Abstract
Multiple classes of cell surface receptors and ion channels participate in the detection of changes in environmental stimuli, and thereby influence animal behavior. Among the many classes of ion channels, Transient Receptor Potential (TRP) cation channels are notable in contributing to virtually every sensory modality, and in controlling a daunting array of behaviors. TRP channels appear to be conserved in all metazoan organisms including worms, insects and humans. Flies encode 13 TRPs, most of which are expressed and function in sensory neurons, and impact behaviors ranging from phototaxis to thermotaxis, gravitaxis, the avoidance of noxious tastants and smells and proprioception. Multiple diseases result from defects in TRPs, and flies provide an excellent animal model for dissecting the mechanisms underlying “TRPopathies.” Drosophila TRPs also function in the sensation of botanically derived insect repellents, and related TRPs in insect pests are potential targets for the development of improved repellents to combat insect-borne diseases.
Keywords: TRP channels, Drosophila, behavior, sensory signaling, thermosensation, taste, smell, vision, mechanosensation, phospholipase C, gustation, olfactory, phototransduction, calcium, proprioception, Transient Receptor Potential, auditory, hearing, thermotaxis
Introduction
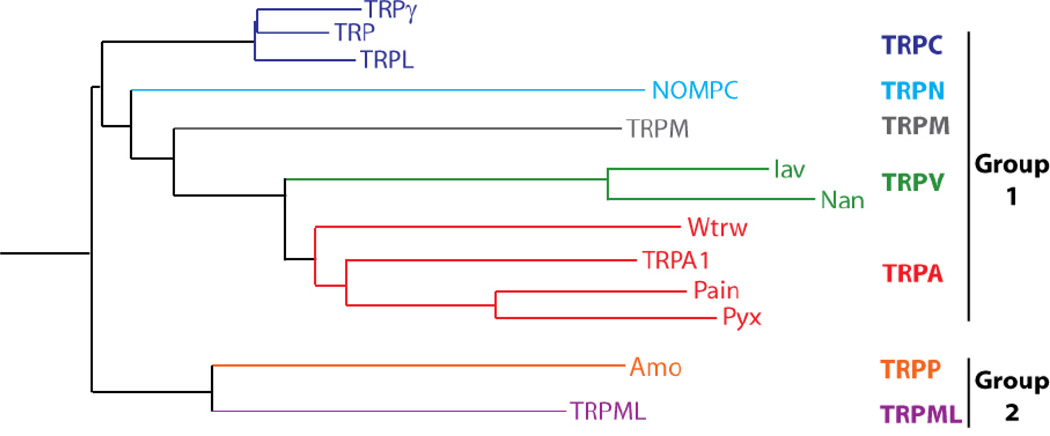
The Transient Receptor Potential (TRP) superfamily of ion channels comprises a collection of cation channels conserved from worms to flies and humans (Ramsey et al., 2006, Venkatachalam and Montell, 2007). The channels are arranged into seven subfamilies based on primary amino acid sequence homology (TRPC, TRPV, TRPA, TRPN, TRPM, TRPML, and TRPP) (Montell, 2005, Montell et al., 2002). TRPs are activated through a wide variety of mechanisms and participate in virtually every sensory modality (Table 1). Since the cloning and characterization of the gene encoding the Drosophila TRP channel, which functions in phototransduction (Montell and Rubin, 1989), twelve other fly TRP channels have been identified (Fig. 1). These channels are critical for sensing the external environment, and function in vision, thermosensation, olfaction, taste, hygrosensation, and mechanosensation. Consequently, these channels have a profound impact on animal behavior. Due to their genetic tractability, flies are an outstanding animal model for studying TRP channel function in the contexts of sensory physiology and animal behavior.
Table 1.
Properties of Drosophila Transient Receptor Potential (TRP) channels.
| TRP Channels with known sensory roles | |||||
|---|---|---|---|---|---|
| Sub Family |
Channel | Abbrev. | Selectivity PCa/PNa |
Physiological modes of activation | Sensory Functions |
| TRPC | Transient Receptor Potential |
TRP | 25–40 | Gq/PLC signaling, PUFA, H+ |
|
| TRP-Like | TRPL | nonselec. cation |
Gq/PLC signaling, PUFA |
|
|
| TRPγ | TRPγ | nonselec. cation |
Gq/PLC signaling, PUFA | - | |
| TRPA | TRPA1 | TRPA1 | - | Heat (>26°C) reactive electrophiles (AITC, NMM, CA) Temp (18–24°C, Rh1/PLC signaling) arist. acid (Gq/PLC sig.), citronellal (Gq/PLC sig.), light |
|
| Painless | Pain | 40 | Heat (~39–42°C) |
|
|
| Pyrexia | Pyx | 0.7 | Heat (~40°C) |
|
|
| Waterwitch | Wtrw | - | - |
|
|
| TRPN | No Mechanoreceptor Potential C |
NOMPC | - | Mechanical stimulation |
|
| TRPV | Inactive | Iav | 2.8 | Hypo-osmotic solution |
|
| Nanchung | Nan | - | Hypo-osmotic solution |
|
|
| TRP Channels without known sensory roles | |||||
| Sub family |
Channel | Abbrev. | Selectivity PCa/PNa |
Physiological modes of activation | Functions |
| TRPM | TRPM | TRPM | - | - |
|
| TRPP | Almost there | Amo | - | - |
|
| TRPML | TRP Mucolipin |
TRPML | - | - |
|
Abbreviations: AITC, allyl isothiocyanate; arist. acid, aristolochic acid; NMM, N-methyl maleimide; CA, cinnamaldehyde; PLC, phospholipase C; PUFA, polyunsaturated fatty acid; Rh1, Rhodopsin 1.
Figure 1.
Phylogenetic tree of the Drosophila TRP channels. The tree was generated with CLC Sequence Viewer 6.4 using the amino acid sequence of the predicted transmembrane domains of the 13 Drosophila TRP channels.
