Abstract
Polychlorinated biphenyls (PCBs) are legacy pollutants that exert toxicities through various mechanisms. In the recent years exposure to PCBs via inhalation has been recognized as a hazard. Those PCBs with lower numbers of chlorine atoms (LC-PCBs) are semi-volatile, and have been reported in the urban air, as well as in the indoor air of older buildings. LC-PCBs are bioactivated to phenols and further to quinone electrophiles with genotoxic/carcinogenic potential. We hypothesized that phenolic LC-PCBs are subject to conjugation and excretion in the urine. PCB3, often present in high concentrations in air, is a prototypical congener for the study of the metabolism and toxicity of LC-PCBs. Our objective was to identify metabolites of PCB3 in urine that could be potentially employed in the estimation of exposure to LC-PCBs. Male Sprague Dawley rats (150–175 g) were housed in metabolism cages and received a single intraperitoneal injection of 600 µmol/kg body weight of PCB3. Urine was collected every four hours; rats were euthanized at 36 h and serum was collected. LC-MS analysis of urine before and after incubation with β-glucuronidase and sulfatase showed that sulfate conjugates were in higher concentrations than glucuronide conjugates and free phenolic forms. At least two major metabolites, and two minor metabolites were identified in urine that could be attributed to mercapturic acid metabolites of PCB3. Quantitation by authentic standards confirmed that approximately 3% of the dose was excreted in the urine as sulfates over 36 hours; with peak excretion occurring at 10–20 h after exposure. The major metabolites were 4’PCB3 sulfate, 3’PCB3 sulfate, 2’PCB3 sulfate, and presumably a catechol sulfate. The serum concentration of 4’PCB3 sulfate was 6.18±2.16 µg/mL. This is the first report that sulfated metabolites of PCBs are formed in vivo. These findings suggest a prospective approach for exposure assessment of LC- PCBs by analysis of phase II metabolites in urine.
Keywords: PCB3, OH-PCBs, phenolic PCBs, PCB sulfate, PCB glucuronide, diconjugate, PCB mercapturic acids, glutathione conjugates, exposure assessment
Introduction
Polychlorinated biphenyls (PCBs) are anthropogenic chemicals that fall in the category of persistent organic pollutants (POPs) since they are not readily degraded by physical or biological processes.1 Environmental persistence of POPs is further enhanced by bio-accumulation and bio-magnification in higher trophic levels of food chain due to their lipophilic properties.2 As PCBs are resistant to heat and degradation, they were widely used in transformer oil in the electrical industry during the first half of twentieth century.3 They were also incorporated into numerous other products for their plasticizing and lubricating properties.4 Although many countries banned the production of PCBs in the late 1970s, their uses continue in closed system applications and legacy pollution persists.5–7 As a result, PCBs are detectable even today in places where point release never occurred.8
Exposure to PCBs occurs by consumption of food, primarily from fish raised in contaminated water.9 An additional exposure via inhalation has been recognized in recent years in both indoor and outdoor settings.10, 11 Concentration of PCBs in the air of Chicago urban area has been reported in the range of 75–5500 pg/m3.12, 13 A pilot study of five public elementary schools conducted by EPA and the New York City School Construction Authority in 2011 and 2012 identified elevated PCB indoor air concentrations in numerous classrooms.14 Students attending school in these classrooms would be inhaling approximately six times more PCBs than they receive from dietary sources (Personal communication-Mark Maddaloni, USEPA). These measurements have raised a new interest in the metabolism and toxicity of airborne PCBs.
Airborne PCBs are mostly mono- to tetra- chlorinated, lower chlorinated congeners (LC-PCBs).11, 15 PCB3, itself found in high concentration in indoor and outdoor air,11, 16 serves as a prototype for studies of metabolism, bioactivation and toxicity of LC-PCBs.17–21 Metabolism of PCB3 to phenolic forms is carried out by cytochrome P450 isoforms.22, 23 Two phenolic metabolites, 4’-OH-PCB3 and 3’,4’-diOH-PCB3, have been reported in urine as major biotransformation products of PCB3 in rats and pigs.18, 19 At least two other mono- and dihydroxylated products have been reported to be generated when PCB3 was incubated with liver microsomes in vitro.22 Oxidative metabolism of PCB3 involves the formation of arene oxide intermediate.21 Further bioactivation may occur by oxidation of dihydroxylated metabolites by peroxidases to quinone and semi-quinone intermediates.24, 25 Both arene oxide and quinone forms of PCB3 are strong eletrophiles with a potential to form adducts to sulfur and nitrogen nucleophiles in proteins and DNA.26–28 This may lead to structural dysfunction, oxidative stress and toxicity.29–31 Overall, exposure of different cell types to mono- and dihydroxylated PCB3 metabolites has resulted in various forms of genotoxicity, such as induction of polyploidy,32 inhibition of cell proliferation by alternation in ROS signaling,33 micronuclei formation, and telomere shortening.34 Genotoxic effects of PCB3 and 4’-OH-PCB3 metabolite have also been demonstrated in vivo.35–39
Exposure assessments for PCBs, in general, are carried out by measuring the serum level of PCBs or their hydroxylated metabolite(OH-PCBs).40, 41 The OH-PCBs most persistent in serum are penta through hepta chlorinated congeners.42 Metabolically active PCBs are less persistent in the serum; and only a few studies have been designed to detect LC-PCBs or their hydroxylated forms in the serum of populations at risk.43 Although bioactivation of LC-PCBs to potential carcinogens has been understood in some detail, the conjugation and detoxification of LC-PCB metabolites has not been as fully explored. Several in vitro studies describe that hydroxylated LC-PCBs may be substrates for glucuronidation,44, 45 and sulfation.46–48 But very few reports exist in the literature of their quantification in vivo.49 As a result, accurate exposure assessments for LC-PCBs remain elusive.
We hypothesized that hydroxylated LC-PCBs are less retained in the serum because they are biotransformed to conjugates and excreted. Our objective was to identify the final metabolites of PCB3 in the urine that could be potentially employed in the analysis of exposure to LC-PCBs. We found that sulfated forms were the major metabolites, and were present in several fold higher concentration than hydroxylated metabolites in both urine and serum.
Materials and Methods
Chemicals and reagents
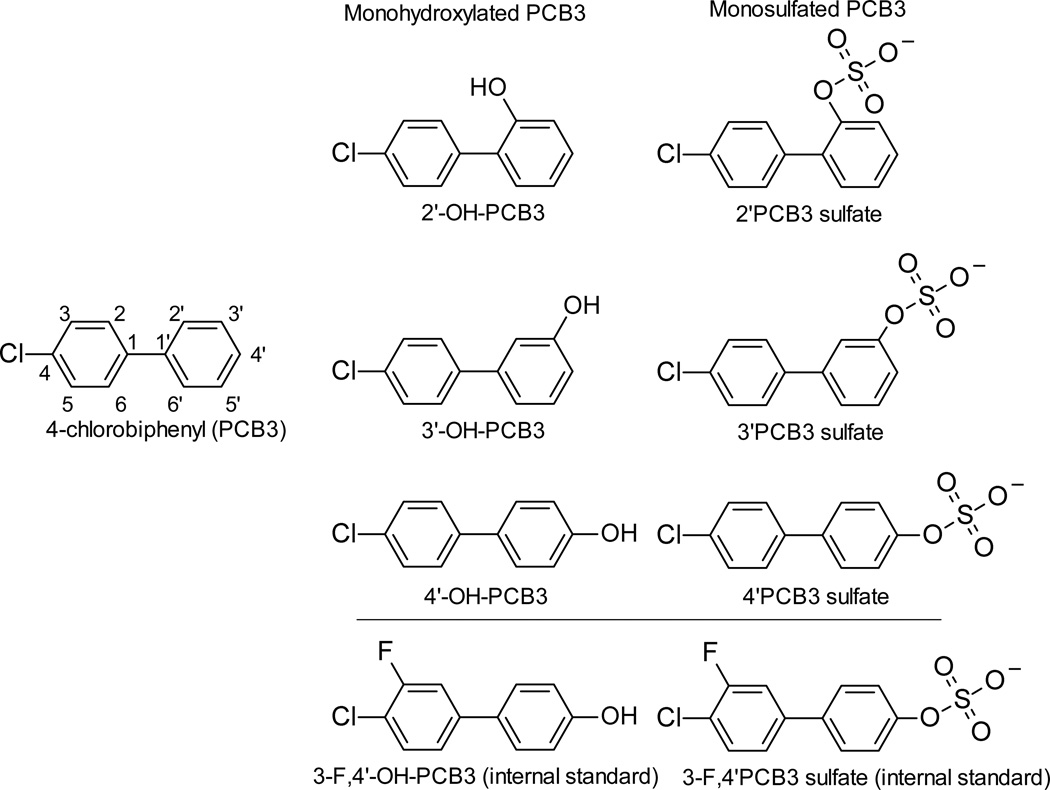
Authentic standards and internal standards (IS) used in this study are shown in Figure 1. Previously described methods were used for the synthesis of PCB3,36 hydroxylated PCB3,22 and sulfated-PCB3.50 HPLC grade solvents used for extraction and LC/MS analysis were obtained from Fisher Scientific Co. (St. Louis, MO). Sulfatase (sulfatase type H-2 crude solution from Helix Pomatia), glucuronidase (β-glucuronidase type XI from Escherichia coli), and D-sachharic-1,4-lactone were obtained from Sigma-Aldrich (St. Louis, MO). Isolute SLE+ 400 array wells were purchase from Biotage LLC (Charlotte, NC). All other chemicals were of the highest purity commercially available.
