Abstract
Background
Hypoplastic left heart syndrome (HLHS) is one of the most common severe congenital cardiac anomalies, characterized by marked hypoplasia of left sided structures of the heart which is commonly accompanied by a thick layer of fibro-elastic tissue, termed endocardial fibroelastosis (EFE). Because human EFE develops only in fetal or neonatal hearts, and often in association with reduced blood flow, we sought to mimic these conditions by subjecting neonatal and 2-week-old rat hearts to variations of the heterotopically transplanted heart model with either no intracavitary or normal flow, and compare endocardium with human EFE tissue.
Methods and Methods
Hearts obtained from neonatal and 2-week-old rats were heterotopically transplanted in young adult Lewis rats in a working (loaded) or non-working (unloaded) mode. After 2 weeks survival, hearts were explanted for histological analysis by staining for collagen, elastin and cellular elements. These sections were compared to human EFE tissue from HLHS.
Results
EFE, consisting of collagen and elastin with scarce cellular and vascular components, developed only in neonatal unloaded transplanted hearts and displayed the same histopathologic findings as EFE from patients with HLHS. Loaded hearts and 2-week-old hearts did not show these alterations.
Conclusions
This animal model for EFE will serve as a tool to study the mechanisms of EFE formation, such as fluid forces, in HLHS in a systematic manner. A better understanding of the underlying cause of EFE formation in HLHS will help develop novel treatment strategies to better preserve growth of the hypoplastic left ventricle.
Keywords: endocardial fibroelastosis, hypoplastic left heart syndrome, animal model
Introduction
Hypoplastic left heart syndrome (HLHS) accounts for 2–3% of all congenital cardiac anomalies. It carries a high early mortality and is characterized by marked hypoplasia of left sided structures of the heart at birth, including the left ventricle, mitral valve and aorta. Furthermore, the left ventricular endocardium is very commonly lined with a thick layer of cellular fibro-elastic tissue, termed endocardial fibroelastosis (EFE). Fetal echocardiographic studies indicate a high association between aortic valve stenosis and the presence of EFE in the fetal heart that eventually becomes hypoplastic (1). Indeed, the association between left ventricular growth in the fetus and the presence of EFE has been highlighted by clinical reports of fetal aortic balloon valvuloplasty where the ability of the left ventricle to grow after fetal valvotomy is highly related to the extent of EFE present at the time of the intervention on the valve (2). Milder form of EFE in the post-natal heart has also been implicated in a restrictive physiology and the inability of the left ventricle to keep up with somatic growth. Surgical interventions removing the thick endocardial layer in young infants has resulted in immediate improvement in the restrictive physiology as well as catch-up growth of the left ventricle (3).
While mechanical removal of the fibroelastic layer from the left ventricular endocardium may be effective, often the left ventricle is already quite hypoplastic at birth and therefore much less amenable to recruitment for use as a systemic ventricle. To elucidate the mechanisms regulating the formation and proliferation of the cells that form the thick endocardial layer seen in HLHS, we sought to develop an animal model that mimicked the conditions under which EFE forms. From clinical observations, a strong relationship has been shown between decreased left ventricular blood flow and the presence of EFE in the fetal state. However, the temporal and mechanistic relationship is not known (1). From these clinical observations we postulated that immaturity or even potentially the fetal state, since in humans we have only observed the formation of this tissue in the fetus or young infant in association with HLHS, is a necessary requirement for EFE formation. The second potentially necessary condition is lack of intracavitary blood flow since in fetal studies, one of the hallmarks of HLHS and EFE formation is when the aortic valve becomes obstructed accompanied by left ventricular distention causing cessation of blood flow through the left ventricular cavity. Therefore, we sought to develop an animal model of EFE, based on our observations of immaturity and lack of blood flow through the left ventricular cavity. To mimic lack of blood flow we used an unloaded version of heterotopic heart transplantation and comparing this to a working heart transplant model, and to mimic immaturity we used neonatal (developmentally very immature) and 2-week-old rat hearts for comparison.