Light sensation
TRP and TRPL and phototransduction in the adult visual systems
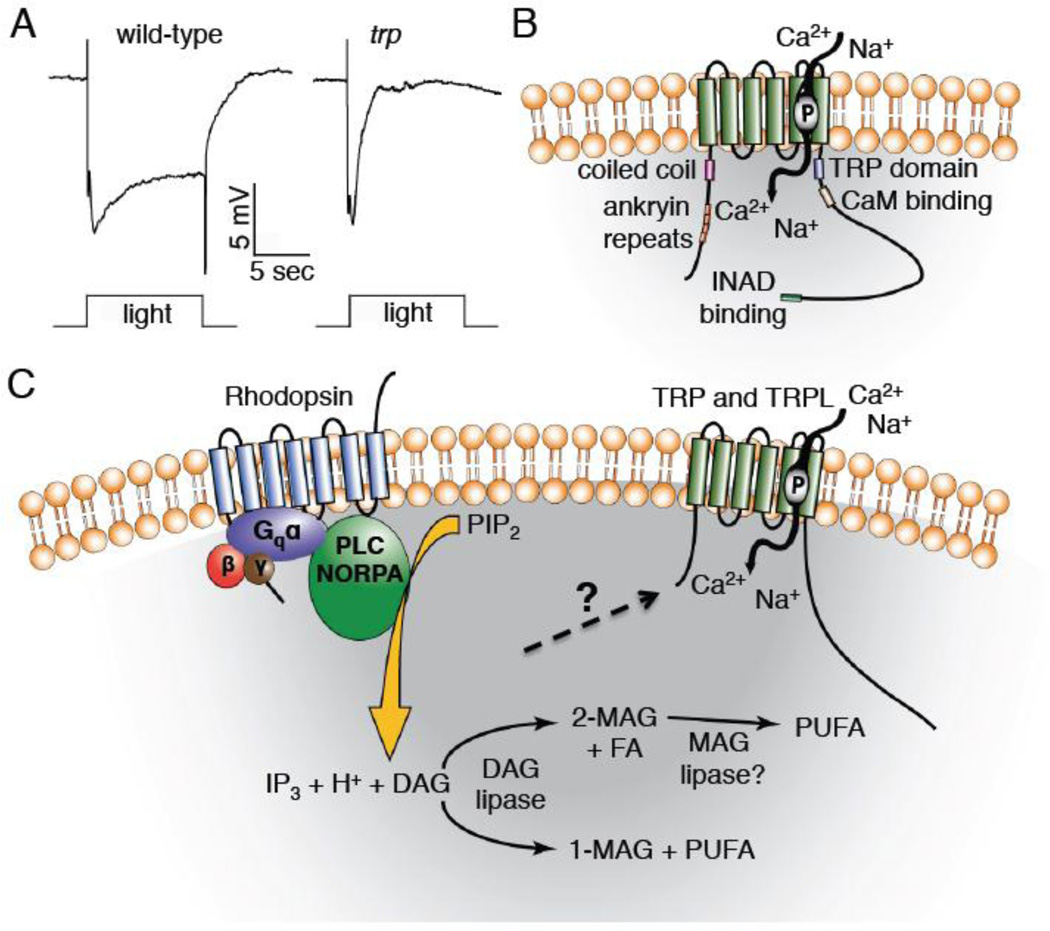
Light sensation in adult fruit flies contributes to a variety of behaviors such as the recognition of visual cues that initiate courtship, circadian-driven activity patterns and the perception of images that are important in navigation through the environment. One of the earliest Drosophila mutations affecting the behavioral response to visual cues during flight was originally referred to as the A-type mutation (Cosens and Perry, 1972). These flies are behaviorally impaired since they display only a transient rather than a sustained response to bright light (Cosens and Manning, 1969) (Fig. 2A). On the basis of this electrophysiological phenotype the mutation was subsequently re-named trp for transient receptor potential (Minke et al., 1975).
Figure 2. Predicted TRP structure and the phototransduction that leads to activation of TRP and TRPL.
A) Electroretinogram (ERG) recordings from trp+ (w1118) and trp343 mutant flies (performed by Dr. Zijing Chen). B) Predicted structure of the Drosophila TRP channel. The channel contains six transmembrane domains, with the pore loop located between the 5th and 6th transmembrane domains. The N-terminal region contains four ankyrin repeat domains and a coiled coil domain, and the C-terminal tail contains a TRP domain, calmodulin (CaM) binding site, and an INAD binding site. C) Model of Drosophila phototransduction. Light capture by rhodopsin initiates a Gq/PLC signaling cascade that culminates in activation of the TRP and TRPL channels and influx of cations. Abbreviations: 1-MAG, monoacylglycerol; 2-MAG, 2-monoacylglycerol; DAG, diacylglycerol; FA, saturated fatty acid; IP3, inositol 1,4,5-trisphosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; MAG, monoacylglycerol; P, pore loop indicated in TRP; PUFA, polyunsaturated fatty acid.
For the first 20 years following the initial description of the A-type/trp mutation, there was no suggestion as to the molecular function of the protein encoded by the wild-type gene until it was cloned and characterized (Montell et al., 1985, Montell and Rubin, 1989). The predicted structural similarity to known ion channels provided the earliest indication that TRP might be a channel (Montell, 2011, Montell and Rubin, 1989) (Fig. 2B). However, if it were a channel, the type of channel was unclear since light-induced quantum bumps were still present in the mutant flies. The subsequent observation that Ca2+ influx was reduced in the mutant animals in response to light stimulation (Hardie and Minke, 1992) suggested that TRP might be a Ca2+ permeable channel required for the light response. Nevertheless, it remained possible that TRP was not a channel per se, but regulated an endogenous channel. Two lines of observations demonstrate that TRP is indeed a pore-forming subunit of a Ca2+ permeable channel. First, expression of TRP in heterologous expression systems results in a novel cation conductance, which is moderately selective for Ca2+ over Na+ (Vaca et al., 1994, Xu et al., 1997). Second, mutation of the pore-loop in TRP reduced the Ca2+ selectivity in vivo (Liu et al., 2007a).
A non-selective cation channel related to TRP, referred to as TRP-Like (TRPL), also participates in phototransduction and is responsible for the remaining light response in the trp mutant (Hardie et al., 1997, Hu et al., 1994, Niemeyer et al., 1996, Phillips et al., 1992, Xu et al., 1997). Loss of TRPL causes a mild electrophysiological phenotype (Leung et al., 2000). However, trpl;trp double mutants are blind (Niemeyer et al., 1996). The transient light response in trp mutant photoreceptor cells arises since the Ca2+ influx through the TRP channels is required for recycling of PIP2, which is necessary for sustaining a visual response (Hardie et al., 2001).
Despite the many years that have elapsed since the identification of TRP and TRPL, the activation mechanism of these channels remains controversial. Light capture by rhodopsin in the adult visual organs (the compound eye, Hofbauer-Buchner eyelet, which is located internally between the retina and the optic lobes, and ocelli; Fig. 3A and B), leads to activation of a G-protein coupled signaling cascade that employs a phospholipase C (PLC) encoded by norpA (Fig. 2C). This results in hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) in the membrane, and formation of diacylglycerol (DAG), inositol 1,4,5-trisphosphate (IP3) and a H+ (Wang et al., 2012) (Fig. 2C). This signaling cascade differs markedly from phototransduction in mammalian rods and cones, which depends on cGMP as the second messenger, and culminates with a light-dependent decline in cGMP levels and closing of cGMP-gated cation channels (Fu and Yau, 2007). However, ~1% of mammalian retinal ganglion cells are intrinsically photosensitive (ipRGCs), and sense light through a TRP-dependent cascade that bears great similarity to Drosophila phototransduction (Perez-Leighton et al., 2011, Provencio et al., 2000, Sekaran et al., 2007, Warren et al., 2006, Xue et al., 2011). These ipRGCs are primarily important in irradiance detection rather than image formation, and contribute circadian rhythm (Berson et al., 2002, Schmidt et al., 2011).
