean boys | 12–19 |
*Lean: 17.4 Obese: 10.4 Obese DM: 6.9 |
not done | Not done |
| Denzer[18] | 582 obese boys | none | 6–18 | 4.4 (age 12–14) 14.8 (age 14–16) |
not done | Not done |
| Taneli[19] | 20 obese tanner stage 2 and 20 obese Tanner stage 4 | 20 lean tanner stage 2 and 20 lean Tanner stage 4 boys | 11–17 | Tanner stage 2:- Lean 4.1 Obese 1.1 Tanner stage 4:- Lean 9.2 Obese 5.6 |
done by inaccurate RIA; FT concentrations were lower in obese boys with Tanner 2 but similar to lean boys in tanner 4 |
Tanner stage 2:- Lean 77 Obese 84 Tanner stage 4:- Lean 92 Obese 94 |
| Reinehr[17] | 81 obese boys tanner stage 1 and 60 obese boys in tanner stage 2 | 24 lean boys tanner stage 1 and 18 lean boys in tanner stage 2 | 4–14 | Tanner stage 1:- Lean 0.1 Obese 0.6 Tanner stage 2:- Lean 3.0 Obese 3.1 |
not done | Not done |
| Mogri (this paper) | 7 obese tanner stage 4 and 18 tanner stage 5 | 8 lean tanner stage 4 and 17 lean Tanner stage 5 | 14–20 | Tanner stage 4:- Lean 17.4 Obese 9.3 Tanner stage 5:- Lean 23 Obese 11 |
cFT concentrations Tanner stage 4:- Lean 0.33 Obese 0.21 Tanner stage 5:- Lean 0.5 Obese 0.28 |
Tanner stage 4:- Lean 40 Obese 44 Tanner stage 5:- Lean 77 Obese 84 |
values extrapolated from graph
It has been assumed that low T concentration in obese males is secondary to enhanced peripheral aromatization of T to estradiol in the adipose tissue[14, 23]. However, we found that males with cFT concentrations <0.23nmol/l had lower estradiol concentrations than those with higher cFT concentrations, in spite of having a higher BMI. T concentrations were directly related to estradiol concentrations. These findings are consistent with those in adult type 2 diabetic men with hypogonadotropic hypogonadism[24]. Thus, the low T concentrations in hypogonadotropic hypogonadism of obesity are not the consequence of estradiol dependent suppression of the hypothalamo-hypophyseal-gonadal axis.
The specific mechanism involved in the pathogenesis of hypogonadotropic hypogonadism in obese males is not known. The selective deletion of the insulin receptor in the neurons of mice leads to hypogonadotropic hypogonadism[25]. Since obesity is an insulin resistant state with an interference with insulin signal transduction and since insulin is known to facilitate GnRH secretion by hypothalamic neurons, in vitro, it is possible that this syndrome is a manifestation of insulin resistance at the neuronal level which results in subnormal secretion of GnRH from the hypothalamus[26]. Leptin resistance in obesity may also contribute to hypogonadotropic hypogonadism[28]. It is now known that kisspeptin stimulates the release of GnRH from hypothalamic neurons[27]. The role of kisspeptin, leptin or other neuropeptides such as ghrelin and neuropeptide Y in obesity associated hypogonadotropic hypogonadism needs to be investigated.
Despite similar Tanner stage, patients with cFT concentrations <0.23 nmol/l were significantly younger than individuals with cFT ≥0.23 nmol/l (15.3 ± 1.4 vs 16.5 ± 1.3years; p= 0.02). Perhaps this factor could explain, to some extent, the difference in cFT concentrations between both groups, particularly taking into account the positive correlation between cFT and age(r=0.39, p=0.005). We found that subjects with cFT concentrations <0.23 nmol/l had higher HOMA-IR independently of BMI and age. These results are consistent with a prior study that demonstrated a direct relation of TT concentrations with insulin sensitivity, estimated by hyperinsulinemic-euglycemic clamp, independently of BMI in males 12–19 years of age[9]. FT or SHBG concentrations were not measured in the study. It is recognized that the mechanism underlying insulin resistance in obesity and type 2 diabetes is dependent upon inflammatory mediators which are significantly increased in this condition at the molecular, cellular and plasma level[29]. Since CRP concentrations are markedly elevated in patients with subnormal FT concentrations and are related inversely to FT concentrations[7, 30], it is possible that there exists an inflammation based interference of insulin signal transduction at the hypothalamic level. This issue requires further investigation of other mediators of inflammation and interference with insulin signal transduction.
It is well known that there is significant day-to-day variability in hormone concentrations, especially T. As in most epidemiologic or cross-sectional studies, the hormone concentrations were measured only once in this study. In view of the variability in T concentrations, this is a limitation. However, it is not likely that the vast difference between the T concentrations of lean and obese males would have altered following repeated measurements since the probability of T concentrations rising or falling with repeated measurements is statistically equal. The issue of repeated measurements is important in the context of diagnosing hypogonadism clinically in individual patients.
Another limitation of our study is that while all subjects had TT measurements carried out by LC-MS/MS, FT concentrations measured by equilibrium dialysis were available only in 36 subjects. However, we could calculate FT in all patients. The calculated FT concentrations differ systematically from those measured by equilibrium dialysis and overestimate FT concentrations by ~15% [22]. While the calculated and directly measured FT are not interchangeable, the calculation has been shown to be a reliable and accurate estimate of FT concentrations when compared with the equilibrium dialysis method[31]. In our own data, there was a highly significant correlation between FT and cFT concentrations. Our results were very similar whether FT or cFT concentrations were compared between the obese and the lean(tables). It is unlikely that the results would have changed significantly if we had FT measured by equilibrium dialysis on all patients. However, our results should be considered preliminary till they are confirmed by a study with a larger sample size.