Figure 1. Structure of standards used in this study.
The atom numbering scheme is indicated for PCB3.
The F-tagged 3-F,4’-OH-PCB3 IS was synthesized by Suzuki-coupling of 1-bromo-3-fluoro-4-chlorobenzene and 4-methoxyphenylboronic acid,51 followed by demethylation with BBr3.52 3-F,4’-OH-PCB3 was subsequently converted via the corresponding 2,2,2-trichloroethyl-protected sulfate diester into the F-tagged 3-F,4’PCB3 sulfate IS.53
4’-Chloro-3’-fluoro-biphenyl-4-yl 2,2,2-trichloroethyl sulfate
White solid. Yield: 87%. mp: 85–86 ºC; 1H NMR (400 MHz, CDCl3): δ/ppm 4.85 (s, 2H, CH2), 7.26 (ddd, 1H, J = 2.0 Hz, J = 8.4 Hz), 7.32 (dd, 1H, J = 2.0 Hz, J = 10.0 Hz), 7.42 (AA´XX´ system, 2H), 7.45 (pseudo t, 1H, J ~ 8 Hz), 7.58 (AA´XX´ system, 2H). 13C NMR (100 MHz, CDCl3): δ/ppm 80.6, 92.4, 115.4 (d, J = 22 Hz), 120.9 (d, J = 18 Hz), 121.8, 123.5 (d, J = 3 Hz), 128.8, 131.2, 138.9, 140.1 (d, J = 7 Hz), 150.0, 158.5 (d, J = 247 Hz). EI-MS m/z (relative intensity, %): 434 (5, C14H9Cl4FO4S•+), 222 (100), 193 (47), 157 (45), 131(18), 96 (38), 61 (56).
Sulfuric acid mono-(4’-chloro-3’-fluoro-biphenyl-4-yl) ester, ammonium salt (3-F,4’PCB3 sulfate)
White solid. Yield: 85%. mp: 140 ºC (dec.); 1H NMR (400 MHz, CDCl3): δ/ppm 7.39 (AA ´XX´ system, 2H), 7.43 (dd, 1H, J = 2.0 Hz, J = 8.4 Hz), 7.49–7.54 (m, 2H), 7.55–7.57 (AA ´XX´ system, 2H). 13C NMR (100 MHz, CDCl3): δ/ppm 115.8 (d, J = 21 Hz), 120.4 (d, J = 17 Hz), 123.0, 124.6 (d, J = 4 Hz), 128.8, 132.0, 136.5, 142.9 (d, J = 6 Hz), 154.2, 159.6 (d, J = 245 Hz). HRMS m/z: calculated for C12H7F35ClS: 300.9748, found: 300.9743.
Animals
All animal experiments were conducted with approval from the Institutional Animal Care and Use Committee of the University of Iowa. Six male Sprague Dawley (SD) rats, weighing 150–175 g, were purchased from Harlan Sprague-Dawley (Indianapolis, IN). They were individually housed in wire-floor metabolism cages in a controlled environment maintained at 22°C with a 12h light-dark cycle, and water and feed ad libitum. After acclimatization for a week, animals were randomly divided into two groups. Three animals received single dose of 600 µmol (112 mg)/kg body weight of PCB3 in corn oil via intraperitoneal injection. This dose has its origin in a study by Espandiari et al,36 in which the parent compound, PCB3, was dosed at this level and the metabolites were administered in diminishing doses. It was further adopted by Lehmann et al,37 for the in vivo mutagenesis assay. Therefore, this dose was adopted in the present study to identify the metabolites. Three control animals received corn oil at 5 mL/ kg body weight. Urine and feces were collected in vials attached to metabolism cages every four hours over 36h. Immediately after collection both urine and fecal samples were transferred to polypropylene tubes (no preservatives added) and kept frozen at −20°C until extraction. Animals were euthanized after 36 h using carbon dioxide asphyxiation followed by cervical dislocation. Blood was collected from the right atrium at time of necropsy in a vacutainer containing clot activators (BD vacutainer, Franklin Lakes, NJ); centrifuged at 5000×g for 10 min; serum was transferred to polypropylene micro-centrifuge tubes; and stored at −20°C until extraction.
Extraction of sulfates from urine, serum and feces
A method for simultaneous extraction of hydroxylated and sulfated metabolites of PCB3 was developed. A composite urine sample was prepared by taking 10% wet weight of urine collected at each time point. A composite or single time point urine sample (50 µL) was spiked with 50 µL (500 ng) internal standard, and diluted with equal volume of 1% formic acid. It was applied to a 400µL capacity SLE+ column and eluted with 750 µL ethyl acetate three times as described in the manufacturer’s instructions. Ethyl acetate extract was blown to dryness and reconstituted in 200 µL acetonitrile: water (35:65, v/v).
Serum (200 µL) was spiked with 50 µL (500 ng) internal standard, and acidified with an equal volume of 1% formic acid. An equal volume of acetonitrile was added followed by incubated for 2 hours at −20°C for protein precipitation. The mixture was centrifuged at 15000×g for 15 minutes at 4°C and the liquid fraction was transferred to a glass vial containing 0.05 g NaCl and 0.15 g MgSO4. After thoroughly mixing by shaking and vortexing for one minute, the mixture was centrifuged again at 5000×g for 5 minutes to visibly separate aqueous and organic phases. Acetonitrile extract was blown to almost dryness under a gentle stream of nitrogen and reconstituted in 30 µL methanol. It was further diluted to 300 µL with 1% Formic acid. Finally, a cleanup was carried out by supported liquid extraction (SLE+) column chromatography as described for urine.
Feces collected at each time point were resuspended in potassium phosphate buffer (0.1 M, pH 6.8) to make a suspension of 20 %, w/v; and centrifuged at 2000×g for 5 minutes. Supernatant was transferred into clean tubes. A composite fecal sample was prepared by taking 10% of wet weight, and 500 µL was processed in the same way as serum.
Enzyme incubation
Urine (50 uL) was diluted 1:1 with 0.1 M potassium phosphate buffer (pH 6.8) and incubated with β-glucuronidase (5 µL, 385 IU) or sulfatase (10 µl, 25 IU) as described previously.54 Sulfatase used in this study has some glucuronidase impurities, and this activity was inhibited by D-sachharic acid-1,4-lactone (20 mM in the final reaction mixture). Enzyme incubation was carried out at 37°C for 4 hours in a water bath. The reaction was stopped by adding an equal volume of 1% formic acid (v/v), followed by addition of internal standards and extraction as described above.
Liquid chromatography-Mass spectrometry
Qualitative analysis was carried out on LCQ Deca (Thermo Scientific, San Jose CA) mass spectrometer connected to an Ulitmate 3000 LC (Dionex). The conjugated metabolites were separated on a Supelcosil™ C18 column (25cm × 4.6 mm, 5µm) using a previously described method with a modification.55 Mobile phases were 10 mM ammonium acetate at pH 6.8 (A) and acetonitrile (B). At a flow rate of 0.5 mL/min, solvent B was initially held at 20% for 5 min, increased to 50% B over 30 min, and finally increased to 85% B for 10 min. MS data were collected in full scan mode from m/z 150 to 700. Negative electrospray ionization was used with capillary temperature at 250°C, capillary voltage of 4.0 kV, and nitrogen sheath and auxiliary gas glows set to 65 and 25 (arbitrary units), respectively.
Quantification of sulfates was carried out in an ultra-performance liquid chromatography interfaced with a triple quadrupole mass spectrometer (Acquity TQD, Waters, USA).The same column and mobile phases were used as described above, but the chromatographic separation was carried out isocratically at 35% B for 15 minutes. The flow rate was 0.8 mL/min. The mass spectrometer was used in single ion recording (SIR) mode to monitor the (M-H)− ions of interest (m/z 283, 299 and 301). Other mass spectrometer settings included the source temperature 120°C, desolvation gas temperature 600°C, capillary voltage 2.6 kV, cone voltage 35 V, cone gas flow 100 L/h and desolvation gas flow 600 L/h.
Monohydroxylated PCB3 was analyzed on an Acquity TQD using a BEH C18 column (2.1 ×10 mm, 1.7 µm) by using a previously described method with a modification.56 Mobile phases were 0.1% ammonium hydroxide in water at pH 10.5 (A) and acetonitrile (B). At a flow rate of 0.150 µL/min, solvent B was initially set to 15% for 3 min then increased to 100% over 5 min. The MS settings were the same as used for sulfates except SIR was set for m/z 203 and 221.