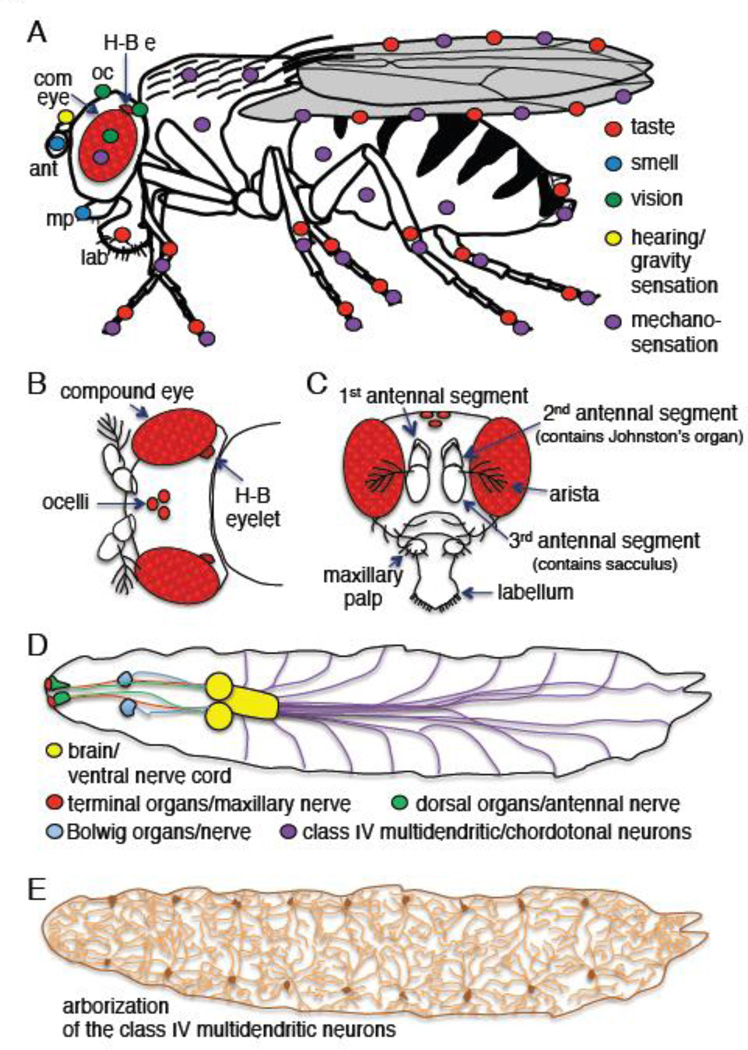
Figure 3. Drosophila sensory organs.
A) Sensory organs in an adult fruit fly. Colored circles indicate the spatial distributions of the sensory organs. Abbreviations: ant, antennae; com. eye, compound eye; H-B e, Hofbauer-Buchner (H-B) eyelet; mp, maxillary palp; lab, labellum; oc, ocelli. The H-B eyelet is located internally in the brain between the retina and lamina, but is shown superficially here and panel B. B) Top view of a fly head showing the visual organs. C) Frontal view of a fly head. D) Larval sensory organs. The cartoon is adapted from previous drawings (Gomez-Marin and Louis, 2012). E) Arborization of the class IV multidendritic neurons that tile the body wall of larvae.
Despite the unresolved question as to the link between PIP2 hydrolysis and TRP and TRPL activation, there is agreement that IP3 does not activate the channels since release of caged IP3 does not induce a light response, and loss of the IP3 receptor has no effect on phototransduction (Hardie, 1995, Raghu et al., 2000). Agonists that might gate the TRP and TRPL channels are polyunsaturated fatty acids (PUFAs), which could be generated through metabolism of DAG (Chyb et al., 1999) (Fig. 2C). In support of this concept, a mutation in inaE, which disrupts a DAG lipase, has a profound effect on phototransduction (Leung et al., 2008). PUFAs might activate the channels through direct interactions with the channels, or through modification of the membrane around TRP and TRPL.
An alternative proposal is that acidification combined with a decline in inhibitory PIP2 activates the channels (Huang et al., 2010). Evidence for this mechanism stems in part from the finding that depletion of phosphoinositides plus addition of the protonophore, 2,4-dinitrophenol to dissociated photoreceptors promotes activation of TRP and TRPL. The channels are also activated by a weak, lipophilic acid (octanoic acid) but not by non-lipophilic acids (Huang et al., 2010). There are at least two possibilities to explain this latter observation. First, non-lipophilic acids are less likely to permeate the cell membrane, and may not cause sufficient intracellular acidification. Second, lipophilic acids might not activate TRP and TRPL through acidification but rather via interaction at the channel/membrane interface. Nevertheless, a recent study concludes that TRPL expressed in HEK293 cells is activated by PUFAs rather than a reduction in PIP2 and acidification (Lev et al., 2011). Thus, no consensus has emerged concerning the link between PIP2 hydrolysis and activation of the channels.
Dual TRP-dependent mechanisms for light sensation in larvae
Light detection in Drosophila larvae also employs TRP channels. In contrast to adults, which are positively phototactic, Drosophila larvae are negatively phototactic. This makes sense, since early to mid-stage larvae burrow into the ground, and light avoidance promotes this behavior. Larvae also do not appear capable of discerning images. As such, they lack a visual system similar to the sophisticated compound eye, which in adults mediates image formation. Instead, larvae are endowed with two types of photoreceptor cells, one of which— the Bolwig organ (Fig. 3D), is in the anterior end of the larvae and is necessary for detecting low levels of light. The 12 photoreceptor cells that comprise the Bolwig organ appear to employ a Drosophila phototransduction cascade that couples rhodopsins to TRPL, but not TRP (Friedrich, 2008). The mechanisms of TRPL activation in the Bolwig organ have not been studied in detail. The photoreceptor cells in the Bolwig organ develop into the extraretinal Hofbauer-Buchner eyelet in adults (Fig. 3B), which retains expression of Rh5, Rh6, and TRPL. The H-B eyelet is situated in the brain between the retina and optic lobes, and has been implicated in modulating circadian rhythm (Helfrich-Forster et al., 2002, Szular et al., 2012). However, a function for TRPL in circadian rhythm has not been described.
Recent work demonstrates that larval detection of bright, potentially damaging levels of light does not require the Bolwig organ, but rather class IV multidendritic (mdIV) neurons, which tile the larval body wall (Fig. 3D and E). These neurons are endowed with extensively branched dendritic arbors that span the surface of the larval body. Ablation or inhibition of these polymodal nociceptors results in deficiencies in the larvae’s ability to sense and avoid bright light, noxious thermal and mechanical stimuli, as well as excessively dry environments (Johnson and Carder, 2012, Xiang et al., 2010, Zhong et al., 2012).
The behavioral avoidance of high intensity light in larvae depends on TRPA1 (Xiang et al., 2010). Surprisingly, a rhodopsin does not appear to be the light receptor that is linked to the activity of TRPA1. One candidate receptor is the gustatory receptor, GR28b, which is related to the light receptor (LITE-1) in C. elegans (Liu et al., 2010). While mutation of Gr28b reduces light-induced firing of md neurons (Xiang et al., 2010), it is not known if behavioral avoidance is impaired. In addition, the link between GR28b and TRPA1 is unclear, since there is no evidence that GR-related proteins are G-protein coupled receptors (GPCRs).
Thermosensation
Fruit flies are poikilothermic animals, and are therefore extremely sensitive to changes in environmental temperature. Moreover, their relatively simple neuronal architecture, and genetic tractability make them an attractive animal model for studying the behavioral and molecular mechanisms underlying thermosensation. The classical “thermoTRP” is mammalian TRPV1, which is activated directly by moderately hot temperatures (≥42°C) (Caterina et al., 1997). Drosophila adults and larvae also use thermoTRPs for avoiding noxious heat and cold and for discriminating small differences in temperature in the comfortable range, which is between 18–24°C. Adults prefer 24°C, while wandering larvae favor 18°C, consistent with the proclivity of these developing animals to burrow into the cool areas beneath the surface of the ground. Drosophila thermoTRPs respond to temperature through two distinct mechanisms, one of which emerged from characterizing the discrimination of small temperature changes in the comfortable range in larvae.