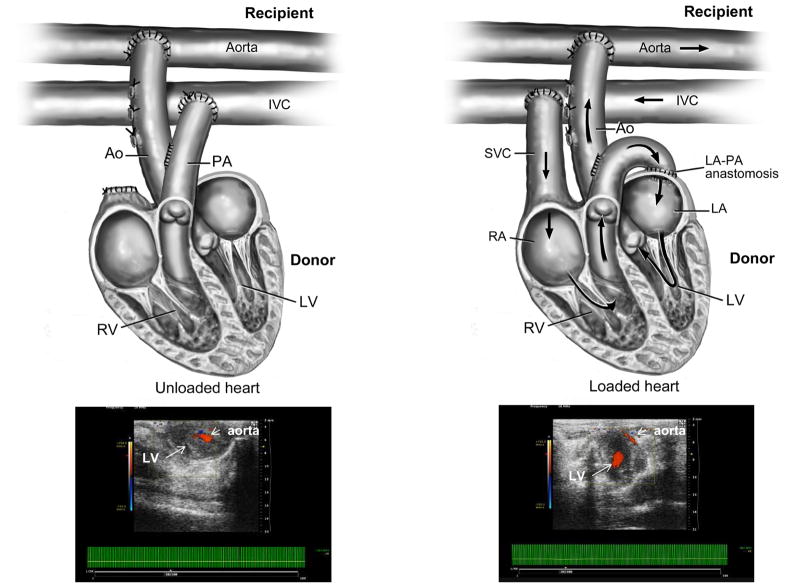
Heterotopic infrarenal transplantation of a mammalian heart has been introduced as experimental model in the 1930s (4) and has later been established for rat heart transplantation studies (5,6). The transplanted heart is histologically normal, and spontaneously beating. More importantly for our studies, two models of heterotopic heart transplantation can be generated depending on the connection of the cardiac chamber and great vessels to the recipient’s vessels (Figure 1). In a non-working, hemodynamically unloaded state, the work of the ventricle as well as intracavitary blood flow is reduced. These are the conditions that we see in human fetal hearts when the aortic valve closes in HLHS. When the transplanted heart is connected to the recipient’s blood vessels such that venous return can fill the left ventricular cavity, then a working heart model (hemodynamically loaded) is generated (7). By combining these two heterotropic rat transplant models, loaded and unloaded, with age differences in the donor heart, we sought to determine the impact of hemodynamic forces and age on the development of EFE.
Figure 1.
The surgical procedure of a non-working/unloaded and a working/loaded heterotopic heart transplant model in a rat is shown. In the lower panel echocardiographic images verify flow through the left ventricular cavity in the case of a loaded heart.
LV=left ventricle, RV=right ventricle, RA=right atrium, LA=left atrium, PA=pulmonary artery, SVC=superior vena cava, IVC=inferior vena cava
Material and Methods
Ethics Statement
All animals received humane care from the Animal Resources of Children’s Hospital Boston and the investigation conforms to the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Children’s Hospital Boston.
Animals Model of Unloaded and Loaded Heart Transplantation
Heterotopic heart transplantation was performed in Lewis rats following a method previously described (5). Young adult rats (100–120g) served as recipients and donor hearts were obtained from neonatal rats (2–4 days of age) and 2-week-old rats (n=7 per experimental groups). Animals were anesthetized with ketamine (40–60mg/kg i.p.)/xylazine (10mg/kg i.p.) and isoflurane via endotracheal tube. The donor animal received 300IU heparin and the heart was explanted through a midline thoracic incision followed by storage in cold high potassium Krebs-Henseleit solution as previously described (8). Implantation of the heart was performed as a heterotopic infra-renal graft unloaded with aorta to aorta and pulmonary artery (PA) to inferior vena cava (IVC) anastomoses. For the loaded hearts, implantation was modified by anastomosing the donor’s PA to the donor’s left atrium (LA) and connecting the donor’s SVC to the recipient’s IVC to allow flow through the ventricles of the transplanted heart (7). The anastomoses were modified in the neonatal group adjusting for small vessel size and the iliac artery and vein of the recipient were used. Analgesia was provided by buprenorphine (0.1–0.5mg/kg s.c.) immediately postoperatively and meloxicam (1mg/kg s.c.) every 24 hours for three days. Flow was visualized through echocardiographic analysis postoperatively. Animals were survived for 2 weeks and were euthanized with inhaled CO2 and exsanguination through excision of the transplanted and native hearts.