Analysis, Quality control, and Quality assurance
Sample analysis was carried out by calibration curve ranging from 10–100 ng/mL for 2’pCB3 sulfate, 20–800 ng/mL for 3’PCB3 sulfate, and 100–2000 ng/mL for 4’PCB3 sulfate with good linearity (R2> 0.995). The limit of detection (LOD) and limit of quantification (LOQ) were calculated from the calibration curve using the formula ,57, 58 where sy is the standard deviation of the predicted y-value for each x-value, and K is the slope of the calibration line of best fit. The LODs were 2, 25, and 150 ng/mL for 2’PCB3 sulfate, 3’PCB3 sulfate and 4’PCB3 sulfate, respectively. The signals for all sulfate metabolites were above LOD in serum, and above LOQ in urine. A set of spiked samples was prepared by spiking known amounts of PCB3 sulfate standards in the urine and serum. Recovery of the each analyte in different matrices are shown in supplemental figure S1. Various approaches for PCB3 sulfates extraction from urine by using different SPE catridges are compared in supplemental figures S2.
Results
Five major and eight minor metabolites of PCB3 were identified in urine
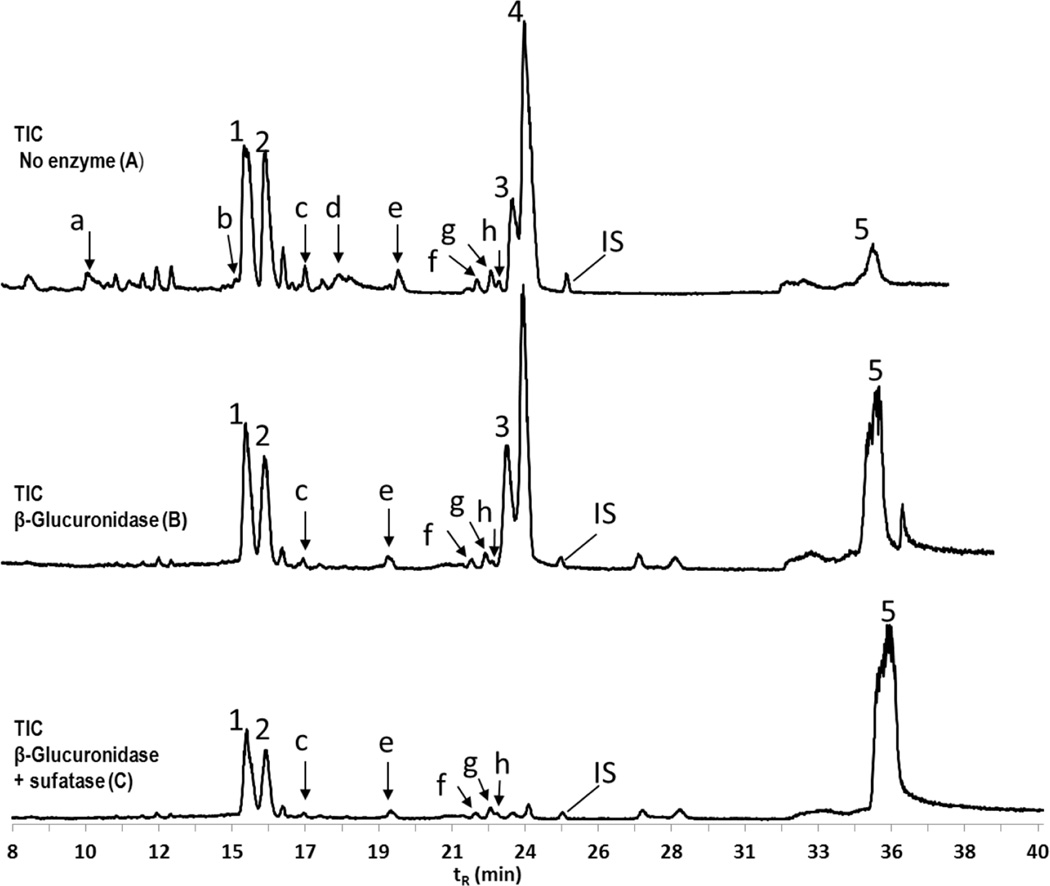
In LC/MS chromatogram, a monochlorinated metabolite of PCB3 was identified by natural isotope abundance ratio of 3:1 for 35Cl/37Cl. Reduced intensity of a peak after incubation of sample with β-glucuronidase or sulfatase further helped to confirm whether it was a glucuronide or sulfate metabolite. Figure 2 shows total ion current (TIC) chromatograms from LC/MS (LCQ Deca) for urine before and after treatment with hydrolyzing enzymes. At least five major metabolites (indicated by peaks 1–5) and eight minor metabolites (indicated by peaks a–h) were identified as potential final metabolites of PCB3 in urine (A). When treated with β-glucuronidase (B), there was no change in the major metabolites, however minor metabolites a, b and d disappeared. The most abundant masses for these peaks were m/z 475, 397, and 379, respectively. When incubated with mixture of β-glucuronidase and sulfatase (C), the major peaks 3 and 4 disappeared. Peak 4 was identified as 4’PCB3 sulfate by comparing with authentic standard. The most abundant mass for peak 3 was m/z 299. These sulfates were further investigated and quantified. All monohydroxylated isomers of PCB3 co-eluted, and are represented by peak 5. The remaining two major peaks and five minor peaks were not affected by hydrolyzing enzymes.
Figure 2. TIC showing metabolites of PCB3 in urine after 10 hours of exposure.
(A) Five major metabolites (peaks 1–5) and eight minor metabolites (peaks a–h) were identified in the urine. The most abundant ions corresponding to the peaks were m/z 475 (a), m/z 397 (b), m/z 366 (1), m/z 366 (2), m/z 461 (c), m/z 379 (d), m/z 329 (e), m/z 348 (f), m/z 348 (g), m/z 313 (h), m/z 299 (3), m/z 283 (4), m/z 203 (5). (B) Incubation with β-glucuronidase resulted in the disappearance of peaks a, b and d, and were attributed to be sulfated-glucuronide diconjugate, dihydrodiol glucuornide conjugate, and monophenol-glucuronide conjugate respectively. (C) Incubation with both β-glucuronidase and sulfatase resulted in additional disappearance of peaks 3 and 4, which were a presumed catechol sulfate, and 4’PCB3 sulfate respectively. All monohydroxylated isomers co-eluted and are indicated by peak 5. All other remaining peaks (1, 2, c, e, f, g, and h) were not affected by hydrolyzing enzymes. MS analysis was carried out by LCQ deca. Mass spectra of all the ions are found in supplemental figure S3.
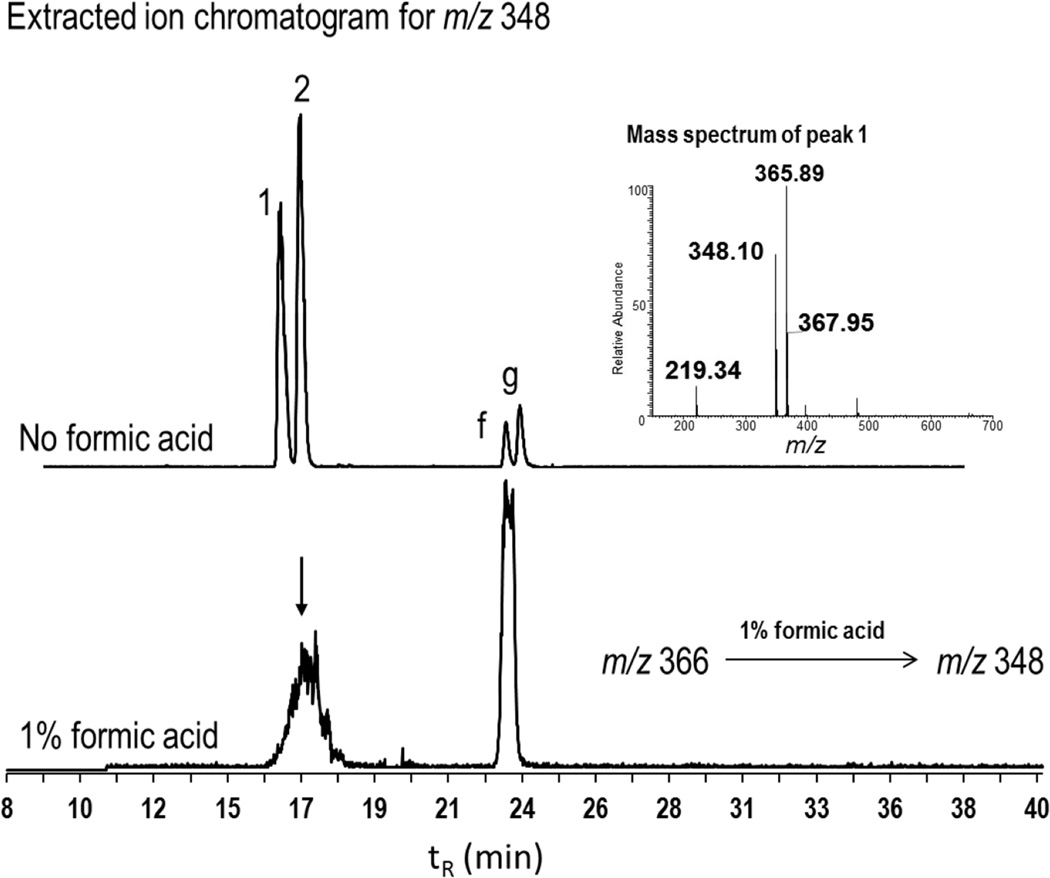
Unknown metabolites 1 and 2 were isomers of m/z 366, and fragmented to m/z 348 in the acidic medium
The mass spectra of peaks 1 and 2 were similar, and consisted of m/z 366 and 348 as most abundant ions. Two other minor peaks f and g were also characterized by m/z 348 as the most abundant ions. In the samples treated with formic acid, we observed degradation of peaks 1 and 2 and increased intensity of peak f or g as a single peak (Figure 3). This indicated that peaks 1 and 2 are metabolite isomers of m/z 366, and their degradation resulted in the formation of minor metabolites f and g. Other minor metabolites were characterized by m/z 461 (peak c), m/z 329 (peak e), and m/z 313 (peak h). The mass spectra of all these unknown metabolites are found in supplemental figure S3.
