Abstract
Study Objectives:
Previous studies have found an inverse association between insomnia and self–reported physical activity, but it is not clear whether insomnia is associated with cardiorespiratory fitness. Our aim was to investigate different insomnia symptoms in relation to the gold standard measure of cardiorespiratory fitness, i.e., peak oxygen uptake (VO2peak).
Design:
Cross-sectional population study.
Setting:
Nord-Trøndelag County, Norway.
Participants:
The group comprised 3,489 men and women who were free from cardiovascular or pulmonary diseases, cancer, and sarcoidosis and who did not use antihypertensive medication. They were included in the fully adjusted model when assessing all insomnia symptoms simultaneously.
Interventions:
N/A.
Measurements and Results:
For insomnia, the participants reported how often they had experienced sleep problems during the past 3 months, including difficulties falling asleep at night, repeated awakenings during the night, early awakenings without being able to go back to sleep, and daytime sleepiness. Response options were “never/almost never,” “sometimes” or “several times a wk.” To measure cardiorespiratory fitness, the participants were asked to walk or run on a treadmill with increasing speed and/or incline until exhaustion, and VO2peak was recorded. We found a modest inverse and graded association of the insomnia symptoms with VO2peak. The association was independent of self-reported physical activity and was apparent for all insomnia symptoms except for early awakenings. We found a dose-response relation for a cumulative combination of insomnia symptoms and VO2peak for experiencing zero, one to two, or three to four symptoms (P for trend < 0.001).
Conclusions:
We found a modest inverse association of insomnia with VO2peak independent of the conventional cardiovascular risk factors and self-reported physical activity.
Citation:
Strand LB; Laugsand LE; Wisløff U; Nes BM; Vatten L; Janszky I. Insomnia symptoms and cardiorespiratory fitness in healthy individuals: the Nord-Trøndelag Health Study (HUNT). SLEEP 2013;36(1):99–108.
Keywords: Cardiorespiratory fitness, insomnia, peak oxygen uptake, sleep
INTRODUCTION
Insomnia is a self-reported condition that includes difficulty falling asleep or remaining asleep.1,2 Insomnia is common in patients with cardiovascular disease and a growing body of evidence suggests that insomnia is associated with an increased risk of coronary heart disease,3–5 but the underlying mechanisms for this association are largely unknown. However, several studies have reported that people with insomnia symptoms tend to have a lower level of physical activity,6–9 which could represent a possible explanation for the increased risk of coronary heart disease in these individuals.
Insomnia could also be inversely associated with cardiorespiratory fitness because its major determinant is physical activity. However, so far only two relatively small studies have assessed the association between sleep and cardiorespiratory fitness using exercise tests.10,11 Whereas physical activity corresponds to any bodily movement produced by skeletal muscles that results in energy expenditure, cardiorespiratory fitness refers to the maximum ability of the circulatory system to supply and extract oxygen during heavy dynamical work with large muscle groups. Both measures are inversely associated with cardiovascular disease morbidity and mortality,12,13 but it has recently been shown that peak oxygen uptake (VO2peak), the gold standard measure of cardiorespiratory fitness, is a stronger determinant of cardiovascular disease risk and longevity than measures of physical activity.13–16
Our aim was to investigate the association of different symptoms of insomnia with VO2peak, in a healthy group of men and women. In our large population-based study we could also take into account the effects of established cardiovascular risk factors, determinants of VO2peak and psychologic distress.
METHODS
Data Collection
The adult population of Nord-Trøndelag County in Norway was invited to participate in the third wave of the countywide health survey “Helseundersøkelsen i Nord-Trøndelag” (HUNT-3) between October 2006 and June 2008. Approximately 51,000 participated (54% of those invited). In the study, information was collected by self-administered questionnaires, clinical measurements, and blood samples. Thus, the self-reported information includes health status; use of tobacco, alcohol, and coffee; responses to some dietary items; use of medication; and information on sleep, physical activity, education, and work history. Anthropometry, including measurements of height, weight, and waist and hip, was recorded, and blood pressure and serum lipids, including total and high-density lipoprotein (HDL) cholesterol, were measured.
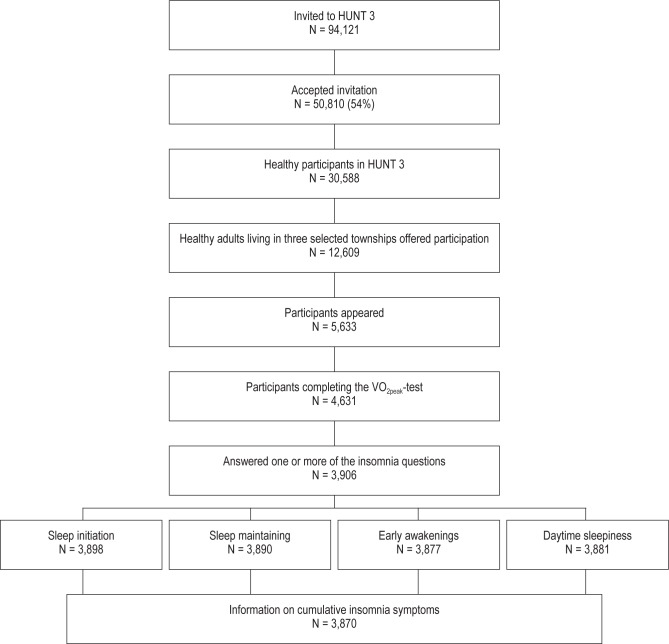
Among the participants in HUNT-3, 30,513 were free from known cardiovascular or pulmonary diseases, cancer, and sarcoidosis and did not use antihypertensive medication at baseline, and were potentially eligible for a separate fitness study. Among them, 12,609 participants were residents in the three townships that were selected for the fitness study, and these participants were invited to the fitness study. A total of 4,631 participants accepted the invitation and completed the fitness test, which measured VO2peak. The participants also responded to a physical activity questionnaire. The process of selecting the participants is illustrated in Figure 1.
Figure 1.