Histological Analysis of Loaded and Unloaded Transplanted Hearts
Paraffin-embedded cross-sections from the midsection of the heart were de-paraffinized, rehydrated with xylene and graded alcohol series, and stained with Hematoxylin & Eosin and van Gieson elastin stain and compared to EFE from HLHS patients.
Determination of Fibrosis by Masson’s Trichrome Staining and Fibroblast Specific Protein (FSP-1) Staining
Separate sections were stained with Masson’s Trichrome, which results in fibrotic (collagen-enriched) areas appearing blue, whereas cellular elements appear red. Slides were visualized using a Zeiss microscope with a Nikon objective and eye-piece for appropriate magnification. Histological sections were analyzed by a blinded observer. Degree of EFE was determined by a grading system with no EFE = Grade 0, islets of EFE = Grade 1, major area of fibrosis = Grade 2, full circumference of endocardium = Grade 3, and ventricular lumen almost obliterated by EFE = Grade 4. In addition, collagen-rich endocardial areas were stained with FSP-1 for confirmation of fibroblast involvement.
Statistical Analysis
Data were analyzed using SPSS software package (version 16.0, SPSS Inc., Chicago, IL) and are reported as mean ± standard error of the mean (SEM). After confirming normal distribution, a two-tailed unpaired student’s t-test or ANOVA with Bonferroni post-hoc analysis where applicable were used for comparison between groups if normality was passed. A value of p≤0.05 was considered statistically significant.
Results
Animal Model of Unloaded and Loaded Heterotopically Transplanted Hearts
Two donor heart age groups were studied neonatal (2–4 day-old) and 2-week-old hearts. Details of the surgical methods are shown in Figure 1. Transplantation was performed in ten animals per group with two deaths in the neonatal heart groups. Blood flow through the loaded ventricles was confirmed by echocardiography (Figure 1). In the unloaded hearts, perfusion was only through the coronary arteries but the loaded hearts receive preload through the ventricular cavity from the recipient’s IVC/iliac vein via the SVC of the graft to the RA. The LA subsequently filled from the right ventricle through a direct anastomosis of the PA to the LA. The graft’s left ventricle then ejects blood through the aorta into the abdominal aorta/iliac artery of the recipient. The recipients were survived for 2 weeks and then euthanized and the donor hearts harvested for analysis. Histological analysis revealed that only neonatal unloaded hearts developed fibroelastic thickening of the endocardium which consisted of collagen and elastin (Figure 2). Loaded hearts did not develop fibroelastic tissue on the endocardium and neither did grafts obtained from older donor animals. The presence of this fibroelastic tissue was dependent on young age and preload. As indicated in Figure 3, no fibroelastic tissue was found in unloaded hearts at any age.
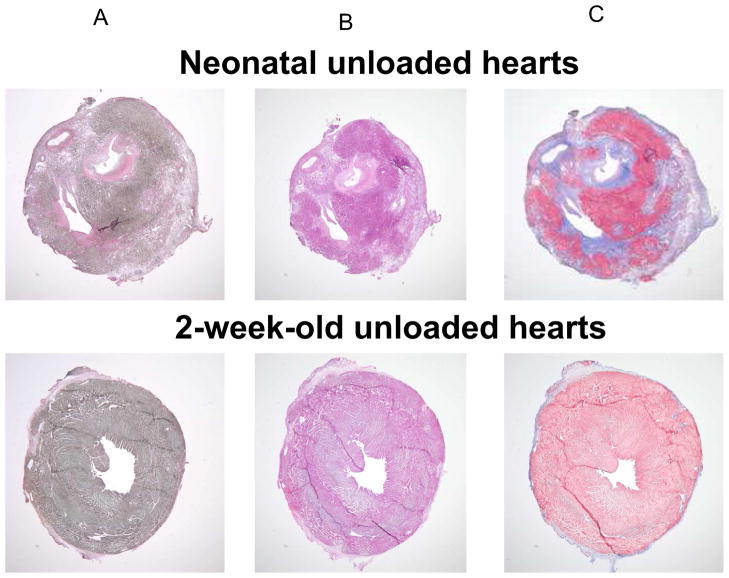
Figure 2.