Figure 3. Effect of formic acid on the fragmentation of m/z 366 (peaks 1 and 2) to m/z 348.
Extracted ion chromatograms for m/z 348 under two conditions of sample preparation in the urine sample. When no acids were used in workup procedure, four peaks 1, 2, f and g were observed (top EIC). In the urine samples diluted with equal volume of 1% formic acid (v/v), peaks 1 and 2 were degraded resulting in a single large peak corresponding to f and g (bottom EIC). A representative mass spectrum for peak 1 showing abundance of masses of ions m/z 366 and m/z 348. The spectra of peak 2 was similar to peak1. This fragmentation pattern suggested that 1, 2, f, and g are putative mercapturic acids metabolites. MS analysis was carried out by LCQ deca.
Less PCB3 was excreted as free phenol or glucuronide conjugate as compared to sulfate conjugates
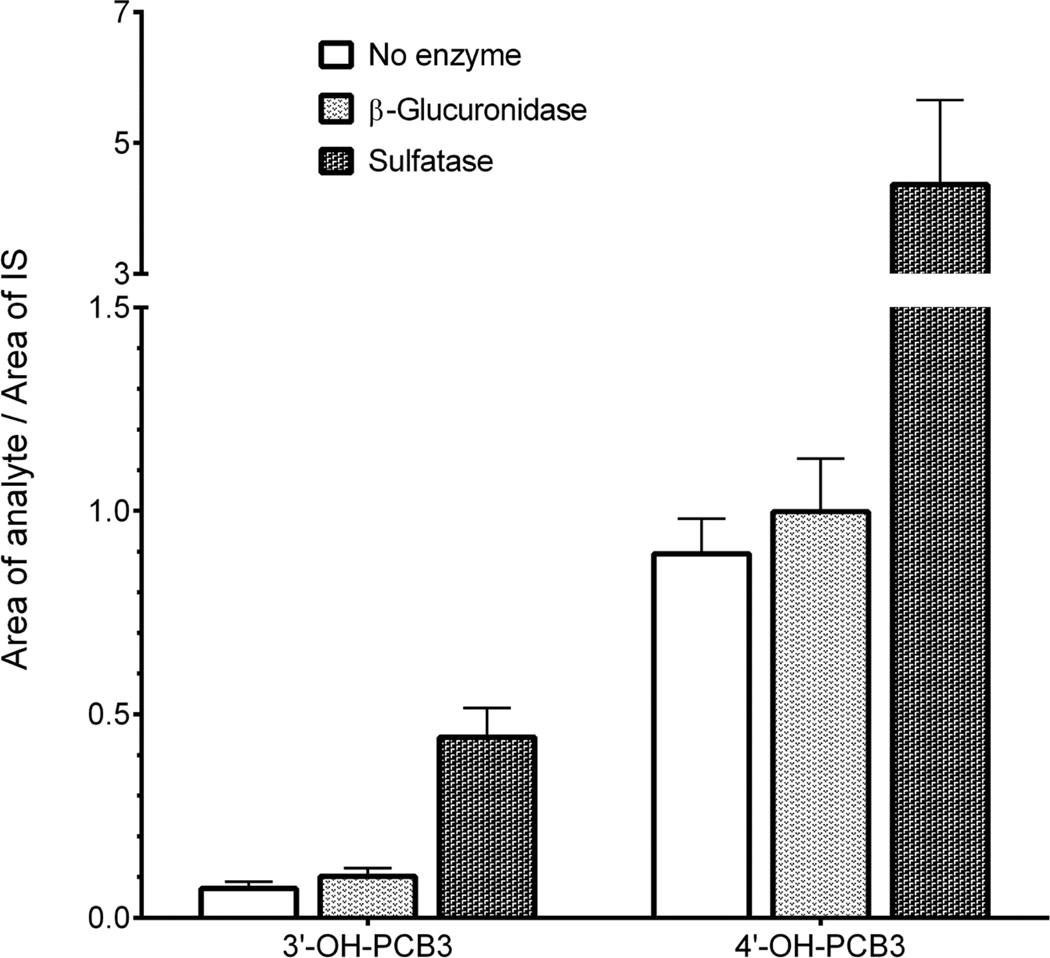
We were further interested in relative proportion of free and conjugated forms of individual phenols. This was achieved with an Acquity C18 column by operating with mobile phases at pH 10.5. A composite urine sample collected over the period of 36 h was analyzed before and after incubation with hydrolyzing enzymes (Figure 4). Two major free phenolic forms were 4’-OH-PCB3 and 3’-OH-PCB3. The concentration of 4’-OH-PCB3 was approximately ten times higher than 3’-OH-PCB3. Incubation with pure β-glucuronidase did not significantly increase the amount of any phenol but the sulfatase released approximately six times more phenols than they were in the free form.
Figure 4. Free and conjugated forms of two major phenols in urine.