Selection process flow chart.
Insomnia
The HUNT-3 questionnaire included nine questions about sleep. The participants answered how often during the past 3 months they had experienced difficulties falling asleep at night, repeated awakenings during the night, early awakenings without being able to go back to sleep, loud snoring, stopping to breath while asleep (sleep disordered breathing), sweating while asleep, daytime sleepiness, waking up with a headache, and having an uncomfortable feeling in the legs. The response categories were “never/almost never,” “sometimes” or “several times a wk.”
In total, 3,870 participants (83.4%) answered all the insomnia questions. The separate response rates for the questions about difficulties falling asleep at night, repeated awakenings during the night, early awakenings without being able to go back to sleep, and daytime sleepiness were 84.2% (n = 3,898), 84.0% (n = 3,890), 83.7% (n = 3,877), and 83.8% (n = 3,881), respectively. These response rates largely reflect the overall response rate of the HUNT-3 questionnaire that included the insomnia questions.
Peak Oxygen Uptake
Before the exercise test, the participants were briefly instructed about the treadmill test, and had 10 min of warming up at a moderate intensity during which they achieved light sweating and a modest shortness of breath, but did not reach subjectively ascertained exhaustion. Before getting on the treadmill (DK City, DK7830, Taichung, Taiwan), a heart rate monitor (Polar S610 or RS400, Polar, Kempele, 8 Finland) was fitted to each participant’s chest and a mask (Hans Rudolph V, Shawnee, Kansas, USA) was fitted to the face.
Portable ergospirometry (Metamax II, Cortex Biophysic Gmbh, Leipzig, Germany) connected to a personal computer was used to measure the cardiorespiratory variables continuously. The test equipment was calibrated routinely by trained personnel. The participants were requested to walk or run on the treadmill at an increasing, individually adjusted speed and/or incline until exhaustion. When the oxygen uptake did not increase more than 2 mL/kg/min despite increased incline or speed, maximum oxygen uptake (VO2max) was considered achieved. As a secondary criterion, the respiratory exchange ratio (i.e., the ratio of carbon dioxide output to oxygen uptake measured at external respiration) should exceed 1.05 to indicate a high level of effort. When the participant reached exhaustion, but not both criteria for VO2max, the term VO2peak was used. Because a considerable proportion of the participants (12.6%) did not achieve the criteria for VO2max, the term VO2peak is used throughout this article.
Physical Activity
The participants answered a questionnaire about weekly physical activity that included questions about frequency, intensity, and duration. The frequency question was stated as “How often do you exercise?” with the response options “Never,” “Less than once a wk,” “Once a wk,” “Two to three times a wk” or “Almost every day.” The question related to intensity was stated as “How hard do you exercise?” with the response options “No sweat or heavy breathing,” “Sweat and heavy breathing,” or “Push myself to exhaustion.” Duration of the exercise was stated as “How long does each session last?” with the response options “Less than 15 min,” “Between 15 and 29 min,” “Between 30 and 60 min,” or “More than 60 min.” Frequency, intensity and duration were combined to form a physical activity index. We recoded the frequency scale to approximate number of times per wk (i.e., “0,” “0.5,” “1,” “2.5,” or “5”), the intensity scale to “1,” “2,” or “3,” and the duration scale to the approximate number of hr per session (i.e. “0.10,” “0.38,” “0.75,” and “1.00”). The physical activity index was the product of the recoded frequency, intensity, and duration scales. This method of calculating physical activity level has been reported and validated previously.17
Clinical Information
Clinical information on resting heart rate, weight, height, and blood pressure was collected by nurses. Resting heart rate was defined as beats per min after 10 min of rest in a dimly lit room. Systolic and diastolic blood pressure was measured three times (Dinamap 845XT Criticon) and the average of the second and third measurement was used in the analysis. Height was measured to the nearest 1 cm and weight to the nearest 0.5 kg. The participants were wearing lightweight clothing and no shoes during these measurements. Body mass index (BMI) was calculated as weight (in kilograms) divided by height squared (in meters).
Sociodemographic (i.e., sex, age, education level, and marital status) and lifestyle factors (i.e., smoking and alcohol intake) were collected by questionnaires. The participants were also asked if they were currently experiencing body pain that had lasted for more than 6 months.
Marital status was categorized into never married, married, or separated/divorced/widowed, and education was categorized into completion of primary and lower secondary school, upper secondary school, or university. The participants were defined as either never smokers, previous smokers, or current smokers. They were asked the question “Do you smoke?” with the alternatives “no, I have never smoked,” “no, I have quit smoking,” “yes, cigarettes occasionally,” “yes, cigars/cigarillos/pipe occasionally,” “yes, cigarettes daily,” and “yes, cigars/cigarillos/pipe daily.” If answering “no, I have never smoked” or “no, I have quit smoking” participants were categorized as never smokers or previous smokers, respectively. Current smoking was defined as currently smoking cigarettes/cigars or cigarillos/pipe, occasionally or daily. In relation to alcohol consumption, the participants reported how many glasses of beer, wine, and spirits they usually consumed over a 2-wk period. To accommodate a nonlinear relationship between alcohol consumption and cardiovascular health where light or moderate drinkers tend to be the healthiest and/or have the most favorable outcomes,18 we categorized the participants into abstainers, light drinkers (zero to one drink per day), moderate drinkers (more than one but fewer than two drinks per day), or heavy drinkers (two or more drinks per day). One drink corresponds to a consumption of 10-15 g of alcohol.
Laboratory Measurements
A nonfasting blood sample was analyzed using a Hitachi 911 Autoanalyzer (Hitachi, Mito, Japan) at the Central Laboratory at Levanger Hospital. Within 2 hr, the blood was centrifuged at the site of the survey and placed in a refrigerator with a constant temperature of 4®C. The sample was sent to the laboratory the same day.
Total cholesterol and HDL cholesterol were analyzed by an enzymatic colorimetric method using reagents from Boehringer Mannheim (Mannheim, Germany). HDL cholesterol was measured after precipitation with phosphotungsten and magnesium ions.