Van Gieson (A), Hematoxylin & Eosin (B) and Masson’s Trichrome (C) staining of unloaded neonatal versus unloaded 2-week-old hearts following 2 weeks of heterotopic transplantation are shown. Unloaded transplantation of neonatal hearts led to the development of massive endocardial thickening by collagen and elastic fibers with scarce cellular or vascular components.
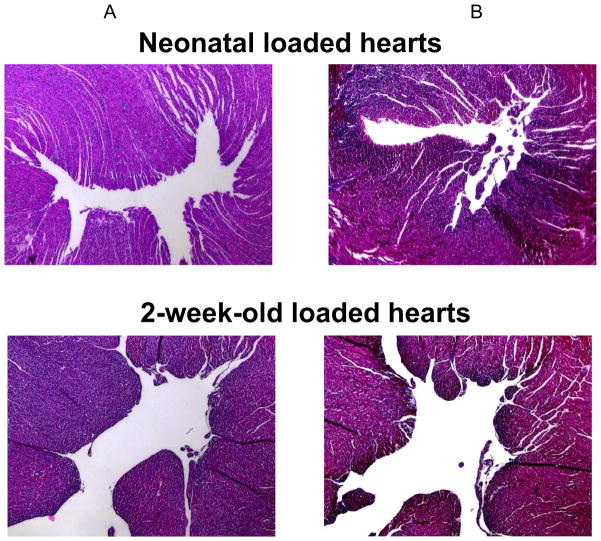
Figure 3.
Hematoxylin & Eosin (A) and Masson’s Trichrome (B) staining of loaded neonatal versus loaded 2-week-old hearts following 2 weeks of heterotopic transplantation are shown. The endocardium does not show any changes.
Histological Comparison of Human EFE and Unloaded Neonatal Rat Hearts
In HLHS, EFE has the gross appearance of whitish-gray endocardial thickening, reducing ventricular volume and leading to functional impairment of the heart. As indicated in Figure 4, the main alteration histologically is a marked increase in collagen fibers (C) and elastic fibers (A). The tissue is cellular but vasculature is scarce (B). The underlying myocardium generally appears normal but hypertrophic.
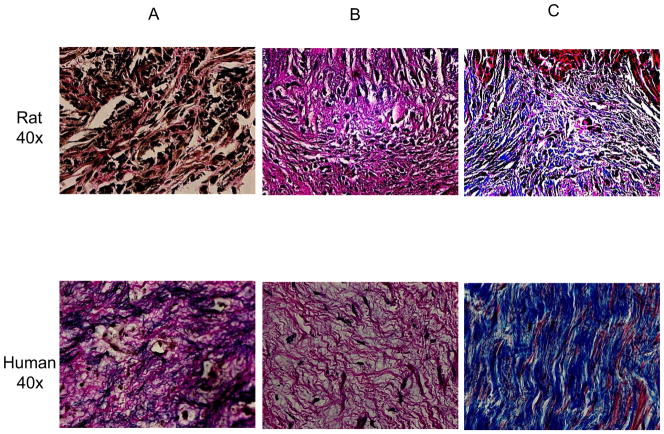
Figure 4.
EFE tissue obtained during surgery for correction of HLHS (bottom panel) was stained with van Gieson for elastic fibers (A), Hematoxylin & Eosin to determine cellularity and vascularity (B), and Masson’s Trichrome for detection of collagen (C). EFE tissue resembles the endocardial fibroelastic tissue in unloaded neonatal rat hearts (top panel).
