Abstract
The benefits of estrogens on bone health are well established; how estrogens signal to regulate bone formation and resorption is less well understood. We show here that 17β-estradiol (E2)-induced apoptosis of bone-resorbing osteoclasts is mediated by cleavage and solubilization of osteoblast-expressed Fas ligand (FasL). U2OS-ERα osteoblast-like cells expressing an EGFP-tagged FasL at the C-terminus showed decreased fluorescence following E2 treatment, indicative of a cleavage event. Treatment of U2OS-ERα cultures with a specific MMP3 inhibitor in the presence of E2 blocked FasL cleavage and showed an increase in the number of EGFP-FasL+ cells. siRNA experiments successfully knocked down MMP3 expression and restored full-length FasL to basal levels. E2 treatment of both human and murine primary osteoblasts showed up-regulation of MMP3 mRNA expression, and calvarial organ cultures showed increased expression of MMP3 protein and co-localization with the osteoblast-specific RUNX2 following E2 treatment. Additionally, osteoblast cell cultures derived from ERαKO mice showed decreased expression of MMP3, but not MMP7 and ADAM10, two known FasL proteases, demonstrating that ERα signaling regulates MMP3. In addition, conditioned media of E2-treated calvarial osteoblasts showed an approximate 6-fold increase in the concentration of soluble FasL indicating extensive cleavage, and soluble FasL concentrations were reduced in the presence of a specific MMP3 inhibitor. Finally, to show the role of soluble FasL in osteoclast apoptosis, human osteoclasts were co-cultured with MC3T3 osteoblasts. Both a specific MMP3 inhibitor and an MMP inhibitor cocktail preserved osteoclast differentiation and survival in the presence of E2 and demonstrate the necessity of MMP3 for E2-induced osteoclast apoptosis. These experiments further define the molecular mechanism of estrogen’s bone protective effects by inducing osteoclast apoptosis through upregulation of MMP3 and FasL cleavage.
Introduction
Estrogen has long been shown to be protective of bone (1). Estrogen increases bone forming osteoblast proliferation and differentiation, while also inducing apoptosis of bone resorbing osteoclasts, leading to an overall increase in bone mineral density (1). Our lab has previously shown that 17β-estradiol (E2) mediates transcriptional upregulation of Fas Ligand (FasL) specifically in osteoblasts to induce E2-mediated apoptosis of osteoclasts (2).
Fas ligand (FasL) is a type II transmembrane protein of the Tumor Necrosis Factor family. The Fas receptor oligomerizes upon ligand binding to recruit caspase 8 and Fas-associated death domain containing protein (FADD)—two key components of the Fas death-inducing signaling complex (DISC) (3). FasL exists in membrane bound and soluble forms (sFasL), both of which are capable of inducing apoptosis (4). FasL has been shown to be cleaved into its soluble form by MMP7 (matrilysin) (4) and ADAM10 (5). In vitro transcribed MMP3 (stromelysin-1) protein is capable of cleaving FasL to the soluble form (4); however this has never been shown in cells.
We, and others, have demonstrated that FasL and the Fas receptor are necessary for E2-induced osteoclast apoptosis (2,6,7). There is however, debate in the field over the cellular source of FasL: osteoblasts or osteoclasts. We have previously shown by several methods that FasL is transcriptionally up-regulated by E2 in osteoblasts (2). First, antibody-purified osteoclasts do not undergo apoptosis when treated with E2 (2). Instead, E2 mediated osteoclast apoptosis must occur in the presence of osteoblasts. Co-cultures of osteoclasts and osteoblasts treated with E2 result in fewer multinuclear, TRAP-positive osteoclasts. Secondly, FasL is detected after E2 treatment in vivo at the growth plate and endosteal surface of osteoblasts, and not in osteoclasts at either location. In agreement with our work, Martin-Milan, et al (7) were unable to demonstrate a stimulatory effect of E2 on FasL production in primary cultures of murine osteoclasts alone. We also demonstrated that E2-induced osteoclast apoptosis is not contact dependent and most likely mediated by a soluble factor, as osteoblast:osteoclast co-cultures at ratios as low as 1:20,000 could induce apoptosis of pre-osteoclasts, and prevent osteoclast differentiation following E2 treatment. Therefore, we hypothesized that osteoblast-derived FasL is cleaved to a soluble form, facilitating osteoclast apoptosis. Our current work demonstrates for the first time that the bone protective effects of E2 are partially mediated by upregulation of MMP3 and subsequent cleavage of FasL from osteoblasts to induce osteoclast apoptosis.
Materials and methods
Reagents
17β-estradiol (E2) and doxycycline were purchased from Sigma-Aldrich Co. The following antibodies were used: FasL (Santa Cruz Biotech, clone N-20), MMP3 (Origene), MMP7 (Santa Cruz Biotech, clone MM0022-4C21), ADAM10 (Santa Cruz Biotech, clone A-3), FLAG (Sigma-Aldrich Co.), RUNX2 (R&D Systems) and β-actin (Sigma-Aldrich Co.). The MMP3 inhibitor (N-[[(4,5-Dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)amino]carbonyl]-L-phenylalanine) and the MMP Inhibitor II (N-Hydroxy-1,3-di-(4–methoxy-benzenesulfonyl)-5,5-dimethyl-[1,3]-piperazine-2-carboxamide) were purchased from Calbiochem. The MMP inhibitor II inhibits MMP1 (IC50 = 24 nM), MMP3 (IC50 = 18.4 nM), MMP7 (IC50 = 30 nM), and MMP9 (IC50 = 2.7 nM).
Cell Culture
U2OS-ERα cells, kindly provided by Drs. Thomas Spelsberg and David Monroe, were maintained as described (8). 24 hours before treatment with E2, ERα expression in U2OS-ERα cells was induced by treatment with 100 ng/mL doxycycline. MC3T3-E1 and MCF7 cells were obtained from ATCC. Human osteoblasts were obtained from ScienCell Research Laboratories. All E2 experiments were performed in media without phenol red and with 5% charcoal dextran- treated fetal bovine serum (CDT-FBS) (Omega Scientific).