Anxiety and Depression
To assess symptoms of anxiety and depression, the Hospital Anxiety and Depression Scale was used. The questionnaire consists of 14 questions (seven for anxiety and seven for depression) with a four-point scale ranging from zero (not at all) to three (very often). Responses are summed to provide separate scores for symptoms of anxiety and depression with possible scores ranging from 0 to 21 for each scale. Higher scores indicate greater likelihood of depression or anxiety.19 The Hospital Anxiety and Depression Scale has been found to be a useful tool in the assessment of symptom severity both in hospital settings and in primary health care.20 The psychometric properties of the scale have been validated previously in the HUNT study.21
Statistical Analysis
Bivariate analyses of the data were done using chi-square tests or one-way analysis of variance for discrete and continuous variables, respectively. Multivariable analyses were performed using general linear models. We assessed the original response categories for each insomnia symptom (i.e., never/almost never, sometimes, several times a wk), and cumulative number of insomnia symptoms (i.e. zero, one to two, three to four symptoms) using indicator (dummy) variables in our regression models to reflect the categoric/ordinal nature of these variables. In the first model (Model 1), we adjusted for age and sex. In the second model (Model 2), we adjusted for age, sex, marital status, education, smoking, alcohol consumption, systolic and diastolic blood pressure, BMI, sleep disordered breathing, snoring, total serum cholesterol and HDL cholesterol, depression score, anxiety score, and chronic pain. In a third model (Model 3), we further adjusted for self-reported physical activity and resting heart rate. We calculated least square means of VO2peak with corresponding 95% confidence intervals for each insomnia symptom. Participants with no insomnia complaints were the reference group. Analyses of trend were conducted separately to quantify the linear trend for the associations of these variables with peak oxygen uptake. We assigned a value from one to three to the insomnia variables representing “never/almost never,” “sometimes,” and “several times a wk,” respectively, and treated these variables as continuous variables in our analyses of trend.
The insomnia symptoms were also dichotomized so we could assess their cumulative effects. Participants with the most frequent symptoms (i.e., those experiencing the symptom several times a wk) were defined as having the respective symptom. To test the cumulative effect of insomnia symptoms, we calculated the risk associated with increasing number of the dichotomized insomnia symptoms sleep initiation problems, repeated awakenings, and early awakenings (i.e. zero, one, two, and three). We did not include daytime sleepiness in the cumulative variable because it is not a core symptom of insomnia. In the analysis of the cumulative effects, we excluded participants with missing information on one or more of the insomnia variables.