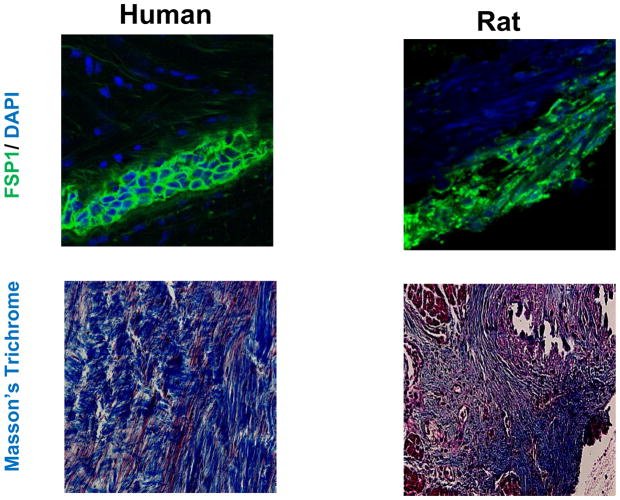
In neonatal unloaded transplanted rat hearts, fibroelastic thickening of the endocardium resembles human EFE with richness in collagen and elastin fibers but atrophy of cardiomyocyte cross-sectional area. FSP1 specific staining indicated that the predominant cell type in this fibroelastic tissue in humans and unloaded neonatal rat hearts are fibroblasts (Figure 5).
Figure 5.
These panels show confocal images of EFE tissue from a child with HLHS (left panel) and fibroelastic tissue from an unloaded neonatal rat heart (right panel), after immunofluorescence staining with an antibody against FSP1 (Fibroblast Specific Protein1, a marker for fibroblasts) shown in green and nuclear counter stain (DAPI) in blue at 63x magnification and corresponding Masson’s Trichrome stains at 40x magnification.
Determination and Grading of Fibroelastosis
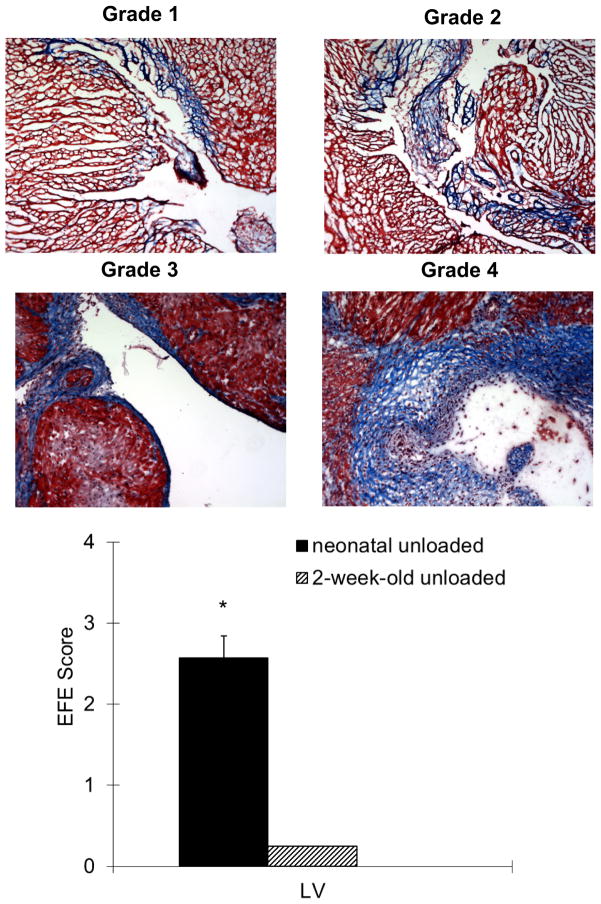
Histological examination showed thickened endocardium with fibroelastosis which appears blue in Masson’s trichrome stains. The amount of blue covering the endocardium was analyzed by establishing a grading system (see Figure 6). Two-week-old donor hearts did not show any signs of fibrosis and were therefore classified as Grade 0. One heart out of ten in the loaded neonatal group showed minimal (Grade 1) endocardial fibrosis but all hearts from the unloaded neonatal donor heart group showed EFE formation from Grade 1–4.