Human osteoclasts
Human osteoclasts were differentiated as follows: peripheral blood mononuclear cells derived from anonymized donors were obtained from the Center for AIDS Research Virology Core/BSL3 Facility that is supported by the National Institutes of Health award AI-28697 and by the UCLA AIDS Institute and the UCLA Council of Bioscience Resource. Since specimens were obtained as anonymous and unidentifiable, the activities of the present research do not involve human subjects and therefore do not require IRB review according to UCLA IRB medical committee standards. Monocytes were purified by negative selection using the Monocyte Isolation Kit II (Miltenyi Biotec) and plated in αMEM with 10 ng/mL M-CSF (R&D Systems) and 30 ng/mL RANKL (R&D Systems) for 2 weeks. TRAP and alkaline phosphatase staining were performed according to the manufacturer’s instructions (Takara Bio Inc.).
Primary calvarial osteoblasts
All animal work was approved by the Animal Research Committee at UCLA. ERα knockout mice were kindly provided by Dr. Pierre Chambon (9). Primary osteoblasts were obtained from the calvaria of wildtype mice or bone marrow of WT and ERαKO as previously described (10). Whole calvariae were isolated from neonatal day 2 wildtype mice for calvarial organ cultures and cultured in osteoblast differentiation media for one week, followed by three days in CDT-FBS. Calvariae were treated with 10 nM E2 or vehicle control for 24 hours and fixed and sectioned according to standard protocols (10).
Co-culture of osteoclasts and osteoblasts
Five MC3T3 cells were plated in each well of a 96-well plate. The following day, 100,000 human monocytes (purified as described above) were plated with the attached MC3T3 cells. The monocytes were cultured for 14 days with or without 10 nM E2 with and without MMP Inhibitor Cocktail or MMP3 inhibitor in αMEM with 10 ng/mL M-CSF, 30 ng/mL RANKL, 5 mM β-glycerophosphate and 100 mg/ml ascorbic acid, then fixed and stained for TRAP (Takara Bio Inc.) according to the manufacturer’s instructions.
Immunofluorescence (IF
Calvarial organ sections were rehydrated according to standard protocols. Slides were incubated in blocking buffer (1X TBST; 3%BSA; 1% normal goat or donkey serum; 0.2% Sodium Azide; 1% Triton X-100), followed by primary antibodies (in blocking buffer) were incubated overnight. Secondary antibodies conjugated to Alexa 488 or Alexa 594 were used to detect the primary antibody. Cells were counterstained with 6-diamidino-2-phenylindole (DAPI) in mounting medium (Vector Laboratories, Inc.) to identify nuclei.
FasL-EGFP
Murine FasL was amplified from pSport-FasL purchased from Open Biosystems using the following primers: caggaattcatgcagcagcccatgaat and cagggatccgctaaaagcttatacaagccgaa. FasL was then sub-cloned into the pEGFP-N1 (EGFP, enhanced green fluorescent protein) vector using standard methods.
Flow Cytometry
U2OS-ERα cells were hormone-deprived by culture for one day in phenol red-free medium (Invitrogen Corporation) supplemented with 5% CDT-FBS. Cells were then electroporated with FasL-EGFP and re-seeded in hormone-free media. The following day cells were treated with 10 nM E2 or ethanol as a vehicle control in the presence or absence of MMP3 inhibitor for 24 hours. Cells were collected and FACS was performed to detect % of cells positive for EGFP.
Protein and Immunoblotting
Cells were hormone-deprived by culture for three days in phenol red-free medium (Invitrogen Corporation) supplemented with 5% CDT-FBS. Cells were treated with 10 nM E2 or ethanol as a vehicle control for 24 hours and then lysed in EBC buffer (50 mM Tris, pH 8, 120 mM NaCl, 0.5% Nonidet P-40) supplemented with a protease inhibitor mixture (Complete, Roche Applied Science) for 30 min on ice. Proteins were subjected to SDS-PAGE and immunoblotting with antisera to the indicated proteins.
RNA and Quantitative PCR (qPCR)
Cells were hormone-deprived by culture for three days in phenol red-free medium (Invitrogen Corporation) supplemented with 5% CDT-FBS. Cells were treated with 10 nM E2 or ethanol as a vehicle control for 3 or 24 hours. Total RNA was converted to cDNA with Superscript III First Strand Synthesis Kit according to the manufacturer’s instructions (Invitrogen Corporation). Primers were selected using Primer3 (11) and the sequences are listed in Supplemental Table 1. cDNA was subjected to quantitative PCR using the Applied Biosystems SYBR Green Mastermix with ROX. Each RNA sample was collected in triplicate and each PCR reaction was amplified in triplicate.
ELISA
Mouse Fas Ligand/TNFSF6 Immunoassay (R&D Systems, Inc.) was used according to manufacturer’s instructions.
siRNA
Cells were hormone-deprived by culture for 24 hours in phenol red-free medium (Invitrogen Corporation) supplemented with 5% CDT-FBS before transfection with 100 nM of siRNA oligonucleotide duplexes directed against MMP3. Two different duplexes were tested and gave identical results. The first was from Santa Cruz Biotech, using pooled siRNA. The second one was from Life Technologies: GCAUAUGAAGUUACUAGCAtt/UGCUAGUAACUUCAUAUGCgg. 24 hours after transfection U2OS-ERα cells were treated with 100 ng/mL doxycycline to induce ERα expression. The following day 10 nM E2 was added for 24 hours before RNA and/or protein was isolated.
Statistical Analysis
All experiments represent both biological and experimental triplicates. Error bars represent mean +/− 1 standard deviation (S.D.).