Thermal nociceptive escape response
Nociceptive responses are essential to allow animals to react very quickly to potentially lethal sensory assaults, such as extremely hot temperatures, noxious mechanical stimuli and dangerous chemicals. Larvae exhibit a rolling and writhing behavior referred to as a nocifensive escape locomotion response when stimulated by either noxious temperatures (>39°C) or forceful mechanical stimulation, and this behavior is dependent on mdIV neurons and the TRPA1 and Painless (Pain) channels (Hwang et al., 2012, Neely et al., 2011, Tracey et al., 2003, Zhong et al., 2012). Because Pain has a temperature activation threshold of ~39–42°C (Sokabe et al., 2008) and is expressed in mdIV neurons (Tracey et al., 2003), it appears to be a direct sensor of noxious heat.
In adults, at least three TRP channels contribute to the nociceptive responses to excessively hot temperatures, all of which belong to the TRPA subfamily. These include TRPA1, Pain and Pyrexia (Pyx) (Lee et al., 2005, Neely et al., 2011). The Pyx channel is directly activated by hot temperatures with a threshold near 40°C, and mutation of pyrexia results in faster paralysis upon exposure to 40°C (Lee et al., 2005). Thus, Pyx appears to help flies function under extreme heat conditions. TRPA1 and Pain also contribute to avoidance of noxious heat (46°C) (Neely et al., 2011).
Thermotaxis away from uncomfortably warm temperatures
In addition to the acute escape response to acutely dangerous temperature, flies and larvae move away from temperatures outside of the preferred 18 – 24°C range. In larvae, thermotactic avoidance of uncomfortably warm temperatures is impaired by RNAi knockdown of trpA1 (Rosenzweig et al., 2005), consistent with the observation that TRPA1 is activated by warm temperatures (Viswanath et al., 2003). This behavior does not appear to require Pain or the md neurons that are required for the nociceptive response to very hot temperatures (46°C) (Rosenzweig et al., 2005). A contribution of TRPA1 to warm thermotaxis was confirmed by analyses of trpA1 mutant flies (Kwon et al., 2008). In adults, TRPA1 also contributes to warm thermotaxis, and does so through functioning in anterior cell (AC) neurons in brain. (Hamada et al., 2008).
There are at least four TRPA1 isoforms (TRPA1-A-D) (Kwon et al., 2010a, Zhong et al., 2012), which display different thresholds for temperature activation and unique expression patterns [note that the TRPA1-A and TRPA1-B isoforms in one study (Kang et al., 2012) correspond to the TRPA-D and TRPA1-A isoforms referred to by other groups (Kwon et al., 2010a, Zhong et al., 2012) and in the current review]. Only TRPA1-A and TRPA1-D are thermally activated, and a 37 amino acid region unique to these isoforms, which is juxtaposed to the N-terminus of the transmembrane domains, appears to contribute to their thermosensitivity (Zhong et al., 2012). TRPA1-D is activated by temperatures starting at ~30- 34°C— too low for it to be a candidate for directly mediating responses to noxious temperatures (≥39°C). Expression of TRPA1-C in mdIV neurons of trpA1 mutant larvae rescues the impairment in thermal escape. However, this observation is perplexing because TRPA1-C is not a thermally activated channel. Thus the question arises as to the mechanism through which this isoform confers thermal sensitivity to mdIV neurons. One possibility is that Pain is the direct temperature sensor, and TRPA1-C is activated downstream, and amplifies the response.
Thermotaxis away from uncomfortably cool temperatures
In mammals, TRPM8 serves as the cold sensor (McKemy et al., 2002, Peier et al., 2002), however, there is no evidence currently that the sole member of the TRPM family in Drosophila contributes to thermosensation. Rather, fly TRPM functions in Mg2+ and Zn2+ homeostasis (Georgiev et al., 2010, Hofmann et al., 2010). A TRPV channel (Inactive; Iav) in the chordotonal neurons (Fig. 3D), as well as TRPL are required for sensing cool temperatures <17.5–18°C, which is the optimal temperature for larvae (Kwon et al., 2010b, Rosenzweig et al., 2008). Neither Iav nor TRPL appear to be activated by cool temperatures in vitro (Kwon et al., 2010b, Rosenzweig et al., 2008). Therefore, the mechanisms underlying cold sensation by TRPL and Iav are not clear.
The direct cool/cold sensor in adult flies remains elusive. However, three related proteins contribute to thermotaxis away from temperature such as 11–19°C in favor of 25°C (Gallio et al., 2011). These proteins, called Brivido1-3 (Brv1-3) share sequence homology with mammalian PKD1 proteins (Gallio et al., 2011), which bind to and may regulate the activity of TRPP2 proteins (also called PKD2s) (Consortium, 1995, Tsiokas, 2009). The Brv proteins contain ten transmembrane domains rather than six. While the last six have homology to TRPP2s, there is currently no evidence that they or mammalian PKD1s are channels, and are therefore not included in the TRP dendrogram (Fig. 1). Consistent with previous evidence that the antenna contribute to cold sensation (Altner and Loftus, 1985, Sayeed and Benzer, 1996), it appears that the brv genes are expressed and function in two parts of the antenna— the sacculus in the 3rd antennal segment, and the arista. In the arista, three neurons robustly respond to cold stimuli, while three other neurons respond specifically to hot stimuli (Gallio et al., 2011). Activation of these latter cells by heat is independent of TRPA1, suggesting the presence of an unknown heat sensor that functions in the antennae in addition to TRPA1, which functions as an internal thermosensor in anterior cell (AC) neurons in the brain (Hamada et al., 2008).
Thermotaxis in the comfortable range through a thermosensory signaling cascade
Animals, including Drosophila adults and larvae, are capable of discerning very small differences in temperature in the comfortable range (18 – 24°C), and seek out their ideal temperature. Surprisingly, TRPA1 is also required in wandering larvae for choosing 18°C over slightly higher temperatures such as 19° – 24°C (Kwon et al., 2008) (Fig. 4A). This finding was initially confusing, since temperatures in the comfortable range fall below the threshold for direct thermal activation of TRPA1 (Viswanath et al., 2003). A resolution of this conundrum is that TRPA1 is both directly and indirectly activated by changes in temperature. In the comfortable range, temperature discrimination depends on a signaling cascade that includes the same PLC (NORPA) and Gq that function in phototransduction (Kwon et al., 2008). These findings implicate a GPCR as the intrinsic thermosensor. Unexpectedly, the GPCR essential for thermotaxis in the comfortable range is the major rhodopsin (Rh1) required for light reception (Shen et al., 2011). This role for rhodopsin is light-independent, as the thermotaxis assays are performed in the dark.