Figure 6.
Masson’s Trichrome identifies fibrosis through blue staining. The degree of EFE formation was determined by establishing a grading system from Grade 0 to 4. Representative slides are shown.
A summary of all groups is depicted. Two-week-old hearts did not show any signs of fibroelastosis. There was a significant difference between the unloaded neonatal hearts and all other groups with regard to degree of fibroelastosis (*p<0.001 unloaded neonatal versus 2-week-old groups).
Discussion
The main finding of our study is the presence of EFE in a flow and age-dependent manner in heterotopically transplanted rat hearts. All unloaded newborn rat hearts, lacking intracavitary flow, develop massive EFE, obliterating the lumen of the ventricles within two weeks after transplantation. In contrast, maintaining intracavitary flow/preload in neonatal transplanted hearts prevents fibroelastosis development. In transplanted hearts from older donors, these fibrotic changes do not occur neither in unloaded nor loaded grafts.
EFE occurs in combination with congenital heart disease such as left ventricular non-compaction (9), primary X-linked isolated EFE (10), HLHS with severe left ventricular outflow tract obstruction (2,3) or as a consequence of maternal lupus with anti-SSA/Ro-SSB/La antibodies, or other maternal infections (11–13), and as autosomal recessive isolated EFE (14). There are also cases of acquired EFE as a consequence of infection in postnatal life (15). Based on these observations, the etiology of EFE can be genetic, hypoxia/ischemia (16,17), infection (11–13), or lack of flow (2,3).
The endocardium consists of several layers with the innermost portion composed of endothelial cells followed toward the myocardium by a middle layer of connective tissue of collagen and elastin with smooth muscle cells, and a subendocardial layer of loose connective tissue with capillaries and nerves (18). The term EFE was first created by Weinberg et al, describing a thick white layer covering the endocardium primarily located in the ventricles (19). Today, EFE is easily identified by echocardiography, magnetic resonance imaging, at surgical intervention or pathological analysis because of a white thickened endocardial layer which is already apparent in utero in cases of HLHS.
Our main interest focuses on the inter-relationship of EFE and HLHS. Not all HLHS hearts develop EFE. No EFE is found in HLHS variants with ventricular septal defect and the equivalent disease on the right side of pulmonary atresia with intact ventricular septum. Histologically, EFE is characterized by low cellularity through myofibroblasts and scarce vasculature, but high amounts of collagen and elastic fibers. There is uncertainty about the origin of EFE tissue. Smooth muscle hyperplasia in children with cardiomyopathy or endothelial cell aberration in HLHS patients has been debated (6,18). Attempts were made to establish animal models but there is currently no model to study HLHS with EFE mimicking the human disease which restricts the study of EFE to histological evaluation with limited possibility of functional and treatment studies (20). In humans the main observation regarding EFE is its presence in developing hearts and its association with hemodynamically unloaded ventricles. The two key elements of immaturity and lack of intracavitary blood flow led us to the development of our animal model which for the first time allows for mechanistic studies of this disease. In a non-working, hemodynamically unloaded state, the work of the ventricle as well as intracavitary blood flow is reduced, cardiac myocytes suffer from rapid atrophy (5), and a thick layer of fibrotic tissue covering the endocardium develops in some hearts, resembling human EFE (6). Our animal model not only shows the development of fibroeloastic tissue obstructing the ventricular cavity but also increased ventricular stiffness, likely caused by increased extracellular matrix deposition and cellular remodeling (21). In parallel, left ventricles in HLHS suffer from diastolic stiffness due to the inelastic fibrotic properties of EFE, but also systolic impairment potentially restricting left ventricular growth (2). Since EFE has several etiologies and develops concomitantly with different congenital and acquired heart diseases, our model is universally applicable and does not restrict EFE to only genetic origin as other models do (22). In a model of monocrotaline-induced pulmonary hypertension, EFE was described in the RV in a non-human primate model which however, has never been reported in more commonly used rodent models (23,24,25,26).