Results
Membrane-bound FasL is cleaved following E2 treatment
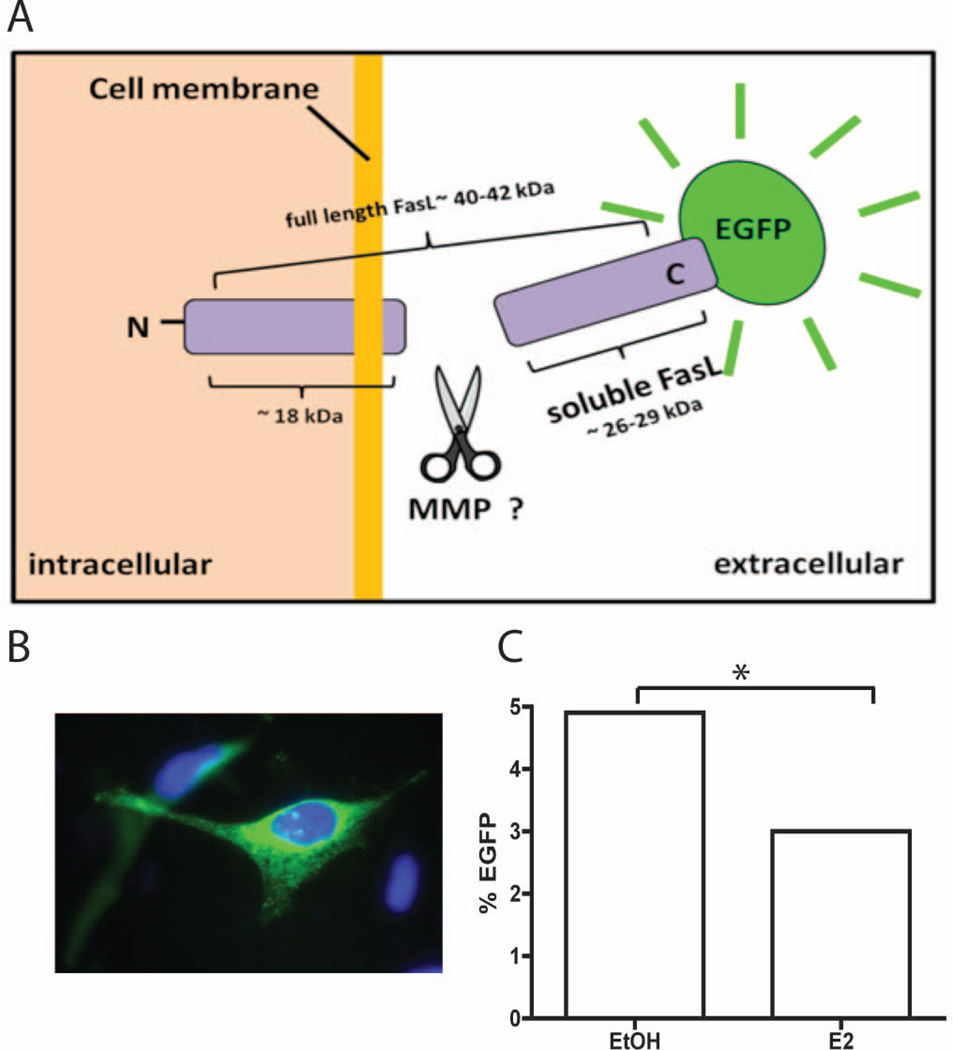
To test if FasL is cleaved and becomes soluble following E2 treatment we created an EGFP (enhanced green fluorescent protein)–tagged FasL vector. EGFP was fused to the extracellular C-terminus of FasL (Fig. 1A) and transfected into U2OS-ERα cells (an osteosarcoma cell line that was stably transfected with doxycycline-inducible expression of ERα). U2OS-ERα cells have osteoblast-like properties and respond well to E2 (12). Under normal conditions EGFP-FasL is membrane-bound and visible by fluorescence microscopy (Fig. 1B). U2OS-ERα cells were treated with 10 nM E2 or vehicle control for 24 hours and analyzed for EGFP fluorescence by flow cytometry. We observed a 40% decrease in the number of EGFP positive cells following E2 treatment (p<0.001) (Fig. 1C), indicating that FasL is cleaved.
Fig. 1.
EGFP-FasL is cleaved after E2 treatment. (A) Schematic of FasL-EGFP construct. See text for details. (B) U2OS-ERα cells were transiently transfected with FasL-EGFP and then the cells were fixed and stained with DAPI to identify the nucleus. (C) U2OS-ERα cells were transfected with FASL-EGFP then treated with vehicle (EtOH) or 10 nM E2 for 24 hours. Cells were analyzed by flow cytometry and the percent of EGFP-positive cells is graphed. * = p-value <0.001.
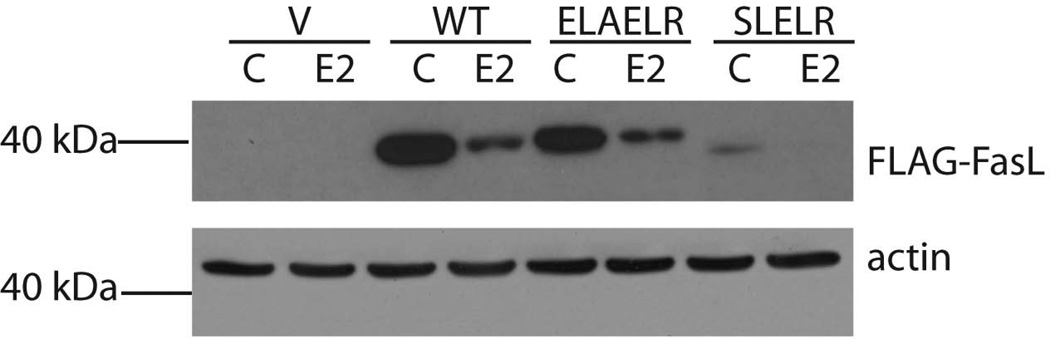
FasL has previously been shown to be cleaved at the leucine residues of the amino acid sequences SL and ELR near the transmembrane region by in vitro transcribed MMP3 and MMP7 (4). Therefore, mutations of these two regions in Flag-FasL were utilized to determine if these same sites are cleaved in vivo in osteoblast-like cells. U2OS-ERα cells were transfected with either wildtype or mutant FasL with an N-terminal Flag-tag. After expression of ERα with doxycycline and 24 hour treatment with vehicle or 10 nM E2, cellular lysates were immunoblotted with an anti-Flag antibody (Fig. 2). After treatment with E2, the amount of full-length FasL was significantly reduced, suggesting that FasL was cleaved. A double mutant, ELAELR, in which the leucine residues are deleted, did not restore full-length FasL after E2 treatment (Fig. 2). Similarly, a second double mutant, SLELR, in which the leucine sites are also deleted in the Flag-FasL construct, was also tested, and full-length FasL was not restored after E2 treatment (Fig. 2). Therefore, the cleavage site(s) in FasL from osteoblast-like cells remains unknown.
Fig. 2.
FasL is cleaved after E2 treatment. U2OS-ERα cells were transfected with Flag-vector alone (V), wildtype (WT) Flag-FasL or mutated Flag-FasL (ELAELR or SLELR) then treated with vehicle control (C) or 10 nM E2 for 24 hours. Cells were lysed and immunoblots were performed with anti-Flag and anti-actin antibodies.