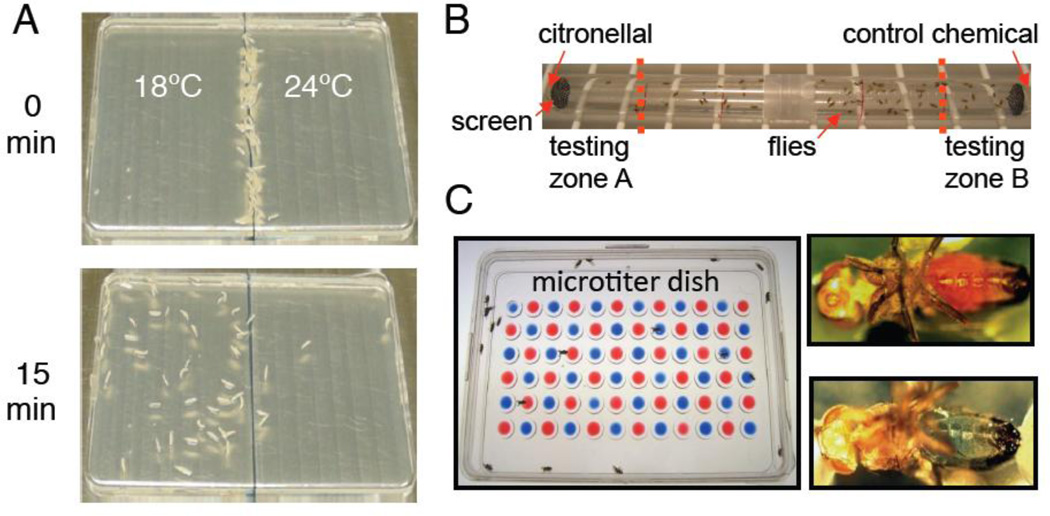
Figure 4. Simple two-way choice behavioral assays for testing temperature, olfactory and gustatory discrimination.
A) Larval temperature two-way choice assay. Larvae are placed along the midline of an agarose-covered plate, which is placed on two aluminum plates kept at different temperatures by circulating water (e.g. 18°C and 24°C). The number of larvae in each temperature zone is scored after 15 minutes. The preference index is calculated using the formula: PI=(N18°C – N24°C)/(N18°C + N24°C). B) Direct airborne repellent test (DART). Two 15 ml polystyrene tubes are prepared by placing 5 µl of odorant (citronellal) or the vehicle onto small pieces of Kimwipe at the bottom of each tube. Mesh screens are positioned to prevent the flies from coming in direct contact with the odorant or the vehicle. Approximately 100 flies are tapped into the tubes and the tubes are taped together along the midline. The number of flies in test zones A and B are counted after 30 minutes and the preference index is calculated using the formula: PI=(NZone B – NZone A)/(NZone B + NZone A) C) Two-way taste choice assay. Flies are starved overnight and placed into a microtiter dish containing two tastants (e.g. sugar versus sugar mixed with a bitter tastant) mixed with either red or blue food dye. Flies are allowed to feed for 2 hours before their abdomens are visually inspected for red, blue, or purple color. The preference index is calculated using the formula: PI= (NBlue + 0.5NPurple)/(NRed + NBlue + NPurple).
The question arises as to the function of a thermosensory signaling cascade, given that thermoTRPs including TRPA1 are capable of being activated directly by changes in temperature. There are at least two possible benefits of the indirect mechanism. First, by analogy to phototransduction, the thermosensory signaling cascade might allow for amplification of small temperatures differences in the comfortable range, and for adaptation to acceptable temperatures, such as 20° and 22°C, if 18°C is not available in the thermal landscape. Thus, the direct mechanism for activation of TRP channels appears to promote survival, since adaptation to noxious conditions might lead to lethality. The indirect mechanism could be considered a mechanism for promoting quality of life—finding the perfect temperature. However, if this is not possible to achieve, it may be okay to adapt.
The mechanism through which Rh1 contributes to thermosensation remains unknown. Rhodopsins are very thermally stable, although they have some intrinsic thermal activity (Baylor et al., 1980, Hardie et al., 2002, Shen et al., 2011). One possibility is that an accessory factor amplifies the thermal sensitivity of Rh1 in larvae. Alternatively, since there is evidence that GPCRs, including rhodopsins are dimers (Maeda et al., 2006), it is possible that a second GPCR subunit binds to Rh1 and contributes to thermal sensitivity.
Mechanosensation
Mechanosensation allows animals to respond to soft touch, noxious touch, sound, and gravity. Sensation of these mechanical stimuli may be carried out by channels that are gated either directly by changes in force, such as Piezo proteins (Coste et al., 2012), or indirectly through signaling cascades that are initiated by GPCRs (Storch et al., 2012).
Mild and noxious touch
The sense of soft touch in adult flies requires receptor neurons in hair-like bristles (mechanosensory sensilla) on the legs, wings, and halteres, which respond to small deformations in the cuticle that occur during flight or movement, and can sense debris and initiate grooming behavior. The TRPN channel, NOMPC (No Mechanoreceptor Potential C), is localized to the tips of the ciliated mechanosensory bristles (Walker et al., 2000), and loss of this channel causes a defect in the electrophysiological response to mechanical stimulation in these sensilla in adults (Walker et al., 2000)
The NOMPC protein includes a tandem array of 29 ankyrin repeats in the N-terminal segment (Walker et al., 2000), which have been proposed to comprise a mechanosensory gating spring (Howard and Bechstedt, 2004). While Drosophila NOMPC has not been shown to be an ion channel, the C. elegans NOMPC is activated by mechanical stimuli in a microsecond timeframe and is therefore likely to be a mechanotransduction channel (Kang et al., 2010b).
In contrast to adults, larvae lack bristle organs for mechanosensation, and instead, sense soft and noxious touch through the mdIV neurons located along the body wall (Fig. 3D and E). Larvae also use light mechanical feedback provided by friction exerted on the body wall to avoid excessively dry environments (Johnson and Carder, 2012). The friction generated during larval pupation as they exit the moist food environment, requires Pain and an unrelated channel that is a member of the degenerin/epithelial family of Na+ channels (Pickpocket) (Johnson and Carder, 2012). Dysregulation of the activity of these channels impairs the ability of larvae to determine proper pupation height.
The TRPA channels, Pain and TRPA1, which function in sensing noxious heat, are also required in mdIV neurons for the escape response to strong mechanical stimuli (Tracey et al., 2003, Zhong et al., 2012). How mdIV neurons contribute to both the nociceptive escape response and light friction is not clear. However, examination of mdIV-mediated behaviors that require TRPA1 or Pain reveals a pattern. Mild avoidance behaviors such as to bright light or dry environments require either TRPA1 or Pain, but not both. More severe behavioral responses such as the nocifensive escape responses to noxious thermal and mechanical stimulation require the activation of both channels, suggesting the possible model that mdIV-mediated behavioral responses are gated by the stimulus intensity and resulting magnitude of neuronal activation. According to this model, a mild stimulus is sufficient to activate a subset of ion channels, while a strong stimulus activates a larger group of ion channels, causing more rapid and/or greater depolarization and increased neuronal firing, resulting in a more severe behavioral response.
Proprioception
Proprioception refers to awareness of the body parts and their relative positions in space, and is essential for coordinated movements. In adult flies, proprioceptive neurons are distributed in joints of appendages, such in the legs, wings and the balancing organ, the halteres (Cheng et al., 2010). In larvae, proprioception appears to require multidendritic neurons referred to as bipolar dendrite (bd) neurons and class 1 dendritic arborization (da) neurons. NOMPC is expressed in the femoral chordotonal neurons and in bd and type 1 da neurons; and loss of NOMPC impairs locomotion and proprioception in both larvae and adults (Cheng et al., 2010).