Incubation with sulfatase released approximately six times more phenol than it was as free-phenol for both 3’-OH-PCB3 and 4’-OHPCB3. Incubation with glucuronidase did not significantly increased the mono-phenols. MS analysis was carried out by Waters Acquity TQD using an Acquity UPLC BEH C18 column, and pH of mobile phase was 10.5. (n=3)
Sulfate conjugates were rapidly excreted in urine
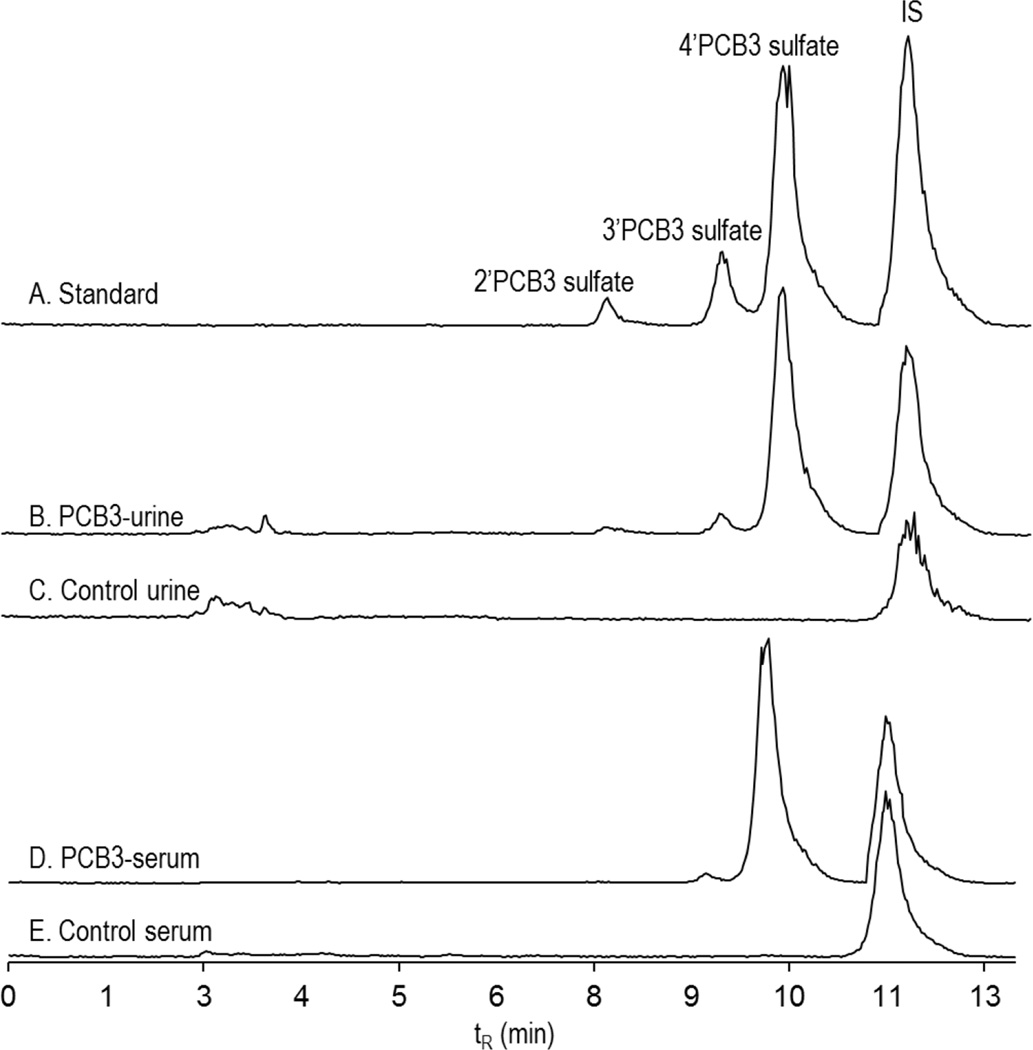
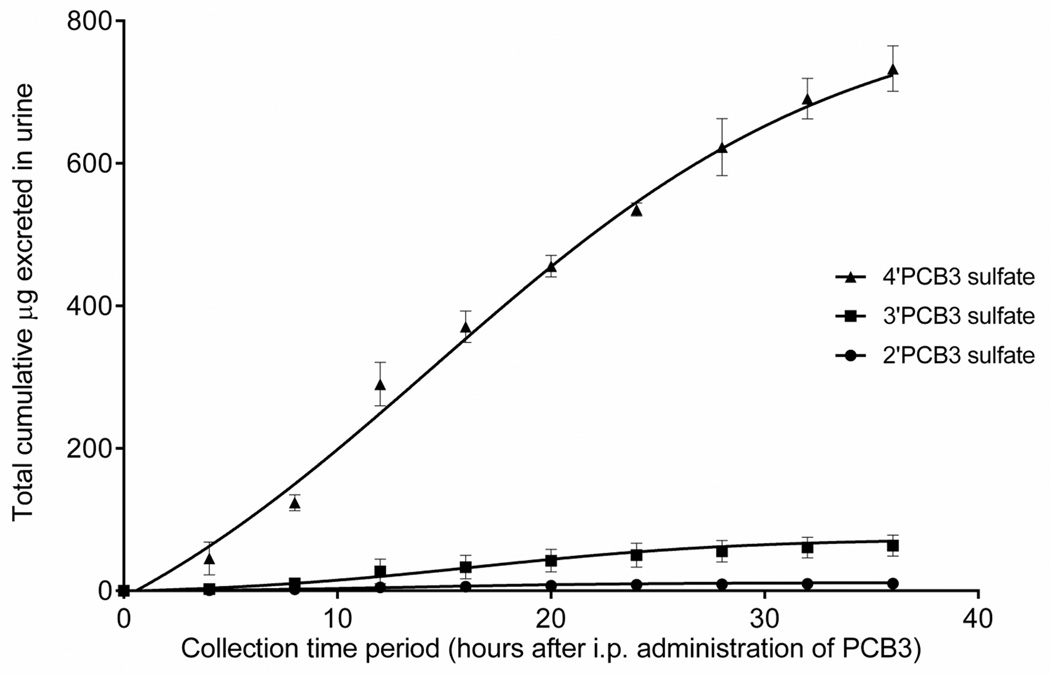
Figure 5 shows the chromatograms of 2’PCB3 sulfate, 3’PCB3 sulfate, and 4’PCB3 sulfate standards at concentration of 0.05, 0.1, o.5 and 0.5 µg/mL (top,chromatogram A). The corresponding peaks in the urine from PCB3 treated and control rats collected after four hours of exposure are shown in chromatograms B and C (middle). The bottom two chromatograms show sulfate metabolite in the serum of PCB3 treated and control rats. The time-course of excretion of sulfated metabolites of PCB3 over the period of 36 h is shown in Figure 6. In urine collected within the first four hours after exposure, the concentrations of 4’PCB3 sulfate, 3’PCB3 sulfate and 2’PCB3 sulfate were as high as 42.0±12, 2.4±1.0 and 0.2 µg/mL, respectively, which represents approximately 0.16% of the dose altogether. A peak excretion occurred between 10–20 hours, followed by sustained elimination up to the end of the collection period. Approximately 3% of the dose was excreted as monosulfated metabolites over this period (Table 1). Since the area under the peak for the unknown sulfate (peak 3) was almost equal to the area of 4’PCB3 sulfate, a significant amount of PCB3 would have been excreted in this form. We did not quantify this metabolite because of the lack of an authentic standard.
Figure 5. Chromatograms showing PCB3 sulfate standard and their peak correspondence in urine and serum.
Chromatograms of 2’ PCB3 sulfate, 3’PCB3 sulfate, 4’PCB3 sulfate, and IS in concentration of 0.05, 0.1, o.5 and 0.5 µg/mL (A), urine from PCB3 treated rats after four hours of exposure (B), urine from control animal (C), serum from PCB3 treated rats (D), and serum from control rats (E). MS analysis was carried out Waters Acquity TQD.
Figure 6. Time-course of the excretion of PCB3 sulfates in urine.
Sulfates were rapidly excreted in urine, reaching a peak excretion within 10–20 h. Urine collected at each time point (50µL) was diluted 1:1 with 1% formic acid and cleaned up in SLE+ chromatography column. Analysis was carried out as described in Figure 5. (n=3)
Table 1.
Disposition of sulfated metabolites in the serum and composite urine over 36 hours.
| Urine (µg/mL) | Feces (µg/g) | Serum (µg/mL) | |
|---|---|---|---|
| 4’PCB3 sulfate | 53.3±4.1 (2.5) | <LOD | 6.18±2.16 |
| 3’PCB3 sulfate | 4.62±1.09 (0.22) | <LOD | 0.137±0.015 |
| 2’PCB3 sulfate | 0.744±0.260 (0.04) | <LOD | 0.0109±0.0029 |
| 4’-OH-PCB3 | 6.68±0.64 (0.43) | 16.2±5.3(0.84) | 0.0955±0.0558 |
Values are means±SD (n=3).
Figures in the parenthesis indicate approximate percentage of dose excreted over 36 hours of exposure.
The signal for sulfate metabolites in 100 µL extract of 100 mg feces was lower than LOD, and not quantified.
Serum concentration of sulfates was several fold higher than hydroxylated forms
The serum concentration of 4’PCB3 sulfate was 6.18±2.2 µg/mL which is about 60 fold higher than 4’-OH-PCB3 in serum (Table 1). We also observed a peak of unknown sulfate (peak 3) as intensive as 4’pCB3 sulfate in the serum.
Metabolites excreted in the feces
We did not see a significant amount of sulfated metabolites excreted in feces, possibly due to the hydrolysis of sulfated metabolites by bacterial sulfatase in the intestine. The amount of hydroxylated metabolite excreted in feces was calculated to be twice as high as that excreted in urine (Table 1). In a preliminary study with two bile cannulated male SD rats (270–300g), which also received a single i.p. injection of 600 µmole/kg body weight PCB3, the ratio of concentration of 4’PCB3 sulfate in bile to urine was approximately 1:10 at 12 hours after exposure (data not shown). Incubation of bile with β-glucuronidase also did not considerably increase the amount of monophenols.