In summary, our animal model showed that the two key regulators of EFE formation are immaturity, since neonatal rats resemble the fetal state of other species, and lack of intracavitary blood flow. A better understanding of the underlying cause of EFE formation in HLHS will help develop novel treatment strategies to better preserve growth of the hypoplastic left ventricle.
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institute HL-075430 (to I. Friehs) and HL-063095 (to P.J. del Nido) and a Pilot Grant from the Harvard Catalyst | The Harvard Clinical and Translational Science Center UL1RR025758–01 (to E. Zeisberg).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharland GK, Chita SK, Nuala F, Anderson RH. Left ventricular dysfunction in the fetus: relation to aortic valve anomalies and endocardial fibroelastosis. Br Heart J. 1991;66:419–424. doi: 10.1136/hrt.66.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElhinney DB, Vogel M, Benson CB, Marshall AC, Wilkins-Haug LE, Silva V, Tworetzky W. Assessment of left ventricular endocardial fibroelastosis in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome. Am J Cardiol. 2010;106:1792–7. doi: 10.1016/j.amjcard.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Emani SM, Bacha EA, McElhinney DB, Marx GR, Tworetzky W, Pigula FA, del Nido PJ. Primary left ventricular rehabilitation is effective in maintaining two-ventricle physiology in the borderline left heart. J Thorac Cardiovasc Surg. 2009;138:1276–1282. doi: 10.1016/j.jtcvs.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann FC, Priestley JT, Markowitz J, Yater WM. Transplantation of the intact mammalian heart. Arch Surg. 1933;26:219–224. [Google Scholar]
- 5.Abbott CP, Dewitt CW, Creech O., Jr The transplanted rat heart: histologic and electrocardiographic changes. Transplantation. 1965;3:432–445. doi: 10.1097/00007890-196505000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Zeisberg EM, Melnychenko I, Zhong Hu T, Friehs I, Kalluri R, del Nido P. Endocardial fibroelastosis in hypoplastic left heart syndrome is caused by aberrant endothelial to mesenchymal transition. Circulation. 2009;120:S603–S604. doi: 10.1161/CIRCRESAHA.116.305629. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asfour B, Hare JM, Kohl T, Baba HA, Kass DA, Chen K, et al. A simple new model of physiologically working heterotopic rat heart transplantation provides hemodynamic performance equivalent to that of an orthotopic heart. J Heart Lung Transplant. 1999;18:927–936. doi: 10.1016/s1053-2498(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 8.Friehs I, del Nido PJ. Increased susceptibility of hypertrophied hearts to ischemic injury. Ann Thorac Surg. 2003;75(2):S678–S684. doi: 10.1016/s0003-4975(02)04692-1. [DOI] [PubMed] [Google Scholar]
- 9.Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation. 2004;109(24):2965–2971. doi: 10.1161/01.CIR.0000132478.60674.D0. [DOI] [PubMed] [Google Scholar]
- 10.Hanukoglu A, Fried D, Somekh E. Inheritance of familial primary endocardial fibroelastosis. Clin Pediatr (Phila) 1986;25(5):272–275. doi: 10.1177/000992288602500508. [DOI] [PubMed] [Google Scholar]
- 11.Buyon JP, Clancy RM. Neonatal lupus syndromes. Curr Opin Rheumatol. 2003;15(5):535–541. doi: 10.1097/00002281-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Silingardi E, Santunione AL, Rivasi F, Gasser B, Zago S, Garagnani L. Unexpected intrauterine fetal death in parvovirus B19 fetal infection. Am J Forensic Med Pathol. 2009;30(4):394–397. doi: 10.1097/PAF.0b013e3181c17b2e. [DOI] [PubMed] [Google Scholar]