Gravity and sound sensation
The gravitational field induces negative geotaxis behavior in adults, while auditory stimulation contributes to courtship and the detection of other organisms and environmental stimuli. Fruit flies respond to gravity and auditory stimuli through distinct neurons housed in the same organ in the 2nd antennal segment—the so-called Johnston’s organ (Fig. 3C) (Göpfert et al., 2006, Sun et al., 2009). This organ includes a greater number of mechanosensory neurons than any other tissue in the fly. The use of separate gravity and sound sensing neurons with different projection patterns into the brain bears similarities to the mammalian vestibular and auditory systems (Göpfert et al., 2006). Fruit flies hear through sound-induced vibrations of the 3rd antennal segment, which in turn stimulates chordotonal neurons located in the Johnston’s organ.
At least five TRP channels participate in gravity and/or sound sensation. These include the two TRPV channels, Nanchung (Nan) and Iav, which are expressed in both types of sensory neurons in the Johnston’s organ and are required for sensing both gravity and sound (Gong et al., 2004, Kim et al., 2003, Sun et al., 2009). Two TRPA channels, Pain and Pyx, participate in just gravitaxis (Sun et al., 2009). However, unlike the other four TRP channels which are expressed in neurons in the Johnston’s organ, Pyx is expressed in non-neuronal cap cells that link the chordotonal neurons with the mobile joint spanning the 2nd and 3rd antennal segments (Sun et al., 2009). One TRP channel, NOMPC, functions in the auditory response only, and has been proposed to be the elusive auditory transduction channel (Effertz et al., 2011, Sun et al., 2009).
Hygrosensation
The ability of flies to monitor humidity levels in their environment is critical for their survival as it not only prevents dehydration, but also allows them to detect moist environments for egg laying. In flies, the hygrosensory receptors are located in bristle organs in the distal antennae; and dry and moist air are detected via different sensilla that express unique channels. The TRPV channel, Nan, is required for sensing moist air, while the TRPA channel, Waterwitch (Wtrw), is necessary to detect dry air (Liu et al., 2007b). However, the mechanism by which hygrosensory cells are activated by moisture levels in the environment is unknown. Two possibilities are that the cells act as chemosensors or mechanosensors. The latter possibility seems the most plausible since Nan and Wtrw-expressing neurons project to mechanosensitive centers in the brain (Liu et al., 2007b).
Chemosensation
The chemosensory modalities, taste and smell, are critical for multiple animal behaviors, ranging from courtship and mating to stimulating aggressive behavior, evaluating sites for egg-laying, for discriminating safe from noxious foods and for detecting toxic odors. While some insects are pollination vectors, in most cases insects are deleterious for plants. Consequently, plants produce both volatile and non-volatile repellent compounds, which are detected through the senses of smell and taste, to ward away insect pests.
Smelling Repellents
One of the most commonly used botanically derived insect repellents is citronellal, and as with other odorants, it is detected through olfactory receptor neurons (ORNs) housed in olfactory sensilla, which are distributed on the 3rd antennal segment and maxillary palp (Fig. 3A and C). Behavioral avoidance to volatile repellents can be assayed using a simple two-way choice test (Fig. 4B), and loss of TRPA1 impairs the aversion to citronellal (Kwon et al., 2010a). Drosophila TRPA1 is activated directly only by very high concentrations of citronellal in vitro (Kwon et al., 2010a), suggesting that there is a signaling cascade that couples citronellal exposure to activation of TRPA1. It turns out that the same Gq and PLC (NORPA) that are required for phototransduction, and for activation of TRPA1 in response to small differences in temperature in the comfortable range (18 – 24 °C), also function in concert with TRPA1 in ORNs for citronellal avoidance (Kwon et al., 2010a). Thus, high concentrations of citronellal may activate TRPA1 directly, while the Gq/PLC/TRPA1 signaling cascade may serve to amply the signal initiated by low concentrations of citronellal.
Surprisingly, while loss of trpA1 abolishes the avoidance response to citronellal, citronellal-evoked action potentials are increased (Kwon et al., 2010a). Rather, the action potentials are eliminated by mutation of a protein that is a co-receptor (ORCO; formerly OR83b) for another type of cation channel encoded by odorant receptors (ORs) (Sato et al., 2008, Smart et al., 2008, Wicher et al., 2008). ORCO and gustatory receptors (GRs) also participate in avoidance of the manmade repellent, DEET (Ditzen et al., 2008, Lee et al., 2010, Syed and Leal, 2008). In the case of citronellal, there are two pathways required for avoidance behavior. It is possible that the amplified response to citronellal following loss of TRPA1 results in rapid depletion of ready releasable pools of neurotransmitters, effectively dampening the overall response to citronellal.
Taste
In contrast to taste receptor cells in humans, which are limited to the tongue, the fly gustatory receptor neurons (GRNs) are contained in sensilla distributed on multiple body parts (Vosshall and Stocker, 2007). These include the labellum situated at the tip of the proboscis, which is the closest fly equivalent to the mammalian tongue (Fig. 3A and C). In addition, sensilla are located on the wing margins, legs, and the ovipositor of females (Fig. 3A) (Singh, 1997, Stocker, 1994). The GRNs in the proboscis project to the suboesophageal ganglion in the fly brain and drive attraction or repulsion behaviors (Thorne et al., 2004, Wang et al., 2004). In flies, the largest class of taste receptors are GRs, which are distantly related to fly ORs (Clyne et al., 2000, Hallem et al., 2006, Montell, 2009, Robertson et al., 2003, Scott et al., 2001). However, GRs are unrelated to mammalian taste receptors (TRs) (Chandrashekar et al., 2006, Hoon et al., 1999). Mammalian TRs are GPCRs, and couple to a Gq/PLC signaling cascade that culminates with activation of TRPM5 (Pérez et al., 2002, Zhang et al., 2003).
Even though GRs represent the major class of taste receptors, flies detect some aversive tastants through a signaling pathway that is quite similar to the mammalian taste transduction pathway. Plants produce an array of non-volatile compounds to repel insect pests, such as aristolochic acid, and the repulsion to feeding on this and other tastants can be assayed using a simple two-way choice test (Fig. 4C). Aristolochic acid activates TRPA1 in GRNs in the proboscis through a PLC-dependent signaling pathway (Kim et al., 2010). TRPA1 is also expressed in GRNs in mouthparts, and can be activated directly by small irritant chemicals such as allyl isothiocyanate (AITC), which is the pungent component in wasabi (Kang et al., 2010a). Pain is also expressed in GRNs and is required for avoidance to AITC (Al-Anzi et al., 2006).
TRP channels and disease
Flies as an animal model for human “TRPopathies”
The genetic tractability of Drosophila makes this organism an appealing animal model for studying human diseases such as autosomal dominant polycystic kidney disease (ADPKD) and mucolipidiosis type IV (MLIV), which result from mutations in TRP channels (Venkatachalam and Montell, 2007). ADPKD is characterized by renal cysts and kidney failure, and occurs as a result of mutations in TRPP2 (PKD2) (Mochizuki et al., 1996) and a large interacting protein with 11 transmembrane domains (PKD1) (Consortium, 1995). TRPP2 appears to localize to primary cilia in renal epithelial cells where it has been proposed to function as a mechanosensitive channel to detect fluid flow (Nauli et al., 2003). The Drosophila TRPP homologue, Almost there (Amo), localizes to the flagellated sperm tail where it is required for sperm storage, indicating evolutionary conservation of TRPP channel function in cilia (Gao et al., 2003, Köttgen et al., 2011, Watnick et al., 2003).