As a sensitivity analysis we repeated the original analysis after excluding participants who reported symptoms of sleep disordered breathing, including cessation of breathing while asleep, and loud snoring several times a wk. In another sensitivity analysis we excluded participants who tended to experience waking up with a headache or nighttime sweating, in addition to participants who several times a wk stopped breathing and snored loudly while asleep. In further sensitivity analyses we excluded 67 participants with diabetes mellitus. We also excluded participants who did not answer all the insomnia questions and re-ran the analyses.
We assessed the association of insomnia symptoms and VO2peak in analyses stratified by sex, age, BMI, education, and systolic blood pressure to investigate effect modification. We also performed formal tests for interactions between sex, age (i.e., younger and older than 50 yr), BMI (i.e., below and above 25 kg/m2), education (i.e., with and without a university degree), systolic blood pressure (i.e., below and above 130 mmHg), and insomnia.
All statistical analyses were conducted using STATA 11 for Windows (Stata Corp., College Station, TX).
RESULTS
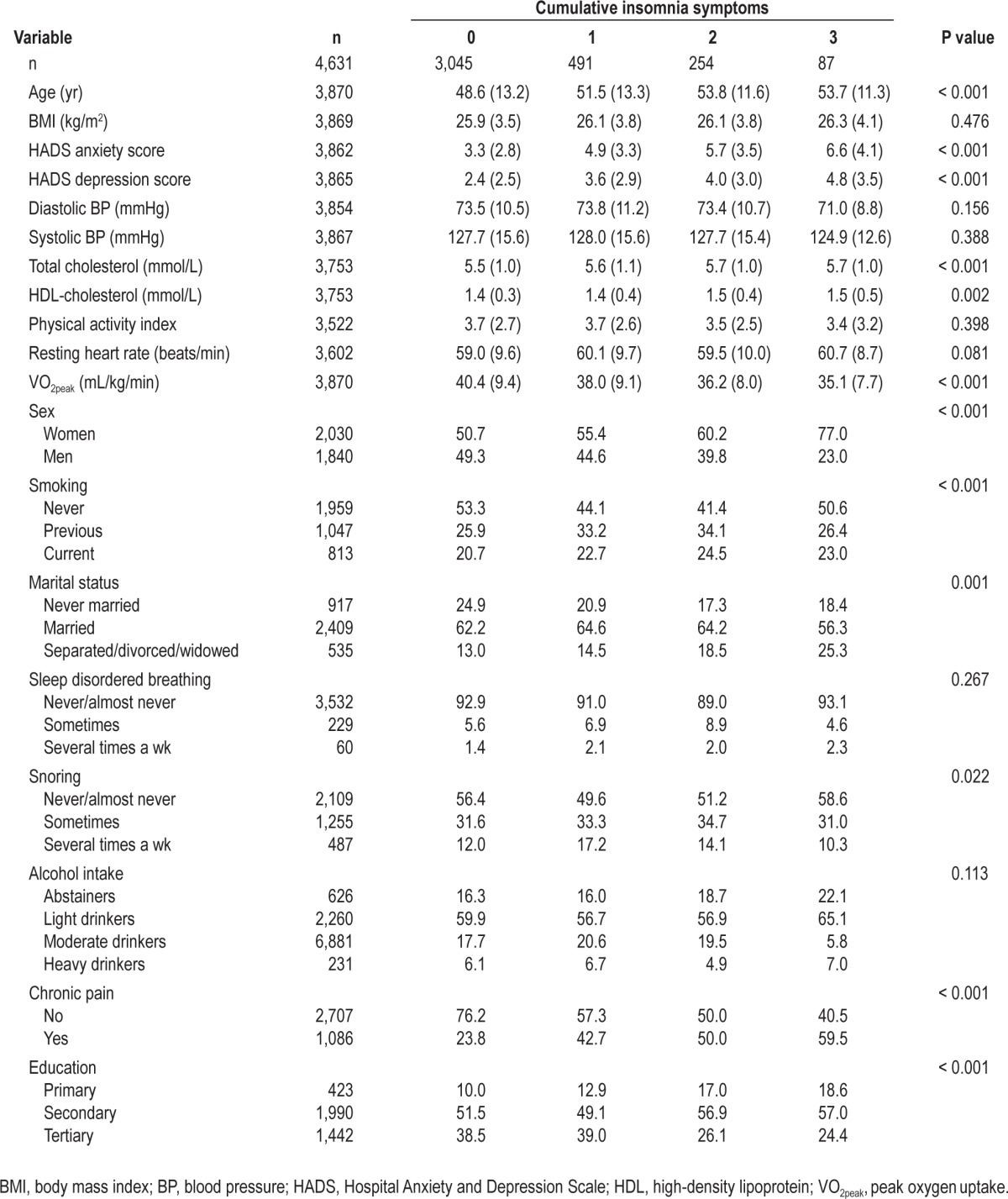
Mean VO2peak was 40.0 mL/kg/min (standard deviation 9.5 mL/kg/min) and the interquartile range was 13.2 mL/kg/min. Sex-specific estimates were 35.9 mL/kg/min and 44.3 mL/kg/min for women and men, respectively. Table 1 shows the frequency of the insomnia symptoms. Frequent awakenings several times a wk were twice as prevalent as problems with sleep initiation and early morning awakenings. In Table 2 we present the main characteristics according to cumulative number of insomnia symptoms. People with insomnia symptoms were older, more depressed, had higher BMI, lower physical fitness, and higher resting heart rate than participants without insomnia symptoms. Women were more likely to experience insomnia symptoms. In addition, participants who were separated, divorced, or widowed and participants with lower education level had an increased number of insomnia symptoms. People with insomnia symptoms also had lower VO2peak. In Table 1, we also present the distribution of participants’ characteristics according to each symptom. In general, the associations between these characteristics and the individual insomnia symptoms were similar to the associations found using the cumulative insomnia symptoms.
Table 1.
Characteristics of the study sample according to the insomnia symptoms
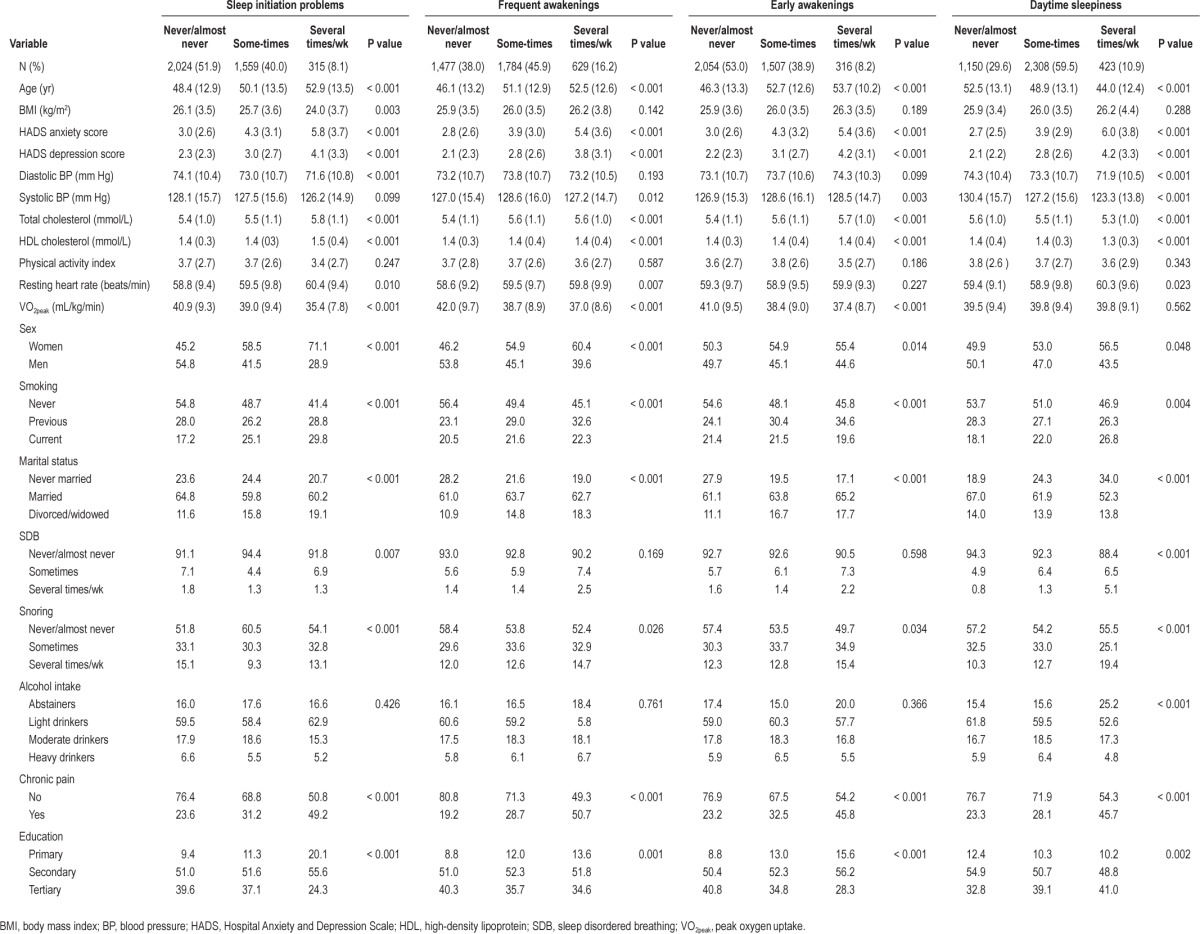
Table 2.