Discussion
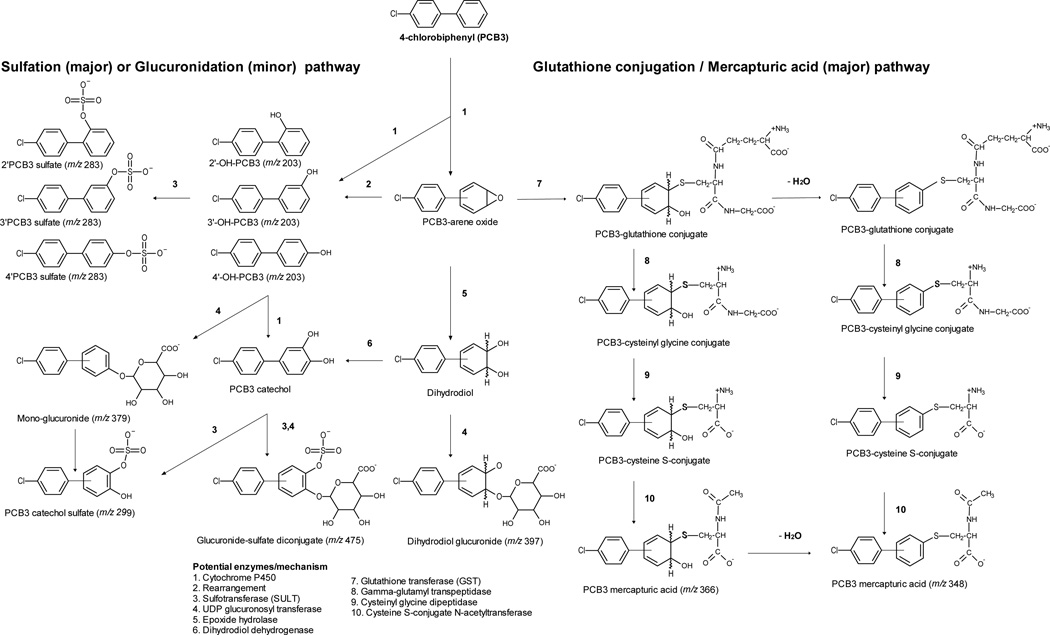
Biotransformation of xenobiotics often involves conversion of lipophilic parent compounds to more polar forms. Hydroxylation of PCB3 by cytochrome P450 isoforms occurs either directly by insertion of oxygen in a C-H bond,59 or indirectly by formation of arene oxide intermediate.60 Relatively little is known about further biotransformation of phenolic PCBs to conjugated metabolites in vivo and their excretion in the urine. In this study we obtained evidence for biotransformation of PCB3 to possible glucuronide, sulfate and glutathione conjugates (Scheme 1). We further quantified 4’PCB3 sulfate, 3’PCB3 sulfate, 2’PCB3 sulfate in the serum and urine.
Scheme 1. Proposed biotransformation pathway for 4-Chlorobiphenyl (PCB3) in rats.
Potential enzymes/mechanisms are indicated by numbers in the legend.
Incubation of urine with β-glucuronidase did not significantly increase the level of mono-phenols. The minor unknown glucuronide of m/z 379, which is most likely a mono-phenol glucuronide, was also not an intense major metabolite. Low glucuronidation of PCB3 metabolites can be explained on the basis of steric hindrance at 4’ position, as noted previously.44
Many hydroxylated LC-PCBs, including OH-PCB3, have been shown to be good substrates for SULT1A1, which is a major sulfotransferase for phenols.46, 48 Expression of rSULT1A1 has been reported to be higher in male adult rats than in the female rats of same age.61, 62 This raises an important question in extrapolating these findings to other species. Human SULT 1A1 has a broad substrate specificity.63, 64 In vitro studies indicate that some hydroxylated LC-PCBs are excellent substrates for hSULT1A1.46
The precursors of reactive quinone and semi-quionone intermediates were observed to be further biotransformed by sulfation and glucuronidation. The unknown major sulfate metabolite (peak 3) has m/z 299, which is 16 units higher than that of 4’PCB3 sulfate. Therefore, it is most likely a catechol sulfate formed by the sulfation of 3’,4’-diOH-PCB3 because this catechol is a major metabolite after 4’-OH-PCB3 in rats.18, 20, 22 In an extracted ion chromatogram for m/z 299, an additional minor peak was also observed which is likely a sulfate of two other dihydroxylated metabolites, namely 2’,3’-diOH-PCB3 and 2’,5’-diOH-PCB3 reported previously (not shown in the chromatogram).22 Two other unknown glucuronides of m/z 397 and m/z 475 can be attributed to dihydrodiolglucuronide, and a sulfated-glucuronide diconjugate respectively. A similar glucuronide metabolite has been reported for many xenobiotic that form arene oxide intermediates.65 Althoug these findings require further confirmation and synthesis of authentic standards, they provide mechanistic insights into the metabolism that bioactivation of PCB3. For instance, it confirms that bioactivation of PCB3 occurs by the formation of dihydrodiol. At the same time, it also suggests that there are mechanisms to detoxify and eliminate these quinone precursors by phase II conjugations.
Glutathione conjugation of an electrophilic metabolite (R-SG) produces a species that undergoes stepwise transformation by hepatic and renal enzymes to form a mercapturic acid (R-SCys-NAC).66 Glutathione conjugation of a PCB arene oxide, and subsequent formation of PCB-mercapturic acids has been described.67 This process proceeds by loss of water from glutathione-PCB arene oxide complex.68 Glutathione conjugation of PCB3 may result in the formation of a final metabolite with a molecular weight of 367; and its (M-H)− would be 366. A loss of water, by mass of 18 units, would form an ion of m/z 348. We observed a mass of m/z 366 fragmented to m/z 348 in the acidic medium. Therefore, the unknown major metabolites 1 and 2 are most likely isomers of PCB3 mercapturic acids of m/z 366 and minor metabolites f and g most likely resulted from their degradation.
We did not observe any ions that could be attributed to mercapturic acids derived from glutathione conjugation with PCB3-quinone. However, minor metabolites of m/z 461, m/z 329 and m/z 313, which were not affected by hydrolyzing enzymes, could possibly have resulted from reaction of PCB3 quinone with endogenous nucleophiles. Metabolism of PCB mercapturates or precursor cysteine conjugates to methyl sulfones has been described.69, 70 No mercapturic acids or methyl sulfone metabolites of PCB3 have been reported. Therefore, we recommend further investigation to the potential formation of PCB3 mercapturates or PCB3-quinone adducts.
Urine collection offers a non-invasive technique of sample collection. Urine is a cleaner matrix than serum for analytical purposes, contains no lipids, and has been widely employed for assessment of exposure to halogenated environmental chemicals and herbicides.71 Conventionally, serum analysis for hydroxylated PCBs is carried out by GC-ECD or GC-MS, which requires derivatization, and subsequent stringent lipid cleanups.72 Hydroxylated LC-PCB are sub-optimally methylated by diazomethane,73 and can be destroyed by treatment with sulfuric acid,74 or acidified silica gels.75 Moreover, it is penta to hepta chlorinated hydroxylated PCBs that have been predominantly studied as persistent metabolites in the serum rather than lower chlorinated hydroxylated PCBs.42 Our study suggests that sulfated metabolites of lower chlorinated PCBs are found at much higher level than hydroxylated metabolites in both serum and urine, and are quantifiable by LC/MS following one-step SPE. Many commercially available SPE cartridges were very efficient in the extraction of sulfated metabolites of PCB3 from urine (Supplemental figure S2). The ease of extraction and quantification of PCB sulfates opens a new avenue for considering this metabolite as a potential biomarker for risk assessment of environmental exposure to lower chlorinated PCBs.
Conclusions
We report the formation of 2’PCB3 sulfate, 3’PCB3 sulfate; 4’PCB3 sulfate in urine and serum of male SD rats exposed to a single dose of 600 µmol PCB3/ kg body weight by i.p. injection. By LC/MS analysis of urine before and after incubation with hydrolyzing enzymes, we have proposed final biotransformation products of PCB3 by sulfation, glucuronidation, and glutathione conjugation. Higher concentrations of monosulfated metabolites than phenolic PCB3 in both urine and serum suggests that this class of metabolites can be an alternative to serum analysis for exposure assessment of LC-PCBs. This is the first report of sulfated PCB metabolites that are formed in vivo.
Supplementary Material
ACKNOWLEDGMENTS
These studies form a portion of the dissertation research of K.D. The authors thank Dr. Xueshu Li for the synthesis of authentic standards standards used in these studies, and thank members of our laboratory for help with the animal studies. K.D. gratefully acknowledges support from the Iowa Superfund Research Program (P42 ES013661) Training Core.
Funding Sources
These studies were supported by funding from NIH (ES 013661, ES 05605). The opinions expressed are solely those of the authors, and do not reflect an official policy of the granting agencies.
ABBREVIATIONS
- CYPs
Cytochrome P450
- SULTs
Sulfotransferases
- POPs
persistent organic pollutant
- 4-chlorobiphenyl
PCB3
- LC-PCBs
lower chlorinated PCBs
- OH-PCBs
hydroxylated PCBs
- i.p.
intraperitoneal
- SLE+
supported liquid extraction
- SPE
solid phase extraction
- SD
Sprague Dawley
- LOD
limit of detection
- LOQ
limit of quantation
Footnotes
Supporting Information. Recovery of sulfates from spiked matrices (S1), different approaches of extraction of sulfates from urine by using SPE cartridges (S2), and mass spectra of metabolites discussed in figures 2 and scheme 1 (S3). This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors state that there is no conflict of interest.