MMP3 cleaves FasL in response to E2 treatment
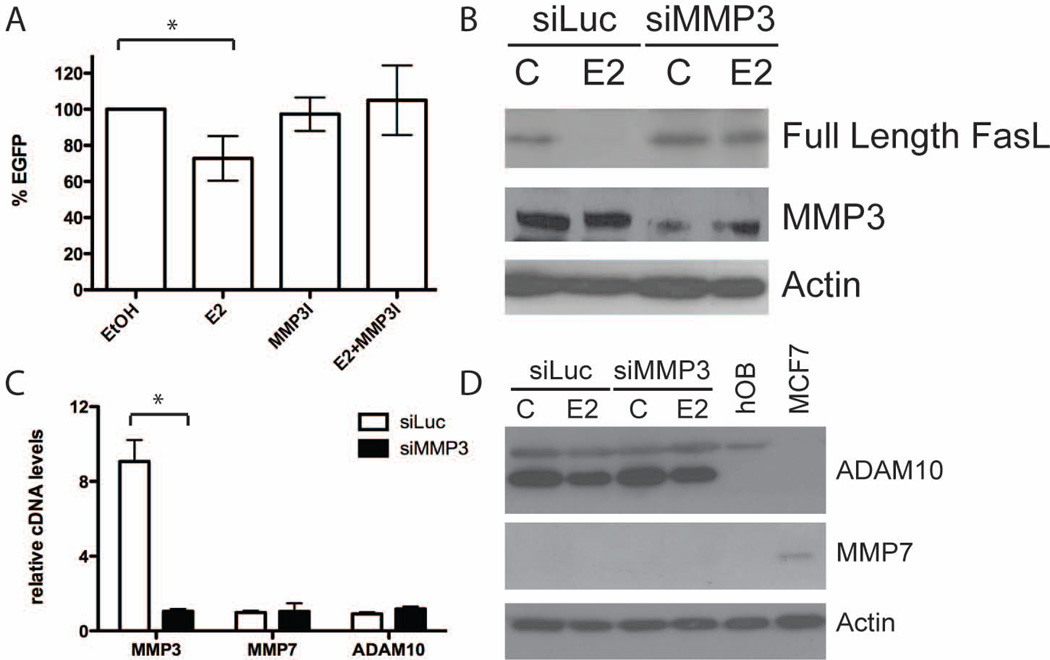
To better understand the nature of E2-mediated FasL cleavage we performed gelatin zymography assays using conditioned media and cell lysates from primary calvarial osteoblasts. Although MMP7 and ADAM10 are two proteases known to cleave FasL, the most intense bands did not correspond to the predicted molecular weights for either one (13,14). The zymography assays demonstrated an intense band in the osteoblast-conditioned media at the predicted molecular weight for MMP3 (Supplemental Fig. 1). Therefore we hypothesized that FasL is preferentially cleaved by MMP3. To test this hypothesis, we treated U2OS-ERα cells transfected with EGFP-FasL with 10 nM E2 in the presence or absence of 1 µM of an MMP3-specific inhibitor. EGFP-FasL cleavage was quantitated by flow cytometry. Cells treated with E2 alone showed a reduction in EGFP+ cells (~30% reduction) compared to vehicle control treated cells. Overall fluorescence was restored in the presence of an MMP3 inhibitor (1 µM), which blocked E2-mediated cleavage of the C-terminal EGFP fragment (Fig. 3A). These data demonstrate that FasL cleavage is mediated by the matrix metalloproteinase MMP3.
Fig. 3.
MMP3 activity is necessary for E2-mediated cleavage of FasL. (A) U2OS-ERα cells were transfected with FASL-EGFP then treated with vehicle control (EtOH), 10 nM E2 and/or 1 µM MMP3 inhibitor (MMP3I) for 24 hours. Cells were analyzed by flow cytometry and the percent change in EGFP compared to EtOH treated cells (set at 100%) is graphed. Four biological replicates are averaged. (B) U2OS-ERα cells were transfected with either an siRNA directed at luciferase (siLUC) or MMP3 (siMMP3). 48 hours after transfection cells were treated for 24 hours with vehicle control (C) or 10 nM E2. Cells were lysed and total cellular protein was immunoblotted for FasL, MMP3 and actin. (C) U2OS-ERα cells were transfected with either an siRNA directed at luciferase (siLUC) or MMP3 (siMMP3). 48 hours after transfection cells were lysed, and cDNA was analyzed by qPCR for MMP3, MMP7 and ADAM10. (D) U2OS-ERα cells were transfected with either an siRNA directed at luciferase (siLUC) or MMP3 (siMMP3). 48 hours after transfection cells were treated for 24 hours with vehicle control (C) or 10 nM E2. Cells were lysed and total cellular protein was immunoblotted for ADAM10, MMP7 and actin. Human osteoblasts (hOB) and MCF7 cells were used as controls. * = p-value <0.05.
To further determine if MMP3 is necessary for FasL cleavage, siRNA directed against MMP3 was transfected into U2OS-ERα cells. Immunoblot analysis demonstrated a decrease in MMP3 protein in the presence of siMMP3 (Fig. 3B). siMMP3 also decreased MMP3 mRNA over 5-fold compared to an siLuciferase (siLuc) negative control (Fig. 3C), confirming successful knockdown. Treatment of U2OS-ERα cells with E2 resulted in cleavage of endogenous full-length FasL protein, virtually eliminating the 40–42 kDa full-length species (Fig. 3B). In contrast, transfection of U2OS-ERα cells with siMMP3 resulted in a failure to reduce full-length FasL, as the full-length species is preserved after E2 treatment. An independent siRNA sequence targeted at MMP3 also inhibited FasL cleavage (Supplemental Fig. 2).
Gel zymography assays (Supplemental Fig. 1) suggested no involvement from the known FasL proteases MMP7 and ADAM10 in osteoblast cultures. To further rule out MMP7 and ADAM10 as FasL proteases in osteoblasts, quantitative PCR and immunoblots were performed after siMMP3 transfection. Following siMMP3 transfection both cDNA levels of MMP7 and ADAM10 remained unchanged (Fig. 3C and 3D). ADAM10 protein is decreased after E2 treatment, but remains unchanged after transfection of siMMP3. MMP7 protein could not be detected in U2OS-ERα cell lysates (Fig. 3D). Taken together, these results suggest that FasL is cleaved specifically by MMP3 in U2OS-ERα cells.