MLIV is a severe childhood neurodegeneration disorder (Wakabayashi et al., 2011) caused by loss-of-function mutations that disrupt human TRPML1 (TRP Mucolipin1; MCOLN1) (Bargal et al., 2000, Bassi et al., 2000, Sun et al., 2000). Unlike most TRP channels that function in the plasma membrane, TRPML1 is primarily localized to late endosomes and lysosomes (Kiselyov et al., 2005, Manzoni et al., 2004, Venkatachalam et al., 2006). The disorder is characterized by severe visual impairment, motor problems and mental retardation, and there is no effective treatment (Wakabayashi et al., 2011). Flies lacking the Drosophila homolog, TRPML, display a phenotype reminiscent of the human disease, including neurodegeneration and motor deficits, and an accumulation of lysosome vesicles (Venkatachalam et al., 2008). The neurodegeneration results from impairment of autophagic removal of damaged mitochondria (Venkatachalam et al., 2008), due to a defect in release of Ca2+ from late endosomes and a diminished fusion of late-endosomes/amphisomes and lysosomes (Wong et al., 2012). Loss of trpml, and the consequent decline in the completion of autophagy reduces autophagic production of amino acids, thereby causing a decrease in activity of the serine/threonine kinase, TORC1 (Wong et al., 2012). As a result, there is diminished pupal survival, and this phenotype is partially suppressed by feeding the mutant larvae an amino acid-rich diet (Wong et al., 2012). This latter finding raises the possibility that an amino-acid rich diet might decrease the severity of the clinical manifestations associated with MLIV.
Surprisingly, the trpml mutant phenotype is rescued not only by expression of trpml+ in neurons, but also by expression of the wild-type gene in phagocytic cells such as glia or hemocytes (Venkatachalam et al., 2008). The basis for this rescue is that trpml function is required in both neurons and phagocytic cells. In the absence of trpml in phagocytic cells, the early-apoptotic neurons are not removed quickly. As a result, there is an accumulation of lateapoptotic cells and release of cytotoxic agents that promote the death of neighboring neurons through a bystander effect (Venkatachalam et al., 2008). The finding that trpml expression in glia suppresses the trpml mutant phenotype in flies raises another therapeutic concept. The microglia in the human brain are bone-marrow derived, and can cross the blood-brain barrier following bone marrow transplantation. This suggests that bone marrow transplantation might provide a potential therapy for MLIV.
TRPs and the control of insect pests
The identification of Drosophila TRP channels as sensors for insect repellents opens the door to exploiting this finding to develop new repellents to control insect-borne disease. While Drosophila TRPA1 is most effectively activated by citronellal through an indirect mode, via a Gq/PLC signaling cascade, TRPA1 from the mosquito vector for malaria, Anopheles gambiae, is potently and directly activated by citronellal (Kwon et al., 2010a). The most effective repellent currently on the market is DEET, which was developed in the 1950s, and has limited potency and duration (Katz et al., 2008). Thus, high-throughput screens for activators of Anopheles TRPA1 offer the potential to identify new classes of repellents to combat insectborne disease.
Acknowledgments
We thank Dr. Z. Chen for performing the ERGs shown in Fig. 2A. Work in C.M.’s laboratory on sensory signaling is supported by the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Anzi B, Tracey WD, Jr, Benzer S. Response of Drosophila to wasabi is mediated by painless the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Altner H, Loftus R. Ultrastructure and function of insect thermo- and hygroreceptors. Annu Rev Entomol. 1985;30:273–295. [Google Scholar]
- Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, et al. Identification of the gene causing mucolipidosis type IV. Nat Genet. 2000;26:118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G. Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet. 2000;67:1110–1120. doi: 10.1016/s0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila lightsensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- Consortium TIPKD. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- Cosens D, Perry MM. The fine structure of the eye of a visual mutant, A-type, of Drosophila melanogaster. J Insect Physiol. 1972;18:1773–1786. doi: 10.1016/0022-1910(72)90109-6. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319:1838–1842. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- Effertz T, Wiek R, Gopfert MC. NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol. 2011;21:592–597. doi: 10.1016/j.cub.2011.02.048. [DOI] [PubMed] [Google Scholar]
- Friedrich M. Opsins and cell fate in the Drosophila Bolwig organ: tricky lessons in homology inference. Bioessays. 2008;30:980–993. doi: 10.1002/bies.20803. [DOI] [PubMed] [Google Scholar]
- Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144:614–624. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ruden DM, Lu X. PKD2 cation channel is required for directional sperm movement and male fertility. Curr Biol. 2003;13:2175–2178. doi: 10.1016/j.cub.2003.11.053. [DOI] [PubMed] [Google Scholar]
- Georgiev P, Okkenhaug H, Drews A, Wright D, Lambert S, Flick M, et al. TRPM channels mediate zinc homeostasis and cellular growth during Drosophila larval development. Cell Metab. 2010;12:386–397. doi: 10.1016/j.cmet.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Gomez-Marin A, Louis M. Active sensation during orientation behavior in the Drosophila larva: more sense than luck. Curr Opin Neurobiol. 2012;22:208–215. doi: 10.1016/j.conb.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, et al. Two interdependent TRPV channel subunits, Inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Albert JT, Nadrowski B, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. 2006 doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Dahanukar A, Carlson JR. Insect odor and taste receptors. Annu Rev Entomol. 2006;51:113–135. doi: 10.1146/annurev.ento.51.051705.113646. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC. Photolysis of caged Ca2+ facilitates and inactivates but does not directly excite light-sensitive channels in Drosophila photoreceptors. J Neurosci. 1995;15:889–902. doi: 10.1523/JNEUROSCI.15-01-00889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Cochrane GW, Juusola M, Georgiev P, Raghu P. Molecular basis of amplification in Drosophila phototransduction: roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron. 2002;36:689–701. doi: 10.1016/s0896-6273(02)01048-6. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P, Moore S, Juusola M, Baines A, Sweeney ST. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron. 2001;30:149–159. doi: 10.1016/s0896-6273(01)00269-0. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Reuss H, Lansdell SJ, Millar NS. Functional equivalence of native light-sensitive channels in the Drosophila trp301 mutant and TRPL cation channels expressed in a stably transfected Drosophila cell line. Cell Calcium. 1997;21:431–440. doi: 10.1016/s0143-4160(97)90054-3. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, et al. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci. 2002;22:9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Chen X, Dietz AS, Gudermann T, Montell C. Drosophila TRPM channel is essential for the control of extracellular magnesium levels. PLoS ONE. 2010;5:e10519. doi: 10.1371/journal.pone.0010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Howard J, Bechstedt S. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol. 2004;14:R224–R226. doi: 10.1016/j.cub.2004.02.050. [DOI] [PubMed] [Google Scholar]