Characteristics of the study sample according to cumulative number of insomnia symptoms
In Model 1, with adjustment for age and sex, there was a modest inverse and graded association between having difficulties falling asleep at night and VO2peak (Table 3). After further adjustment for marital status, education, smoking, alcohol consumption, systolic and diastolic blood pressure, BMI, sleep disordered breathing, snoring, total serum cholesterol and HDL cholesterol, depression and anxiety score, and chronic pain (Model 2), the strength of the association somewhat weakened. Additional adjustment for the physical activity index and resting heart rate (Model 3) further attenuated the association. After adjustment for the potentially confounding factors in Model 2 and Model 3, there was also a graded inverse association between experiencing repeated awakenings during the night and VO2peak (Table 3). For those who reported early awakenings, VO2peak tended to be lower with increasing frequency of the symptom, but there was little statistical evidence for a linear trend.
Table 3.
Least square means and 95% confidence intervals for the insomnia symptoms and VO2peak (mL/kg/min)
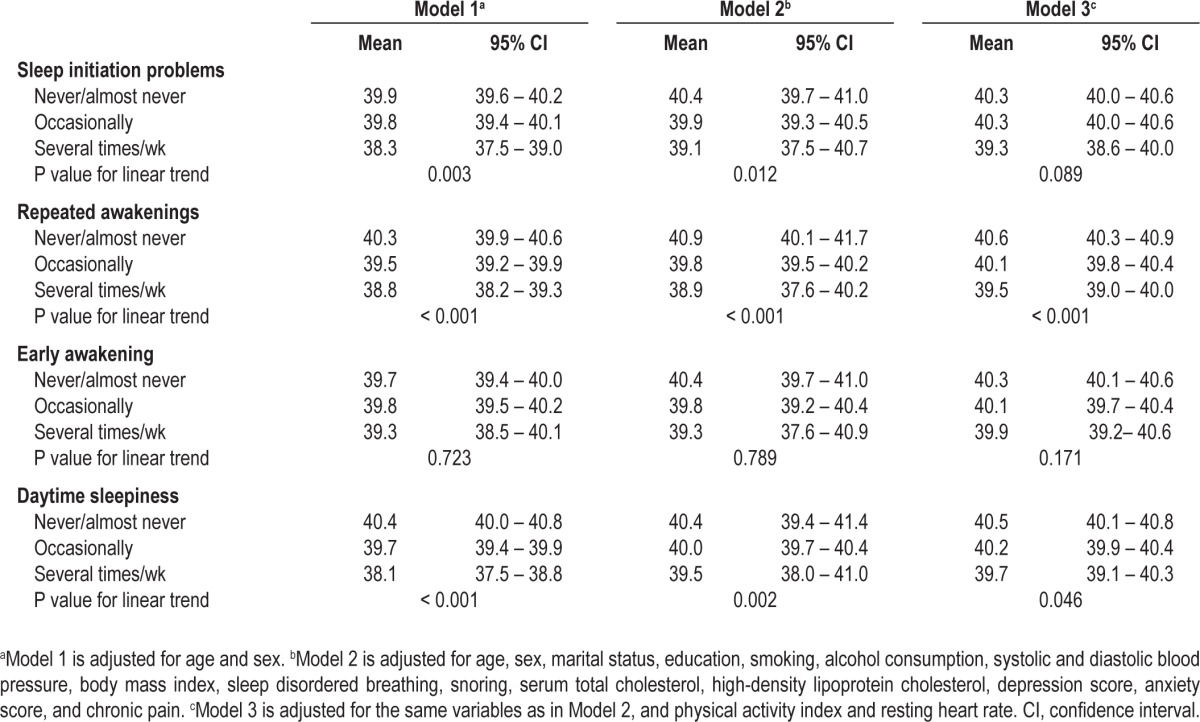
For daytime sleepiness, we found an inverse association with VO2peak that persisted after adjustment for the potential confounders in Model 2. With additional adjustment for the physical activity index and resting heart rate, the association was further attenuated.
We also used a combination of insomnia symptoms in the analysis, and found that increasing number of symptoms was inversely associated with VO2peak in a dose-dependent manner (Table 4). Model 2 and Model 3 without adjustment for BMI provided essentially the same estimates (data not shown).
Table 4.
Least square means and 95% confidence intervals for the cumulative insomnia symptoms (sleep initiation problems, repeated awakenings and early awakenings) and VO2peak (mL/kg/min)

In a separate analysis, excluding 60 participants who reportedly stopping breathing while asleep and 489 participants reporting loud snoring several times a wk, the results remained essentially unchanged in all models (see Supplementary Tables S1 and S2). Additional exclusion of participants experiencing waking up with a headache or experiencing nighttime sweating resulted in no appreciable changes. Likewise, we did not observe any considerable differences when excluding 67 participants with diabetes mellitus (Supplementary Tables S3 and S4). Undertaking the analysis on participants who answered all the insomnia questions did not change the results (Supplementary Table S5).
We found no statistical evidence for effect modification of the association between insomnia symptoms and VO2peak by sex, age, BMI, systolic blood pressure, or education, based on stratified analyses and by formal testing of possible interactions.
DISCUSSION
In this large population study, we found a modest inverse and graded association between insomnia symptoms and cardiorespiratory fitness, as indicated by peak oxygen uptake (VO2peak). To the best of our knowledge, a similar analysis, using directly measured VO2peak, has not been previously published. The association was largely independent of self-reported physical activity and it was apparent for all insomnia symptoms. By combining the symptoms in a cumulative manner, there was also an inverse dose-response association with VO2peak.