ERα signaling regulates MMP3 expression in human and murine osteoblasts
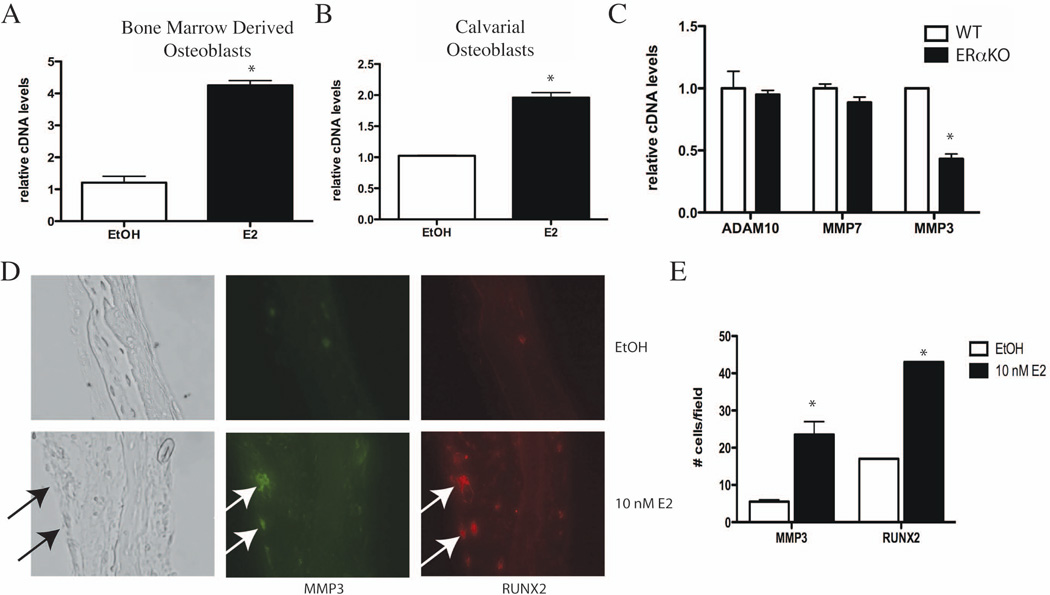
Although MMP3 expression in osteoblasts has been demonstrated (15,16), it has not been shown to be regulated by E2. To test what role E2 has in regulating MMP3 expression, primary calvarial osteoblasts and bone marrow-derived osteoblasts were differentiated for two weeks in osteoblast differentiation media, and then treated with 10 nM E2 for 3 hours. mRNA was isolated and cDNA was constructed for gene expression analysis. Compared to vehicle control, E2 treatment increased MMP3 mRNA in both bone marrow derived osteoblasts and primary calvarial osteoblasts (Fig. 4A and B). Similar differentiation and E2 time courses also increased the expression of FasL in osteoblasts (2). In addition, MMP3 expression is regulated by E2 in primary human osteoblasts: human perivascular cells were cultured under osteoblastic conditions in the presence or absence of E2 (10) and analyzed for gene expression differences. qPCR analysis demonstrated that MMP3 mRNA is significantly increased in human osteoblasts following E2 treatment (Supplemental Fig. 3) (10). These data show that MMP3 is regulated by E2 in both human and murine osteoblast cells.
Fig. 4.
E2 induces MMP3 expression in primary murine osteoblasts. (A) Bone-marrow derived osteoblasts were differentiated for two weeks and then treated for 3 hours with vehicle control (EtOH) or 10 nM E2. MMP3 mRNA was analyzed by quantitative PCR and normalized to actin mRNA. (B) Primary calvarial osteoblasts were differentiated for 10 days and then treated for 3 hours with vehicle control (EtOH) or 10 nM E2. RNA was obtained and MMP3 mRNA was analyzed by quantitative PCR and normalized to actin mRNA. (C) Bone-marrow derived osteoblasts from wildtype (WT) and estrogen receptor knockout (ERαKO) mice were differentiated for 10 days. RNA was obtained and MMP7, ADAM10 and MMP3 mRNA was analyzed by quantitative PCR and normalized to actin mRNA. (D) Calvariae from neonatal day 2 mice were grown as organ cultures for seven days and then treated with vehicle control (EtOH) or 10 nM E2 for 24 hours. Calvariae were fixed and paraffin-embedded. Immunofluorescence for MMP3 (green) and RUNX2 (red) was performed. (E) Quantification of MMP3 and RUNX2 immunofluorescence in part (D). The number of positive cells per field was counted and graphed. Error bars in all panels represent +/− 1 SD.
To better understand the nature and extent of ERα-regulated MMP3 transcription we investigated the expression of known FasL proteases in wildtype and ERα knockout (ERαKO) mice. Expression levels of ADAM10 and MMP7 in osteoblasts isolated from ERαKO mice did not differ from their wildtype littermates (Fig. 4C). In contrast, osteoblasts isolated from ERαKO mice show a significant reduction in the basal level of MMP3 when compared to osteoblasts from wildtype littermates, indicating that ERα signaling regulates MMP3 expression.
To determine if MMP3 is regulated by E2 in vivo we used a calvarial organ culture system to look at MMP3 expression (17). Whole calvariae were isolated from neonatal day 2 wildtype mice and cultured in osteoblast differentiation media for one week. Calvariae were treated with 10 nM E2 or vehicle control for 24 hours and then fixed and sectioned according to standard protocols (10). Immunofluorescence analysis of calvarial organs treated with vehicle control revealed co-localization of MMP3 and RUNX2 in osteoblasts. Following E2 treatment, MMP3 and RUNX2 double-positive cells increased over 3 fold (Fig. 4D and 4E), indicating that ERα signaling can regulate MMP3 expression in osteoblasts in vivo. ERα-regulated MMP3 expression is specific to osteoblasts as nearby chondrocytes are negative for MMP3 (Fig. 4D). Together, these experiments demonstrate that MMP3 expression occurs specifically in osteoblasts and is regulated by E2 through ERα in vivo. In addition, expression of RUNX2, a bona fide ERα transcriptional target (10,18,19), is up-regulated by E2 in primary calvarial osteoblasts and decreased in ERαKO osteoblasts (Supplemental Fig. 4). These data indicate a direct relationship between E2 treatment and the expression of MMP3 and RUNX2 in osteoblasts.