We conclude that obese pubertal and post-pubertal males with Tanner staging ≥4 have significantly lower TT, FT and SHBG concentrations in whom LH and FSH concentrations are also inappropriately low. Subjects with subnormal testosterone concentrations also had significantly lower total and free estradiol concentrations and thus hypogonadotrophic hypogonadism in the obese is not secondary to an increase in estradiol concentrations. In view of the rising prevalence of obesity, the association of significantly lower T concentrations with obesity in males is alarming and points to major public health problem.
Acknowledgements
We are thankful to the endocrinology fellows who helped M.M. in the collection of blood samples. P.D. is supported by grants from NIH(R01 DK075877-01A2) and the American Diabetes Association(708CR13). PD is also supported by grants from Sanofi-Aventis, Merck, Amylin and Abbott Laboratories. S.D. is supported by a grant from the American Diabetes Association(1 10 JF 13).
Abbreviations
- T
Testosterone
- TT
Total Testosterone
- FT
Free Testosterone
- cFT
Calculated Free Testosterone
- LC-MS/MS
Liquid chromatography and tandem mass spectrometry
- CV
coefficient of variation
- CRP
C-reactive protein
- HOMA-IR
homeostasis model of insulin resistance
- SHBG
sex hormone binding globulin
Footnotes
Declaration of Interest
S.D. has received speaker honoraria from Abbott Laboratories. P.D. has received consulting fees and research support from Abbott Laboratories.
Contribution statement
M.M.: Acquisition of data, analysis and interpretation of data, wrote manuscript
S.D.: Acquisition of data, analysis and interpretation of data, wrote manuscript
T.Q.: Interpreted data, reviewed manuscript critically
H.G.: Interpreted data, reviewed manuscript critically
P.D. put forth the hypothesis, planned the study, interpreted data and critically reviewed the manuscript.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, D'Agostino RB, Jr, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 3.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462–5468. doi: 10.1210/jc.2004-0804. [DOI] [PubMed] [Google Scholar]
- 4.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–1192. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96:2643–2651. doi: 10.1210/jc.2010-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandel A, Dhindsa S, Topiwala S, Chaudhuri A, Dandona P. Testosterone concentration in young patients with diabetes. Diabetes Care. 2008;31:2013–2017. doi: 10.2337/dc08-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care. 2006;29:2289–2294. doi: 10.2337/dc06-0637. [DOI] [PubMed] [Google Scholar]
- 8.Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–868. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 9.Moriarty-Kelsey M, Harwood JE, Travers SH, Zeitler PS, Nadeau KJ. Testosterone, obesity and insulin resistance in young males: evidence for an association between gonadal dysfunction and insulin resistance during puberty. J Pediatr Endocrinol Metab. 2010;23:1281–1287. doi: 10.1515/jpem.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salameh WA, Redor-Goldman MM, Clarke NJ, Reitz RE, Caulfield MP. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75:169–175. doi: 10.1016/j.steroids.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 12.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 13.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 14.Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66:103–109. [PubMed] [Google Scholar]
- 15.Kushnir MM, Blamires T, Rockwood AL, et al. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56:1138–1147. doi: 10.1373/clinchem.2010.143222. [DOI] [PubMed] [Google Scholar]
- 16.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 17.Reinehr T, de Sousa G, Roth CL, Andler W. Androgens before and after weight loss in obese children. J Clin Endocrinol Metab. 2005;90:5588–5595. doi: 10.1210/jc.2005-0438. [DOI] [PubMed] [Google Scholar]
- 18.Denzer C, Weibel A, Muche R, Karges B, Sorgo W, Wabitsch M. Pubertal development in obese children and adolescents. Int J Obes (Lond) 2007;31:1509–1519. doi: 10.1038/sj.ijo.0803691. [DOI] [PubMed] [Google Scholar]
- 19.Taneli F, Ersoy B, Ozhan B, et al. The effect of obesity on testicular function by insulin-like factor 3, inhibin B, and leptin concentrations in obese adolescents according to pubertal stages. Clin Biochem. 2010;43:1236–1240. doi: 10.1016/j.clinbiochem.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Rosner W. An extraordinarily inaccurate assay for free testosterone is still with us. J Clin Endocrinol Metab. 2001;86:2903. doi: 10.1210/jcem.86.6.7643. [DOI] [PubMed] [Google Scholar]
- 21.Winters SJ, Kelley DE, Goodpaster B. The analog free testosterone assay: are the results in men clinically useful? Clin Chem. 1998;44:2178–2182. [PubMed] [Google Scholar]
- 22.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 23.Cohen PG. The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt--a major factor in the genesis of morbid obesity. Med Hypotheses. 1999;52:49–51. doi: 10.1054/mehy.1997.0624. [DOI] [PubMed] [Google Scholar]
- 24.Dhindsa S, Furlanetto R, Vora M, Chaudhuri A, Ghanim H, Dandona P. Low Estradiol Concentrations in Males with subnormal testosterone concentrations and Type 2 Diabetes. Diabetes Care. 2011;34:1–6. doi: 10.2337/dc11-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 26.Salvi R, Castillo E, Voirol MJ, et al. Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insulin: implication of the mitogen-activated protein kinase pathway. Endocrinology. 2006;147:816–826. doi: 10.1210/en.2005-0728. [DOI] [PubMed] [Google Scholar]
- 27.Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 28.Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 31.Morley JE, Patrick P, Perry HM., 3rd Evaluation of assays available to measure free testosterone. Metabolism. 2002;51:554–559. doi: 10.1053/meta.2002.31975. [DOI] [PubMed] [Google Scholar]