- Hu Y, Vaca L, Zhu X, Birnbaumer L, Kunze DL, Schilling WP. Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient potential-like (trpl) protein of Drosophila. Biochem Biophys Res Commun. 1994;201:1050–1056. doi: 10.1006/bbrc.1994.1808. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol. 2010;20:189–197. doi: 10.1016/j.cub.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Hwang RY, Stearns NA, Tracey WD. The ankyrin repeat domain of the TRPA protein Painless is important for thermal nociception but not mechanical nociception. PLoS ONE. 2012;7:e30090. doi: 10.1371/journal.pone.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WA, Carder JW. Drosophila Nociceptors Mediate Larval Aversion to Dry Surface Environments Utilizing Both the Painless TRP Channel and the DEG/ENaC Subunit, PPK1. PLoS ONE. 2012;7:e32878. doi: 10.1371/journal.pone.0032878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, Jenkins AM, et al. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010a;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010b;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz TM, Miller JH, Hebert AA. Insect repellents: historical perspectives and new developments. J Am Acad Dermatol. 2008;58:865–871. doi: 10.1016/j.jaad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci USA. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynikov N, et al. TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage. J Biol Chem. 2005;280:43218–43223. doi: 10.1074/jbc.M508210200. [DOI] [PubMed] [Google Scholar]
- Köttgen M, Hofherr A, Li W, Chu K, Cook S, Montell C, et al. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS ONE. 2011;6:e20031. doi: 10.1371/journal.pone.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, et al. Drosophila TRPA1 channel Is required to avoid the naturally occurring insect repellent citronellal. Curr Biol. 2010a;20:1672–1678. doi: 10.1016/j.cub.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shen WL, Shim HS, Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J Neurosci. 2010b;30:10465–10471. doi: 10.1523/JNEUROSCI.1631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim SH, Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong ST, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- Leung HT, Geng C, Pak WL. Phenotypes of trpl mutants and interactions between the transient receptor potential (TRP) and TRP-like channels in Drosophila. J Neurosci. 2000;20:6797–6803. doi: 10.1523/JNEUROSCI.20-18-06797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, et al. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron. 2008;58:884–896. doi: 10.1016/j.neuron.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Katz B, Tzarfaty V, Minke B. Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: Implications on gating of Drosophila TRPL (Transient Receptor Potential-Like) channel. J Biol Chem. 2011;287:1436–1447. doi: 10.1074/jbc.M111.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Wang T, Postma M, Obukhov AG, Montell C, Hardie RC. In vivo Identification and manipulation of the Ca2+ selectivity filter in the Drosophila Transient Receptor Potential channel. J Neurosci. 2007a;27:604–615. doi: 10.1523/JNEUROSCI.4099-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ward A, Gao J, Dong Y, Nishio N, Inada H, et al. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat Neurosci. 2010;13:715–722. doi: 10.1038/nn.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007b;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Imanishi Y, Sun W, Jastrzebska B, Hatala DA, et al. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni M, Monti E, Bresciani R, Bozzato A, Barlati S, Bassi MT, et al. Overexpression of wild-type and mutant mucolipin proteins in mammalian cells: effects on the late endocytic compartment organization. FEBS Lett. 2004;567:219–224. doi: 10.1016/j.febslet.2004.04.080. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Nenhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Minke B, Wu C-F, Pak WL. Isolation of light-induced responses of the central retina cells from the electroretinogram of Drosophila. J Comp Physiol. 1975;98:361–385. [Google Scholar]
- Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, et al. PKD2 a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. The history of TRP channels, a commentary and reflection. Pflugers Arch. 2011;461:499–506. doi: 10.1007/s00424-010-0920-3. [DOI] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Neely GG, Keene AC, Duchek P, Chang EC, Wang QP, Aksoy YA, et al. TrpA1 Regulates Thermal Nociception in Drosophila. PLoS ONE. 2011;6:e24343. doi: 10.1371/journal.pone.0024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Peier A, Moqrich A, Hergarden A, Reeve A, Andersson D, Story G, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Perez-Leighton CE, Schmidt TM, Abramowitz J, Birnbaumer L, Kofuji P. Intrinsic phototransduction persists in melanopsin-expressing ganglion cells lacking diacylglycerol-sensitive TRPC subunits. Eur J Neurosci. 2011;33:856–867. doi: 10.1111/j.1460-9568.2010.07583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P, Colley NJ, Webel R, James T, Hasan G, Danin M, et al. Normal phototransduction in Drosophila photoreceptors lacking an InsP3 receptor gene. Mol Cell Neurosci. 2000;15:429–445. doi: 10.1006/mcne.2000.0846. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig M, Brenman KM, Taylor TD, Phelps P, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2008;105:14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci USA. 1996;93:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, et al. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Sekaran S, Lall GS, Ralphs KL, Wolstenholme AJ, Lucas RJ, Foster RG, et al. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J Neurosci. 2007;27:3981–3986. doi: 10.1523/JNEUROSCI.4716-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- Singh RN. Neurobiology of the gustatory systems of Drosophila and some terrestrial insects. Microsc Res Tech. 1997;39:547–563. doi: 10.1002/(SICI)1097-0029(19971215)39:6<547::AID-JEMT7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Smart R, Kiely A, Beale M, Vargas E, Carraher C, Kralicek AV, et al. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Mol Biol. 2008;38:770–780. doi: 10.1016/j.ibmb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila Painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Storch U, Mederos y Schnitzler M, Gudermann T. G protein-mediated stretch reception. Am J Physiol Heart Circ Physiol. 2012;302:H1241–H1249. doi: 10.1152/ajpheart.00818.2011. [DOI] [PubMed] [Google Scholar]
- Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu L, Ben-Shahar Y, Jacobs JS, Eberl DF, Welsh MJ. TRPA channels distinguish gravity sensing from hearing in Johnston's organ. Proc Natl Acad Sci USA. 2009;106:13606–13611. doi: 10.1073/pnas.0906377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci USA. 2008;105:13598–13603. doi: 10.1073/pnas.0805312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szular J, Sehadova H, Gentile C, Szabo G, Chou WH, Britt SG, et al. Rhodopsin 5- and Rhodopsin 6-mediated clock synchronization in Drosophila melanogaster is independent of retinal phospholipase C-beta signaling. J Biol Rhythms. 2012;27:25–36. doi: 10.1177/0748730411431673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Wilson RI, Laurent G, Benzer S. painless a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Tsiokas L. Function and regulation of TRPP2 at the plasma membrane. Am J Physiol Renal Physiol. 2009;297:F1–F9. doi: 10.1152/ajprenal.90277.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol. 1994;266:C1501–C1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Hofmann T, Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J Biol Chem. 2006;281:17517–17527. doi: 10.1074/jbc.M600807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Long A, Elsaesser R, Nikolaeva D, Broadie K, Montell C. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135:838–851. doi: 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Gustafson AM, Sidransky E, Goldin E. Mucolipidosis type IV: An update. Mol Genet Metab. 2011;104:206–213. doi: 10.1016/j.ymgme.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang T, Ni J, Von Lintig J, Montell C. The Drosophila visual cycle and de novo chromophore synthesis depends on rdhB. J Neurosci. 2012;32:3485–3491. doi: 10.1523/JNEUROSCI.5350-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN projecting rat retinal ganglion cells. Eur J Neurosci. 2006;23:2477–2487. doi: 10.1111/j.1460-9568.2006.04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol. 2003;13:2179–2184. doi: 10.1016/j.cub.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Wong CO, Li R, Montell C, Venkatachalam K. Drosophila TRPML is required for TORC1 activation. Curr Biol. 2012 doi: 10.1016/j.cub.2012.06.055. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XZ, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, et al. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhong L, Bellemer A, Yan H, Honjo K, Robertson J, Hwang RY, et al. Thermosensory and non-thermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat sensor domains of a thermoTRP channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]