ERα-mediated MMP3 expression increases soluble FasL and leads to increased osteoclast apoptosis
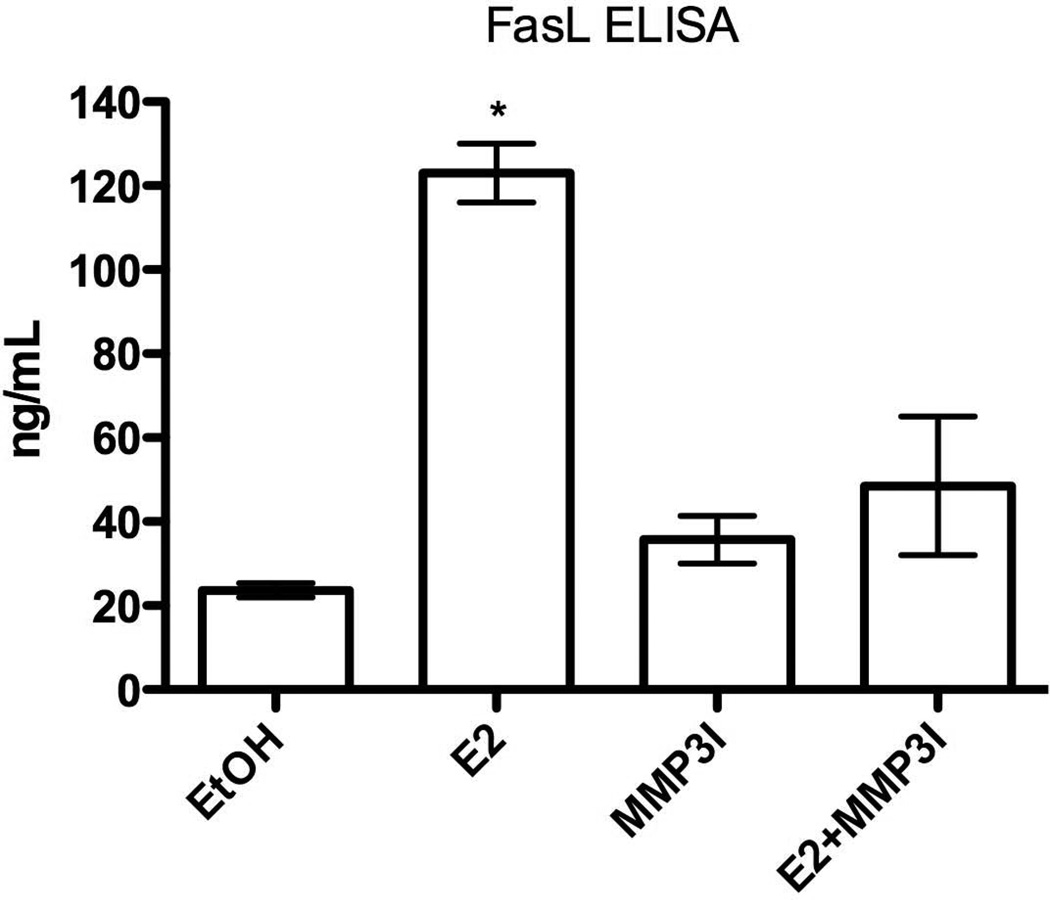
Because sFasL can induce apoptosis, we set out to determine if E2 treatment induced cleavage of FasL and increased the soluble fragment concentration in osteoblast-conditioned media. ELISA assays were performed using the conditioned media from primary calvarial osteoblasts cultured in differentiation media for two weeks. A 24 hour treatment of 10 nM E2 increased the sFasL concentration by approximately 6-fold (Fig. 5). Given that our previous results demonstrated MMP3 expression and activity were necessary for FasL cleavage (Fig. 3), we hypothesized that addition of the specific MMP3 inhibitor would reduce the amount of sFasL. Indeed, addition of the MMP3 inhibitor, in the presence of 10 nM E2, blocked the cleavage event and decreased sFasL concentration (Fig. 5). This demonstrates that MMP3, through ERα signaling, cleaves membrane bound FasL in osteoblasts and increases the concentration of the soluble C-terminal domain which leads to osteoclast apoptosis.
Fig. 5.
An MMP3 inhibitor blocks E2-mediated FasL cleavage in primary calvarial osteoblasts. Primary murine calvarial osteoblasts were differentiated for 10 days, followed by treatment with vehicle control (EtOH), 10 nM E2 and/or 1 µM MMP3 inhibitor for 24 hours. The conditioned media was removed and subjected to ELISA for FasL. * = p-value <0.01 E2 vs. vehicle, MMP3I and E2+MMP3I. (MMP3I=specific MMP3 inhibitor)
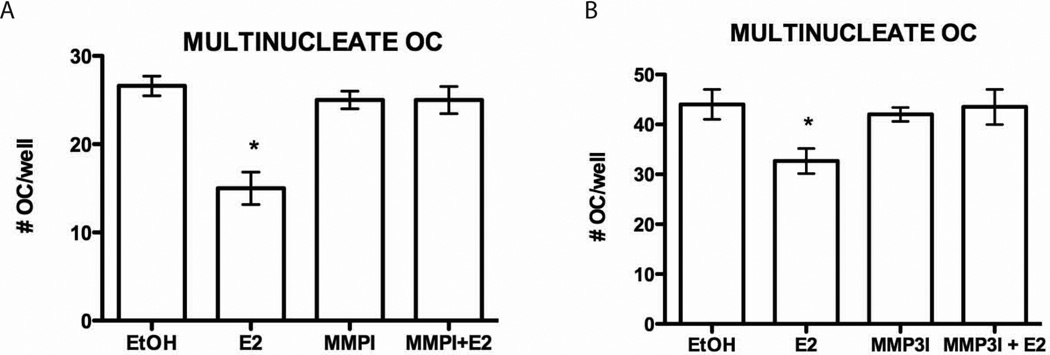
Next, we set out to determine if MMP3-mediated sFasL cleavage is indeed necessary for osteoclast apoptosis. Our lab has previously shown that osteoblasts are necessary for E2-mediated induction of apoptosis of human monocytes differentiated to osteoclasts (2). This co-culture system was again used to demonstrate that MMP3 cleaves FasL, leading to osteoclast apoptosis, and ultimately lack of osteoclasts. MC3T3 murine osteoblast cells were incubated for fourteen days with purified human monocytes under conditions to differentiate both osteoblasts and osteoclasts. Cultures lacking E2 were able to form fully differentiated osteoclasts in the presence of MC3T3 osteoblasts (Fig. 6A and B). Co-cultured cells treated with 10 nM E2 for the entire course of differentiation resulted in a significant decrease in the number of multinucleated, TRAP-positive osteoclasts (Fig. 6A and B). Our previous publications have shown this to be due to osteoclast apoptosis (2). Addition of 50 nM of an MMP inhibitor cocktail restored multi-nucleated osteoclast cell numbers, demonstrating that MMPs are necessary for E2-mediated apoptosis of osteoclasts (Fig. 6A). A 1 µM specific MMP3 inhibitor also restored osteoclast cell number to near basal levels, further demonstrating the necessity for MMP3 in E2-mediated osteoclast apoptosis (Fig. 6B).