REFERENCES
- 1.Jones KC, de Voogt P. Persistent organic pollutants (POPs): state of the science. Environ. Pollut. 1999;100:209–221. doi: 10.1016/s0269-7491(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 2.Burreau S, Zebuhr Y, Broman D, Ishaq R. Biomagnification of PBDEs and PCBs in food webs from the Baltic Sea and the northern Atlantic ocean. Sci. Total Environ. 2006;366:659–672. doi: 10.1016/j.scitotenv.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Nisbet IC, Sarofim AF. Rates and Routes of Transport of PCBs in the Environment. Environ. Health Perspect. 1972;1:21–38. doi: 10.1289/ehp.720121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breivik K, Sweetman A, Pacyna JM, Jones KC. Towards a global historical emission inventory for selected PCB congeners--a mass balance approach. 1. Global production and consumption. Sci. Total Environ. 2002;290:181–198. doi: 10.1016/s0048-9697(01)01075-0. [DOI] [PubMed] [Google Scholar]
- 5.Consonni D, Sindaco R, Bertazzi PA. Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: A review, 1989–2010. Environ. Int. 2012;44:151–162. doi: 10.1016/j.envint.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Wania F, Mackay D. Peer reviewed: tracking the distribution of persistent organic pollutants. Environ. Sci. Technol. 1996;30:390A–396A. doi: 10.1021/es962399q. [DOI] [PubMed] [Google Scholar]
- 7.Iwata H, Tanabe S, Sakai N, Nishimura A, Tatsukawa R. Geographical distribution of persistent organochlorines in air, water and sediments from Asia and Oceania, and their implications for global redistribution from lower latitudes. Environ. Pollut. 1994;85:15–33. doi: 10.1016/0269-7491(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 8.Arellano L, Fernandez P, Tatosova J, Stuchlik E, Grimalt JO. Long-Range Transported Atmospheric Pollutants in Snowpacks Accumulated at Different Altitudes in the Tatra Mountains (Slovakia) Environ. Sci. Technol. 2011;45:9268–9275. doi: 10.1021/es202111n. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg M, Soler L, Contenot S, Verger P. Assessment of seasonality in exposure to dioxins, furans and dioxin-like PCBs by using long-term food-consumption data. Food Addit. Contam. A. 2011;28:502–512. doi: 10.1080/19440049.2011.553844. [DOI] [PubMed] [Google Scholar]
- 10.Currado GM, Harrad S. Comparison of polychlorinated biphenyl concentrations in indoor and outdoor air and the potential significance of inhalation as a human exposure pathway. Environ. Sci. Technol. 1998;32:3043–3047. [Google Scholar]
- 11.Ludewig G, Lehmann L, Esch H, Robertson LW. Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environ. Toxicol. Pharmacol. 2008;25:241–246. doi: 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persoon C, Peters TM, Kumar N, Hornbuckle KC. Spatial distribution of airborne polychlorinated biphenyls in Cleveland, Ohio and Chicago, Illinois. Environ. Sci. Technol. 2010;44:2797–2802. doi: 10.1021/es901691s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu D, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC. Atmospheric PCB congeners across Chicago. Atmos. Environ. 2010;44:1550–1557. doi: 10.1016/j.atmosenv.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Environmental Protection Agency. Public Health Levels for PCBs in Indoor School Air. 2011 Available at http://www.epa.gov/pcbsincaulk.
- 15.Hu D, Martinez A, Hornbuckle KC. Discovery of non-aroclor PCB (3,3'-dichlorobiphenyl) in Chicago air. Environ. Sci. Technol. 2008;42:7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa Y, Noma Y, Mori Y, Sakai S. Congener profiles of PCB and a proposed new set of indicator congeners. Chemosphere. 2007;67:1838–1851. doi: 10.1016/j.chemosphere.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 17.Block WD, Cornish HH. Metabolism of Biphenyl and 4-Chlorobiphenyl in the Rabbit. J. Biol. Chem. 1959;234:3301–3302. [Google Scholar]
- 18.Safe S, Ruzo LO. The metabolism of 4-chlorobiphenyl in the pig. Can. J. Physiol. Pharmacol. 1975;53:392–396. doi: 10.1139/y75-056. [DOI] [PubMed] [Google Scholar]
- 19.Safe S, Hutzinger O, Ecobichon DJ, Grey AA. The metabolism of 4'chloro-4-biphenylol in the rat. Can. J. Biochem. 1975;53:415–420. doi: 10.1139/o75-057. [DOI] [PubMed] [Google Scholar]
- 20.Hass JR, Jao LT, Wilson NK, Matthews HB. Metabolism of 4-chlorobiphenyl and 4,4'-dichlorobiphenyl in the rat: qualitative and quantitative aspects. J. Agric. Food Chem. 1977;25:1330–1333. doi: 10.1021/jf60214a005. [DOI] [PubMed] [Google Scholar]
- 21.Wyndham C, Safe S. In vitro metabolism of 4-chlorobiphenyl by control and induced rat liver microsomes. Biochem. 1978;17:208–215. doi: 10.1021/bi00595a002. [DOI] [PubMed] [Google Scholar]
- 22.McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem. Res. Toxicol. 1996;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- 23.Parkinson A, Safe S. Cytochrome P-450-mediated metabolism of biphenyl and the 4-halobiphenyls. Biochem. Pharmacol. 1982;31:1849–1856. doi: 10.1016/0006-2952(82)90487-7. [DOI] [PubMed] [Google Scholar]
- 24.Song Y, Wagner BA, Lehmler HJ, Buettner GR. Semiquinone radicals from oxygenated polychlorinated biphenyls: Electron paramagnetic resonance studies. Chem. Res. Toxicol. 2008;21:1359–1367. doi: 10.1021/tx8000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wangpradit O, Mariappan SVS, Teesch LM, Duffel MW, Norstrom K, Robertson LW, Luthe G. Oxidation of 4-Chlorobiphenyl Metabolites to Electrophilic Species by Prostaglandin H Synthase. Chem. Res. Toxicol. 2009;22:64–71. doi: 10.1021/tx800300t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW. Metabolic activation of PCBs to quinones: reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem.Res. Toxicol. 1996;9:623–629. doi: 10.1021/tx950117e. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Narang A, Ding X, Eadon G. Characterization and quantitative analysis of DNA adducts formed from lower chlorinated PCB-derived quinones. Chem. Res. Toxicol. 2004;17:502–511. doi: 10.1021/tx034245b. [DOI] [PubMed] [Google Scholar]
- 28.Oakley GG, Robertson LW, Gupta RC. Analysis of polychlorinated biphenyl-DNA adducts by 32P-postlabeling. Carcinogenesis. 1996;17:109–114. doi: 10.1093/carcin/17.1.109. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan A, Robertson LW, Ludewig G. Sulfhydryl binding and topoisomerase inhibition by PCB metabolites. Chem. Res. Toxicol. 2002;15:497–505. doi: 10.1021/tx010128+. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan A, Lehmler HJ, Robertson LW, Ludewig G. Production of DNA strand breaks in Vitro and reactive oxygen species in Vitro and in HL-60 cells by PCB metabolites. Toxicol. Sci. 2001;60:92–102. doi: 10.1093/toxsci/60.1.92. [DOI] [PubMed] [Google Scholar]
- 31.Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Polychlorinated biphenyl quinone metabolites poison human topoisomerase IIalpha: altering enzyme function by blocking the N-terminal protein gate. Biochem. 2006;45:10140–10152. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- 32.Flor S, Ludewig G. Polyploidy-induction by dihydroxylated monochlorobiphenyls: Structure-activity-relationships. Environ. Int. 2010;36:962–969. doi: 10.1016/j.envint.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhuri L, Sarsour EH, Goswami PC. 2-(4-Chlorophenyl)benzo-1,4-quinone induced ROS-signaling inhibits proliferation in human non-malignant prostate epithelial cells. Environ. Int. 2010;36:924–930. doi: 10.1016/j.envint.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobus JA, Flor S, Klingelhutz A, Robertson LW, Ludewig G. 2-(4'-Chlorophenyl)-1,4-Benzoquinone Increases the Frequency of Micronuclei and Shortens Telomeres. Environ. Toxicol. Pharmacol. 2008;25:267–272. doi: 10.1016/j.etap.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereg D, Tampal N, Espandiari P, Robertson LW. Distribution and macromolecular binding of benzo[a]pyrene and two polychlorinated biphenyl congeners in female mice. Chem. Biol. Interact. 2001;137:243–258. doi: 10.1016/s0009-2797(01)00256-3. [DOI] [PubMed] [Google Scholar]
- 36.Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Initiating activity of 4-chlorobiphenyl metabolites in the resistant hepatocyte model. Toxicol. Sci. 2004;79:41–46. doi: 10.1093/toxsci/kfh097. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann L, Esch HL, Kirby PA, Robertson LW, Ludewig G. 4-monochlorobiphenyl (PCB3) induces mutations in the livers of transgenic Fisher 344 rats. Carcinogenesis. 2007;28:471–478. doi: 10.1093/carcin/bgl157. [DOI] [PubMed] [Google Scholar]