Overall, the fitness study population attained higher levels of VO2peak than participants in most of the other studies with comparable data.22–26 There are no widely accepted reference values for VO2peak, but others have reported mean sex-specific levels ranging from 32 mL/kg/min to 42 mL/kg/min in young women (i.e., age 20-29 yr) to 21 mL/kg/min to 26 mL/kg/min in older women (older than 69 yr) based on population data. Among young men, estimates have ranged from 38 mL/kg/min to 48 mL/kg/min, and to 25 mL/kg/min to 31 mL/kg/min in older men.22–26 In another study of healthy people that may be similar to that of the participants of our study, VO2peak levels were also similar to our findings.27 Thus, VO2peak was 33.0 mL/kg/min in women and 42.4 mL/kg/min in men. The difference between these levels and our study results seems to be attributable to differences in the older age groups.
Other cross-sectional population studies have assessed the association of some insomnia symptoms with self-reported physical activity and generally found an inverse association.6–8 Only two studies have evaluated insomnia in relation to cardiorespiratory fitness.10,11 Thus, in a study of 552 adolescent girls the 20-m shuttle-run test was used to estimate fitness, and girls who were classified as fit were more likely to report better sleep quality than other girls.10 In another study of 291 female college students, a 800-m run/walk test was used to estimate fitness, and there was a positive correlation between the test result and the total Pittsburg Sleep Quality Index score (Pearson correlation coefficient 0.43).11 Also, they found that daytime sleepiness was more strongly correlated with cardiorespiratory fitness (Pearson correlation coefficient 0.43) than was sleep initiation (Pearson correlation coefficient 0.24).
We found that reporting frequent awakenings several times a wk was twice as prevalent as problems with sleep initiation and early morning awakenings. Several previous studies reported similar prevalence for all insomnia symptoms.28–30 We speculate that sunlight during the night in summer and spring (due to the high latitude of Nord-Trøndelag County) could have facilitated repeated awakenings in our study population. However, some of the previous studies actually found a higher prevalence for frequent awakening than for sleep initiation or early morning awakening, which is in line with our results.31,32
Although acute sleep deprivation and insomnia are distinctly different conditions, it should be mentioned that a few small-scale experimental studies have measured VO2peak in healthy volunteers to estimate the effect of acute sleep withdrawal on cardiorespiratory fitness.33,34 The results showed that VO2peak decreased after the loss of 3 hr of sleep in seven male cyclists,33 with a gradual reduction in VO2peak with increasing sleep deprivation during a 64-hr sleep deprivation period among 12 participants.34
In addition to the direct measurement of VO2peak, we could also adjust for self-reported physical activity in the analysis of insomnia symptoms and cardiorespiratory fitness. Thus, we found that the association of insomnia with VO2peak was independent of self-reported physical activity. VO2peak measures a person’s peak aerobic capacity, which is considered the most important determinant of aerobic and endurance performance.35 Nevertheless, the association with physical activity is modest at the population level. Self-reported physical activity is more prone to misclassification than VO2peak.36 Such a misclassification can result in a weaker association with its correlates. In contrast, VO2peak is a considered to be the gold standard measurement of cardiorespiratory fitness, which is determined by physical activity and other factors including genes.37–39 Several studies have also proposed that VO2peak is more strongly associated with cardiovascular disease risk and survival than physical activity.13–15
Our results suggest that repeated awakenings were more strongly associated with VO2peak (P for linear trend < 0.001) than sleep initiation, early awakenings, and daytime sleepiness. The tendency for lower VO2peak with increasing severity of the insomnia seems clear, however, for all four insomnia symptoms (Model 3). Somewhat similar results were reported by Japanese investigators who found no clear association of early awakenings with self-reported physical activity, but an inverse association with the other symptoms.7 Nevertheless, more research is needed to establish whether different insomnia symptoms have differential associations with cardiorespiratory fitness and physical activity.
Although the nature of the association between insomnia and VO2peak remains unclear, there are several plausible underlying mechanisms for the observed inverse association. One possible explanation is that people who suffer from insomnia are less likely to engage in physical activity,40 but it is also possible that a sedentary lifestyle could lead to poorer quality of sleep.41 Moreover, insomnia may share some common risk factors with poor fitness or may affect VO2peak via physiologic hyperarousal or metabolic changes.42 Several studies have shown that insomnia is associated with increased body temperature,43 increased heart rate,44 increased metabolic rate,45 and increased activity of the hypothalamic-pituitary-adrenal (HPA) axis,46 all of which also are associated with physical inactivity in a similar fashion.47 More recently it has also been reported that insomnia is associated with cardiovascular risk factors such as hypertension or diabetes,48–50 both of which are correlates of cardiorespiratory fitness.51,52
Women are more prone to insomnia than men,2 and there is a documented sex difference in peak oxygen uptake.53,54 However, our study and previous studies have failed to provide evidence for any sex difference related to the association of insomnia symptoms with physical activity or cardiorespiratory fitness.6,7
Limitations
Despite the relatively large sample size, the population-based design, and the use of directly measured VO2peak, the current study also has some limitations.
Most importantly, this study may be subject to bias because of self-selection due to the low participation rate (44.6%). However, almost all of those who were invited to the current study from the large HUNT study agreed to participate in the fitness test. Due to limited capacity at the test sites resulting in long lines, many potential participants chose to withdraw their participation from the study. Those who finally participated in the study could be healthier than those who quit or declined participation. A comparison of the participants in the fitness study with a healthy sample of the total HUNT population (i.e., free from cardiovascular or pulmonary diseases, cancer, or sarcoidosis) confirmed that the fitness participants did not considerably differ from other healthy HUNT participants although they had slightly lower mean weight (69.8 kg compared with 71.5 kg in women, and 85.6 kg compared with 86.2 kg in men), and BMI (25.4 compared with 26.1 in women, and 26.6 compared with 27.0 in men). In female fitness study participants, mean waist-to-hip ratio was 0.85 compared with 0.86, systolic blood pressure was 123.5 mm Hg compared with 124.3 mm Hg, diastolic blood pressure was 69.7 mm Hg compared with 69.8 mm Hg, and cholesterol levels was 5.44 mmol/L compared with 5.52 mmol/L in the healthy HUNT population. In men, mean waist-to-hip ratio was 0.92 in both groups, whereas mean systolic and diastolic blood pressure was 131.9 mm Hg and 76.2 mm Hg in the fitness study population compared with 131.4 mm Hg and 75.4 mm Hg in the healthy HUNT population. Cholesterol levels were 5.48 mmol/L and 5.50 mmol/L in the two groups, respectively.55
The response rate to the insomnia questions was 83.4%, which largely reflects the overall response rate to the questionnaire with the insomnia items. Thus, a nonresponse bias specifically caused by avoidance of questions targeting insomnia is not likely in the current study.