Fig. 6.
MMP3 activity is required for estrogen-mediated apoptosis. (A) Human monocytes were differentiated with M-CSF and RANKL to osteoclasts in a co-culture with osteoblasts (MC3T3 cells) for 14 days. The cells were treated with vehicle control (EtOH) or 10 nM E2 in the presence or absence of the 50 nM MMP Inhibitor Cocktail (MMPI) or 1 µM MMP3 inhibitor (MMP3I) (B) for days 3–14 of differentiation. The cells were then fixed and stained for TRAP. The number of multinucleated cells was counted. Each treatment was performed in triplicate and error bars represent the mean +/− 1 SD. * = p-value <0.05 E2 vs. vehicle, MMPI, MMPI+E2 or MMP3I and MMP3I+E2.
Discussion
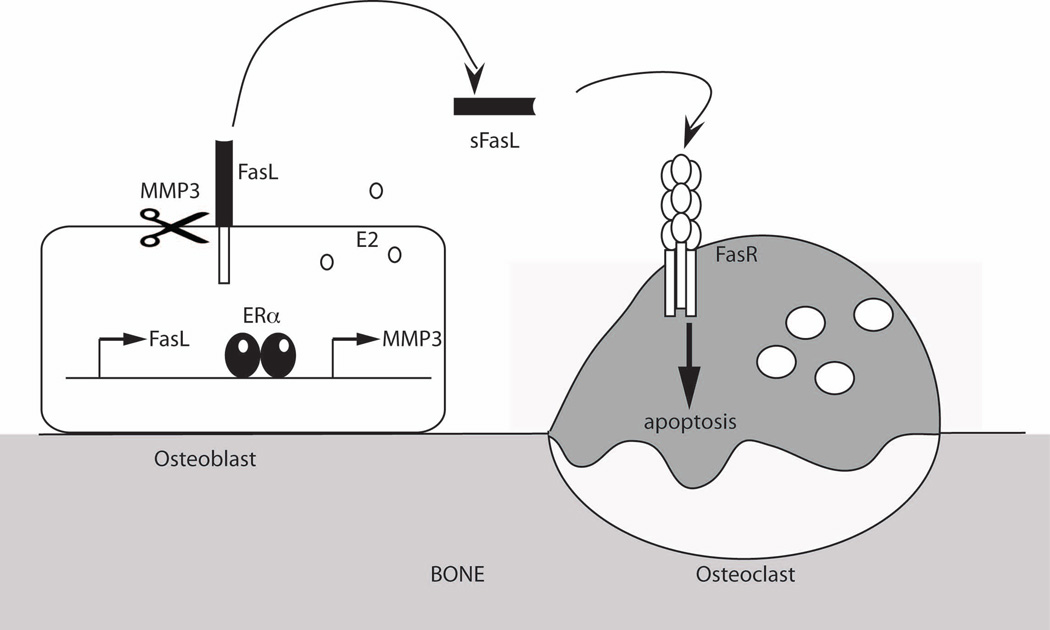
We have previously shown that E2, via ERα, induces transcription of Fas Ligand (FasL) in osteoblasts, resulting in a paracrine signal that induces osteoclast apoptosis (2,20). These data contradict the work of Nakamura et al., (6) where it is suggested that FasL is transcriptionally induced by E2 in osteoclasts. Nakamura et al., used an osteoclast-specific ERα knockout mouse (ERαΔOC/ΔOC) and showed a lack of regulation of FasL expression in bones. However, their work did not determine if the specific localization of FasL expression was in either osteoblasts or osteoclasts. Their experiments used whole bones instead of purified cell types, making it difficult to determine which cells expressed FasL. Conversely, we showed, using multiple approaches, that FasL is transcriptionally up-regulated by E2 specifically in osteoblasts (2). First, E2 induces FasL mRNA in a murine osteoblast cell line (MC3T3), a human cell line expressing ERα (U2OS-ERα), and in primary murine calvarial osteoblasts. Second, E2 induces FasL expression in osteoblasts but not in osteoclasts, as shown by co-localization of FasL with RUNX2. Third, antibody-purified osteoclasts fail to undergo E2-induced apoptosis in the absence of osteoblasts. Finally, co-cultures of MC3T3 osteoblasts and ERαKO bone marrow-derived osteoclasts demonstrate that ERα in osteoblasts is necessary and sufficient for osteoclast apoptosis. We have extended these findings here to further explain how ERα signaling induces osteoclast apoptosis. These experiments, and that of other groups (7), support our hypothesis that E2 induces FasL transcription in osteoblasts, induces MMP3 expression and subsequent sFasL generation, which leads to apoptosis of osteoclasts (Figure 7). ERα is necessary in osteoclasts (6), and as such, additional experiments must be performed to identify the ERα targets specifically in osteoclasts.
Fig. 7.
Model of E2 regulation of FasL transcription and cleavage. E2 increases transcription of FasL and MMP3 in osteoblasts. FasL is cleaved by MMP3 to the soluble form and then induces apoptosis of osteoclasts.
Soluble FasL has been shown to both induce (21) and antagonize apoptosis (22) depending on cell type and stimulus. It has been postulated that different isoforms of FasL, with different amino-termini, have different biological functions or are induced differentially in a tissue-specific manner (4). Here, we propose that MMP3 cleavage of FasL in osteoblasts is induced by E2 and results in osteoclast apoptosis. However, the exact site of FasL cleavage is unknown in this system, as mutations in three locations (ELA, ELR and SL) near the transmembrane region of FasL failed to block E2-mediated cleavage. Many different substrates have been reported for MMP3 and there is no known consensus motif for MMP3 cleavage (23).
Recently, Schiltz et al., showed that a transgenic mouse over-expressing TIMP-1 (Tissue Inhibitor of Metalloproteinases-1) in osteoblasts exhibited no bone loss induced by ovariectomy (24). Specifically, the TIMP-1 transgenic had a decrease in osteoclast activity after ovariectomy, as compared to wildtype mice. As an inhibitor of metalloproteinases, TIMP-1 is able to inhibit MMP2, MMP3 and MMP13. It was demonstrated that the TIMP-1 modulation of MMPs plays a role in TGFβ activation and/or the RANKL/OPG ratio in bone leading to reduced osteoclast activity but not numbers. However, this may not be the only mechanism for osteoclast survival modulation, as we hypothesize a direct regulation of osteoclast survival via paracrine FasL signaling, which would also be altered in the TIMP-1 transgenic mouse.