- 38.Maddox C, Wang B, Kirby PA, Wang K, Ludewig G. Mutagenicity of 3-methylcholanthrene, PCB3, and 4-OH-PCB3 in the lung of transgenic BigBlue (R) rats. Environ. Toxicol. Pharmacol. 2008;25:260–266. doi: 10.1016/j.etap.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobus JA, Wang B, Maddox C, Esch H, Lehmann L, Robertson LW, Wang K, Kirby P, Ludewig G. 3-Methylcholanthrene (3-MC) and 4-chlorobiphenyl (PCB3) genotoxicity is gender-related in Fischer 344 transgenic rats. Environ. Int. 2010;36:970–979. doi: 10.1016/j.envint.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hovander L, Malmberg T, Athanasiadou M, Athanassiadis I, Rahm S, Bergman A, Wehler EK. Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma. Arch. Environ. Contam. Toxicol. 2002;42:105–117. doi: 10.1007/s002440010298. [DOI] [PubMed] [Google Scholar]
- 41.Dirtu AC, Jaspers VL, Cernat R, Neels H, Covaci A. Distribution of PCBs, their hydroxylated metabolites, and other phenolic contaminants in human serum from two European countries. Environ. Sci. Technol. 2010;44:2876–2883. doi: 10.1021/es902149b. [DOI] [PubMed] [Google Scholar]
- 42.Bergman A, Klasson-Wehler E, Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ. Health Perspect. 1994;102:464–469. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrick RF, Meeker JD, Altshul L. Serum PCB levels and congener profiles among teachers in PCB-containing schools: a pilot study. Environ. Health. 2011;10:56. doi: 10.1186/1476-069X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tampal N, Lehmler HJ, Espandiari P, Mahnberg T, Robertson LW. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs) Chem. Res. Toxicol. 2002;15:1259–1266. doi: 10.1021/tx0200212. [DOI] [PubMed] [Google Scholar]
- 45.Volp RP. Glucuronidation in 4-chlorobiphenyl metabolism by rat and human liver microsomes. Drug Metab. Dispos. 1988;16:650–652. [PubMed] [Google Scholar]
- 46.Wang LQ, Lehmler HJ, Robertson LW, James MO. Polychlorobiphenylols are selective inhibitors of human phenol sulfotransferase 1A1 with 4-nitrophenol as a substrate. Chem. Biol. Interact. 2006;159:235–246. doi: 10.1016/j.cbi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol. 2006;19:1420–1425. doi: 10.1021/tx060160+. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Smart JT, Song Y, Lehmler HJ, Robertson LW, Duffel MW. Structure-Activity Relationships for Hydroxylated Polychlorinated Biphenyls as Substrates and Inhibitors of Rat Sulfotransferases and Modification of These Relationships by Changes in Thiol Status. Drug Metab. Dispos. 2009;37:1065–1072. doi: 10.1124/dmd.108.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oikari A, Anas E. Chlorinated Phenolics and Their Conjugates in the Bile of Trout (Salmo-Gairdneri) Exposed to Contaminated Waters. Bull. Environ. Contam. Toxicol. 1985;35:802–809. doi: 10.1007/BF01636591. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Parkin S, Duffel MW, Robertson LW, Lehmler HJ. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ. Int. 2010;36:843–848. doi: 10.1016/j.envint.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joshi SN, Vyas SM, Duffel MW, Parkin S, Lehmler HJ. Synthesis of Sterically Hindered Polychlorinated Biphenyl Derivatives. Synthesis (Stuttg) 2011;7:1045–1054. doi: 10.1055/s-0030-1258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem. Res. Toxicol. 1996;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Parkin S, Duffel MW, Robertson LW, Lehmler H-J. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ. Int. 2010;36:843–849. doi: 10.1016/j.envint.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am. J. Clin. Nutr. 2002;76:588–594. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- 55.Daidoji T, Gozu K, Iwano H, Inoue H, Yokota H. UDP-glucuronosyltransferase isoforms catalyzing glucuronidation of hydroxy-polychlorinated biphenyls in rat. Drug Metab. Dispos. 2005;33:1466–1476. doi: 10.1124/dmd.105.004416. [DOI] [PubMed] [Google Scholar]
- 56.Zhai GS, Lehmler HJ, Schnoor JL. Hydroxylated Metabolites of 4-Monochlorobiphenyl and Its Metabolic Pathway in Whole Poplar Plants. Environ. Sci. Technol. 2010;44:3901–3907. doi: 10.1021/es100230m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.St'avova J, Stahl DC, Seames WS, Kubatova A. Method development for the characterization of biofuel intermediate products using gas chromatography with simultaneous mass spectrometric and flame ionization detections. J. Chromatogr. A. 2012;1224:79–88. doi: 10.1016/j.chroma.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 58.University of North Dakota. Chemistry Department. How to use linest function to calculate LOD. Available at http://www.und.edu/dept/chromatography/Docs/Determination%20of%20LODs.pdf.
- 59.Tomaszewski JE, Jerina DM, Daly JW. Deuterium isotope effects during formation of phenols by hepatic monoxygenases. Evidence for an alternative to arene oxide pathway. Biochem. 1975;14:2024–2031. doi: 10.1021/bi00680a033. [DOI] [PubMed] [Google Scholar]
- 60.Jerina DM, Daly JW. Arene oxides: a new aspect of drug metabolism. Science. 1974;185:573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- 61.Klaassen CD, Liu L, Dunn RT., 2nd Regulation of sulfotransferase mRNA expression in male and female rats of various ages. Chem. Biol. Interact. 1998;109:299–313. doi: 10.1016/s0009-2797(97)00141-5. [DOI] [PubMed] [Google Scholar]
- 62.Chen G, Baron J, Duffel MW. Enzyme-and sex-specific differences in the intralobular localizations and distributions of aryl sulfotransferase IV (tyrosine-ester sulfotransferase) and alcohol (hydroxysteroid) sulfotransferase a in rat liver. Drug Metab. Dispos. 1995;23:1346–1353. [PubMed] [Google Scholar]
- 63.Berger I, Guttman C, Amar D, Zarivach R, Aharoni A. The molecular basis for the broad substrate specificity of human sulfotransferase 1A1. PloS one. 2011;6:e26794. doi: 10.1371/journal.pone.0026794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hempel N, Gamage N, Martin JL, McManus ME. Human cytosolic sulfotransferase SULT1A1. Internat. J. Biochem. Cell Biol. 2007;39:685–689. doi: 10.1016/j.biocel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Webb L, Miles K, Kessler F, Ritter JK. Activity of rat UGT1A1 towards benzo[a]pyrene phenols and dihydrodiols. Environ. Toxicol. Pharmacol. 2006;21:224–230. doi: 10.1016/j.etap.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 66.Hinchman CA, Ballatori N. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J. Toxicol. Environ. Health. 1994;41:387–409. doi: 10.1080/15287399409531852. [DOI] [PubMed] [Google Scholar]
- 67.Bakke JE, Bergman AL, Larsen GL. Metabolism of 2,4',5-Trichlorobiphenyl by the Mercapturic Acid Pathway. Science. 1982;217:645–647. doi: 10.1126/science.6806905. [DOI] [PubMed] [Google Scholar]
- 68.Letcher R, Klasson-Wehler E, Bergman A. In: Methyl Sulfone and Hydroxylated Metabolites of Polychlorinated Biphenyls. Hutzinger O, Paasivirta J, editors. Volume 3. Berlin / Heidelberg: Springer; 2000. pp. 315–359. Anthropogenic Compounds Part K. [Google Scholar]
- 69.Gustafsson JA, Rafter JJ, Bakke JE, Gustafsson BE. The effect of intestinal microflora on the enterohepatic circulation of mercapturic acid pathway metabolites. Nutr. Cancer. 1981;2:224–231. doi: 10.1080/01635588109513687. [DOI] [PubMed] [Google Scholar]
- 70.Bergman A, Larsen GL, Bakke JE. Biliary-Secretion, Retention and Excretion of 5 C-14-Labeled Polychlorinated-Biphenyls in the Rat. Chemosphere. 1982;11:249–253. [Google Scholar]
- 71.Jurewicz J, Hanke W, Sobala W, Ligocka D. Exposure to phenoxyacetic acid herbicides and predictors of exposure among spouses of farmers. Ann. Agric. Environ. Med. 2012;19:51–56. [PubMed] [Google Scholar]
- 72.Hovander L, Athanasiadou M, Asplund L, Jensen S, Wehler EK. Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. J. Anal. Toxicol. 2000;24:696–703. doi: 10.1093/jat/24.8.696. [DOI] [PubMed] [Google Scholar]
- 73.van 't Erve TJ, Rautiainen RH, Robertson LW, Luthe G. Trimethylsilyldiazomethane: a safe nonexplosive, cost effective and less-toxic reagent for phenol derivatization in GC applications. Environ. Int. 2010;36:835–842. doi: 10.1016/j.envint.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sundström G, Jansson B. The metabolism of 2,2′,3,5′,6-pentachlorobiphenyl in rats, mice and quails. Chemosphere. 1975;4:361–370. [Google Scholar]
- 75.Kania-Korwel I, Zhao H, Norstrom K, Li X, Hornbuckle KC, Lehmler HJ. Simultaneous extraction and clean-up of polychlorinated biphenyls and their metabolites from small tissue samples using pressurized liquid extraction. J. Chromatogr. A. 2008;1214:37–46. doi: 10.1016/j.chroma.2008.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.