The cross-sectional study design did now allow us to assess the direction of the association of insomnia with cardiorespiratory fitness. Although insomnia may also share some determinants with cardiorespiratory fitness, we adjusted for a large set of covariates in the analysis, and the association of insomnia symptoms with VO2peak was largely independent of these covariates.
This study is based on self-reported measures of sleep, and validation with objective measures (i.e., by polysomnography) was not available. However, insomnia is not routinely evaluated by polysomnography,56 and the condition is typically defined as “a self-reported condition which includes having difficulties falling asleep, or remaining asleep.”1,2 Thus, insomnia may be present even if a polysomnographic evaluation shows no sign of it.
Self-report is always prone to individual interpretation when no anchors are given and this approach does not allow quantitative or objective assessment of the degree of insomnia. However, there might also be some disadvantages with anchored questions. Insomnia is a subjective feeling and the same sleep problem might be perceived differently by different individuals. For example, lying awake in bed for 20 min might be a significant problem for some, whereas others might not be bothered by this. In contrast, our approach reflects the degree of subjective problems perceived by the individual.
Hormone replacement therapy has well-known positive effects on insomnia.57 Regrettably, we did not have information on this variable in our study. We have, however, adjusted for strong correlates of hormonal replacement therapy such as age and socio-demographic characteristics and therefore our multiadjusted estimates are not likely to be substantially confounded by the use of hormone replacement therapy among women entering menopause.
We also did not have information about ethnicity in our study. However, Nord-Trøndelag County in Norway has a very homogenous population with fewer than 3% non-Caucasians.58
Our study was performed in an apparently healthy, socioeconomically homogenous population and our results cannot be directly generalized to less healthy populations or to countries on different latitudes, with a different socioeconomic status, or with different sleeping habits.
CONCLUSION
We found a modest inverse association of insomnia symptoms with VO2peak, which is the gold standard measure of cardiorespiratory fitness, and the association was independent of self-reported physical activity. VO2peak is an important determinant of cardiovascular disease,16 and our findings may therefore be important in the complex interrelation between sleep and the cardiovascular system. Insomnia is a prevalent and manageable condition, and increased focus on adverse health effects of insomnia could be helpful in the prevention of cardiovascular disease. Future studies with a prospective design are needed to confirm our findings and to establish the direction of the relation of insomnia symptoms with cardiorespiratory fitness.
ACKNOWLEDGMENTS
Linn B. Strand received a research fellowship grant from the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology. Dr. Janszky is supported by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, by the Swedish Research Council and by the Swedish Council of Working Life and Social Research. Bjarne Nes is supported by grants from the K.G. Jebsen Foundation, Norwegian Council of Cardiovascular Disease and Professor Wisløff is supported by the Norwegian Research Council Funding for Outstanding Young Investigators and the K.G. Jebsen Foundation, Norwegian Council of Cardiovascular Disease. The Nord-Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord-Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health.
Footnotes
A commentary on this article appears in this issue on page 11.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Least square means and 95% confidence intervals for the insomnia symptoms and VO2peak (mL/kg/min) when excluding participants reporting sleep disordered breathing and snoring several times per wk
Least square means and 95% confidence intervals for the cumulative insomnia symptoms and VO2peak (mL/kg/min) when excluding participants reporting sleep disordered breathing and snoring several times per wk
Least square means and 95% confidence intervals for the insomnia symptoms and VO2peak (mL/kg/min) when excluding participants with diabetes
Least square means and 95% confidence intervals for the cumulative insomnia symptoms and VO2peak (mL/kg/min) when excluding participants with diabetes
Least square means and 95% confidence intervals for the insomnia symptoms and VO2peak (mL/kg/min) when excluding participants that did not respond to all the insomnia questions
REFERENCES
- 1.American Psychiatric Association. Arlington, the United States: 2004. Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV) [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Elwood P, Hack M, Pickering J, Hughes J, Gallacher J. Sleep disturbance, stroke, and heart disease events: evidence from the Caerphilly cohort. J Epidemiol Commun H. 2006;60:69–73. doi: 10.1136/jech.2005.039057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction. Circulation. 2011;124:2073–81. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 6.Sherrill DL, Kotchou K, Quan SF. Association of physical activity and human sleep disorders. Arch Intern Med. 1998;158:1894–8. doi: 10.1001/archinte.158.17.1894. [DOI] [PubMed] [Google Scholar]
- 7.Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000;23:41. [PubMed] [Google Scholar]
- 8.Ohida T, Kamal A, Uchiyama M, et al. The influence of lifestyle and health status factors on sleep loss among the Japanese general population. Sleep. 2001;24:333. doi: 10.1093/sleep/24.3.333. [DOI] [PubMed] [Google Scholar]
- 9.Ortega FB, Chillon P, Ruiz JR, et al. Sleep patterns in Spanish adolescents: associations with TV watching and leisure-time physical activity. Eur J Appl Physiol. 2010;110:563–73. doi: 10.1007/s00421-010-1536-1. [DOI] [PubMed] [Google Scholar]
- 10.Mota J, Vale S. Associations between sleep quality with cardiorespiratory fitness and BMI among adolescent girls. Am J Hum Biol. 2010;22:473–5. doi: 10.1002/ajhb.21019. [DOI] [PubMed] [Google Scholar]