- 13.Ni J, Bowles NE, Kim YH, Demmler G, Kearney D, Bricker JT, Towbin JA. Viral infection of the myocardium in endocardial fibroelastosis. Molecular evidence for the role of mumps virus as an etiologic agent. Circulation. 1997;95(1):133–139. doi: 10.1161/01.cir.95.1.133. [DOI] [PubMed] [Google Scholar]
- 14.Rios B, Castaneda P, Simpson JW. Endocardial fibroelastosis occurring in a mother and daughter. Tex Heart Inst J. 1984;11(3):296–300. [PMC free article] [PubMed] [Google Scholar]
- 15.Factor SM. Endocardial fibroelastosis: myocardial and vascular alterations associated with viral-like nuclear particles. Am Heart J. 1978;96(6):791–801. doi: 10.1016/0002-8703(78)90012-1. [DOI] [PubMed] [Google Scholar]
- 16.Johnson FR. Anoxia as a cause of endocardial fibroelastosis in infancy. Arch Pathol. 1952;54:237–247. [PubMed] [Google Scholar]
- 17.Hutchins GNI, Bannayan GA. Development of endocardial fibroelastosis following mvocardial infarction. Arch Pathol. 1971;91:113–118. [PubMed] [Google Scholar]
- 18.Neustein HB, Lurie PR, Fugita M. Endocardial fibroelastosis found on transvascular endomyocardial biopsy in children. Arch Pathol Lab Med. 1979;103:214–219. [PubMed] [Google Scholar]
- 19.Weinberg T, Himelfarb AJ. Endocardial fibroelastosis (so-called foetal endocarditis) Bull Johns Hopkins Hosp. 1943;72:299–306. [Google Scholar]
- 20.Eghtesady P, Michelfelder E, Altaye M, Ballard E, Hirsh R, Beekman RH. Revisiting animal models of aortic stenosis in the early gestation fetus. Ann Thorac Surg. 2007;83:631–369. doi: 10.1016/j.athoracsur.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Brinks H, Tevaearai H, Muhlfeld C, Bertschi D, Gahl B, Carrel T, et al. Contractile function is preserved in unloaded hearts despite atrophic remodeling. J Thorac Cardiovasc Surg. 2009;137:742–746. doi: 10.1016/j.jtcvs.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Kamisago M, Schmitt JP, McNamar D, Seidman C, Seidman JG. Sarcomere protein gene mutations and inherited heart disease: a beta-cardiac myosin heavy chain mutation causing endocardial fibroelstosis and heart failure. Novartis Found Symp. 2006;274:176–189. [PubMed] [Google Scholar]
- 23.Chesney CF, Allen JR. Animal model: pulmonary hypertension, cor pulmonale and endocardial fibroelastosis in monocrotaline-intoxicated nonhuman primates. Am Pathol. 1973;70(3):489–492. [PMC free article] [PubMed] [Google Scholar]
- 24.De Man FS, Handoko ML, van Ballegoij JJ, Scahlij I, bogaards SJ, Postmus PE, van der Velden J, Westerhof N, Paulus WJ, Vonk-Noordegraaf A. Bisoprolol delays progression toward right heart failure in experimental pulmonary hypertension. Circ Heart Fail. 2012;5(1):97–105. doi: 10.1161/CIRCHEARTFAILURE.111.964494. [DOI] [PubMed] [Google Scholar]
- 25.Hadi AM, Mouchaers KT, Schalij I, Grunberg K, Meijer GA, Vonk-Noordegraaf A, van der Laarse WJ, Belien JA. Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol (Dordr) 2011;34(4):343–354. doi: 10.1007/s13402-011-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campian ME, Verberne HJ, Hardziyenka M, de Bruin K, Selwaness M, van den Hoff MJ, Ruijter JM, van Eck-Smit BL, de Bakker JM, Tan HL. Serial noninvasive assessment of apoptosis during right ventricular disease progression in rats. J Nucl Med. 2009;50(8):1371–1377. doi: 10.2967/jnumed.108.061366. [DOI] [PubMed] [Google Scholar]