MMP3 is thought to play an important role in degrading cartilage in rheumatoid arthritis and osteoarthritis, although the MMP3 knockout mouse shows no difference in a collagen-induced arthritis model (25). Furthermore, no gross bodily changes are observed in the MMP3 knockout mouse, although it is unknown if a skeletal phenotype was analyzed. Based on our results, we would hypothesize that these mice would show mild to severe osteoporosis due to an inability of E2 to induce FasL cleavage and subsequent osteoclast apoptosis without MMP3.
In summary, our results further define the molecular mechanism of how E2 and ERα signaling protect bone and induce osteoclast apoptosis via an up-regulation of both MMP3 and soluble FasL. These data would further support the suggestion that FasL could be a target for osteoporosis therapies (26). Our results provide a new therapeutic target —MMP3— that could be activated to alleviate or prevent post-menopausal osteoporosis.
Supplementary Material
Acknowledgements
Authors’ roles: Study design: AG, GAM-C, SAK. Study conduct: AG, CT, MG, GP, MEM, KW, GAM-C, and SAK. Drafting manuscript: AG, CT and SAK. Revising manuscript content: AG, CT, KW, GAM-C, and SAK. Approving final version of manuscript: AG, CT, MG, GP, MEM, KW, GAM-C, and SAK. SAK takes responsibility for the integrity of the data analysis. MEM was supported by NIH IMSD Grant # GM 55052. This work was supported by a K12 BIRCWH Grant from the National Institutes of Health Office of Research on Women’s Health (HD001400-08) and 1R56DK090231-01 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to SAK. GAM-C is currently supported by a K22 Grant from the National Cancer Institute (CA137168-01A1). Human monocytes were obtained from UCLA CFAR (grant 5P30 AI028697). The authors thank Edgar Anaya for technical assistance. Flag-FasL vectors were kindly provided by Barbara Fingleton, Ph.D. (Vanderbilt University) (4).
Footnotes
All authors state that they have no conflicts of interest
References
- 1.Krum SA. Direct transcriptional targets of sex steroid hormones in bone. J Cell Biochem. 2011;112(2):401–408. doi: 10.1002/jcb.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27(3):535–545. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10(1):26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 4.Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys. 2002;408(2):155–161. doi: 10.1016/s0003-9861(02)00525-8. [DOI] [PubMed] [Google Scholar]
- 5.Kirkin V, Cahuzac N, Guardiola-Serrano F, Huault S, Luckerath K, Friedmann E, Novac N, Wels WS, Martoglio B, Hueber AO, Zornig M. The Fas ligand intracellular domain is released by ADAM10 and SPPL2a cleavage in T-cells. Cell Death Differ. 2007;14(9):1678–1687. doi: 10.1038/sj.cdd.4402175. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24(2):323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem. 2003;90(2):315–326. doi: 10.1002/jcb.10633. [DOI] [PubMed] [Google Scholar]
- 9.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 10.Miranda-Carboni GA, Guemes M, Bailey S, Anaya E, Corselli M, Peault B, Krum SA. GATA4 Regulates Estrogen Receptor-{alpha}-Mediated Osteoblast Transcription. Mol Endocrinol. 2011;25(7):1126–1136. doi: 10.1210/me.2010-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 12.Krum SA, Miranda-Carboni GA, Lupien M, Eeckhoute J, Carroll JS, Brown M. Unique ER{alpha} cistromes control cell type-specific gene regulation. Mol Endocrinol. 2008;22(11):2393–2406. doi: 10.1210/me.2008-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulte M, Reiss K, Lettau M, Maretzky T, Ludwig A, Hartmann D, de Strooper B, Janssen O, Saftig P. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ. 2007;14(5):1040–1049. doi: 10.1038/sj.cdd.4402101. [DOI] [PubMed] [Google Scholar]
- 14.Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol. 1999;9(24):1441–1447. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- 15.Peeters-Joris C, Hammani K, Singer CF. Differential regulation of MMP-13 (collagenase-3) and MMP-3 (stromelysin-1) in mouse calvariae. Biochim Biophys Acta. 1998;1405(1–3):14–28. doi: 10.1016/s0167-4889(98)00094-9. [DOI] [PubMed] [Google Scholar]
- 16.Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, Kamoi K, Suda T. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology. 1998;139(3):1338–1345. doi: 10.1210/endo.139.3.5818. [DOI] [PubMed] [Google Scholar]
- 17.Mohammad KS, Chirgwin JM, Guise TA. Assessing new bone formation in neonatal calvarial organ cultures. Methods Mol Biol. 2008;455:37–50. doi: 10.1007/978-1-59745-104-8_3. [DOI] [PubMed] [Google Scholar]
- 18.Plant A, Samuels A, Perry MJ, Colley S, Gibson R, Tobias JH. Estrogen-induced osteogenesis in mice is associated with the appearance of Cbfa1-expressing bone marrow cells. J Cell Biochem. 2002;84(2):285–294. doi: 10.1002/jcb.10021. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy TL, Chang WZ, Liu Y, Centrella M. Runx2 integrates estrogen activity in osteoblasts. J Biol Chem. 2003;278(44):43121–43129. doi: 10.1074/jbc.M306531200. [DOI] [PubMed] [Google Scholar]
- 20.Krum SA, Brown M. Unraveling estrogen action in osteoporosis. Cell Cycle. 2008;7(10):1348–1352. doi: 10.4161/cc.7.10.5892. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14(6):1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4(1):31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 23.Nagase H, Fields CG, Fields GB. Inhibition of osteoblastic metalloproteinases in mice prevents bone loss induced by oestrogen deficiency. J Biol Chem. 1994;269(33):20952–20957. [Google Scholar]
- 24.Schiltz C, Marty C, de Vernejoul MC, Geoffroy V. Inhibition of osteoblastic metalloproteinases in mice prevents bone loss induced by oestrogen deficiency. J Cell Biochem. 2008;104(5):1803–1817. doi: 10.1002/jcb.21747. [DOI] [PubMed] [Google Scholar]
- 25.Mudgett JS, Hutchinson NI, Chartrain NA, Forsyth AJ, McDonnell J, Singer II, Bayne EK, Flanagan J, Kawka D, Shen CF, Stevens K, Chen H, Trumbauer M, Visco DM. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998;41(1):110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Imai Y, Kouzmenko A, Kato S. Targeting Fas/FasL signaling, a new strategy for maintaining bone health. Expert Opin Ther Targets. 2011;15(10):1143–1155. doi: 10.1517/14728222.2011.600690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.