- 11.Lee A, Lin W. Association between sleep quality and physical fitness in female young adults. J Sports Med Phys Fitness. 2007;47:462. [PubMed] [Google Scholar]
- 12.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA. 2009;301:2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 13.Blair S, Kampert J, Kohl H, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:5. [PubMed] [Google Scholar]
- 14.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33:754–61. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D-C, Sui X, Ortega FB, et al. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Brit J Sport Med. 2010 doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- 16.Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA. 2005;294:2981–8. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]
- 17.Kurtze N, Rangul V, Hustvedt B-E, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study — HUNT 1. Scand J Public Health. 2008;36:52–61. doi: 10.1177/1403494807085373. [DOI] [PubMed] [Google Scholar]
- 18.Janszky I, Ljung R, Ahnve S, Hallqvist J, Bennet AM, Mukamal KJ. Alcohol and long-term prognosis after a first acute myocardial infarction: the SHEEP study. European heart journal. 2008;29:45–53. doi: 10.1093/eurheartj/ehm509. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond A, Snaith R. The HADS: Hospital Anxiety and Depression Scale. Windsor: NFER Nelson, ; 1994. [Google Scholar]
- 20.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 21.Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Brit J Psychiat. 2001;179:540. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Talbot LA, Metter EJ, Fleg JL. Leisure-time physical activities and their relationship to cardiorespiratory fitness in healthy men and women 18-95 years old. Med Sci Sports Exerc. 2000;32:417. doi: 10.1097/00005768-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Shvartz E, Reibold R. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviation, space, and environmental medicine. 1990;61:3. [PubMed] [Google Scholar]
- 24.Jackson AS, Wier LT, Ayers GW, Beard EF, Stuteville JE, Blair SN. Changes in aerobic power of women, ages 20-64 yr. Med Sci Sports Exerc. 1996;28:884. doi: 10.1097/00005768-199607000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol. 1997;83:160–5. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 26.Inbar O, Oren A, Scheinowitz M, Rotstein A, Dlin R, Casaburi R. Normal cardiopulmonary responses during incremental exercise in 20-to 70-yr-old men. Med Sci Sports Exerc. 1994;26:538. [PubMed] [Google Scholar]
- 27.Matthews C, Heil D, Freedson P, Pastides H. Classification of cardiorespiratory fitness without exercise testing. Med Sci Sports Exerc. 1999;31:486–8. doi: 10.1097/00005768-199903000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12 778 adults in France. J Sleep Res. 2000;9:35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 29.Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Medicine. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS) Sleep. 2011;34:997. doi: 10.5665/SLEEP.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohayon MM, Hong S-C. Prevalence of insomnia and associated factors in South Korea. J Psychosom Res. 2002;53:593–600. doi: 10.1016/s0022-3999(02)00449-x. [DOI] [PubMed] [Google Scholar]
- 32.Ohayon MM, Sagales T. Prevalence of insomnia and sleep characteristics in the general population of Spain. Sleep Medicine. 2010;11:1010–8. doi: 10.1016/j.sleep.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Mougin F, Simon-Rigaud M, Davenne D, et al. Effects of sleep disturbances on subsequent physical performance. Eur J Appl Physiol. 1991;63:77–82. doi: 10.1007/BF00235173. [DOI] [PubMed] [Google Scholar]
- 34.Plyley M, Shephard R, Davis G, Goode R. Sleep deprivation and cardiorespiratory function. Eur J Appl Physiol. 1987;56:338–44. doi: 10.1007/BF00690902. [DOI] [PubMed] [Google Scholar]
- 35.Vanhees L, Lefevre J, Philippaerts R, et al. How to assess physical activity? How to assess physical fitness? J Cardiovasc Risk. 2005;12:102–14. doi: 10.1097/01.hjr.0000161551.73095.9c. [DOI] [PubMed] [Google Scholar]
- 36.van Poppel MNM, Chinapaw MJM, Mokkink LB, Willem van M, Terwee CB. Physical Activity Questionnaires for Adults: A Systematic Review of Measurement Properties. Sports Med. 2010;40:565–600. doi: 10.2165/11531930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Jackson AS, Sui X, Hebert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169:1781. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laukkanen JA, Laaksonen D, Lakka TA, et al. Determinants of cardiorespiratory fitness in men aged 42 to 60 years with and without cardiovascular disease. The American journal of cardiology. 2009;103:1598–604. doi: 10.1016/j.amjcard.2009.01.371. [DOI] [PubMed] [Google Scholar]
- 39.Radom-Aizik S, Hayek S, Shahar I, Rechavi G, Kaminski N, Ben-Dov I. Effects of aerobic training on gene expression in skeletal muscle of elderly men. Med Sci Sports Exerc. 2005;37:1680–96. doi: 10.1249/01.mss.0000181838.96815.4d. [DOI] [PubMed] [Google Scholar]
- 40.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006;91:881–4. doi: 10.1136/adc.2005.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foti KE, Eaton DK, Lowry R, McKnight-Ely LR. Sufficient sleep, physical activity, and sedentary behaviors. Am J Prev Med. 2011;41:596–602. doi: 10.1016/j.amepre.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Lushington K, Dawson D, Lack L. Core body temperature is elevated during constant wakefulness in elderly poor sleepers. Sleep. 2000;23:504. [PubMed] [Google Scholar]
- 44.Stepanski E, Glinn M, Zorick F, Roehrs T, Roth T. Heart rate changes in chronic insomnia. Stress Medicine. 1994;10:261–6. [Google Scholar]
- 45.Radomski MW, Hart LE, Goodman JM, Plyley MJ. Aerobic fitness and hormonal responses to prolonged sleep deprivation and sustained mental work. Aviation, space, and environmental medicine. 1992;63:101–6. [PubMed] [Google Scholar]
- 46.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 47.Tsatsoulis A, Fountoulakis S. The Protective Role of Exercise on Stress System Dysregulation and Comorbidities. Ann N Y Acad Sci. 2006;1083:196–213. doi: 10.1196/annals.1367.020. [DOI] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep durationis associated with type 2 Diabetes. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansson JK, Kronholm E, Jula AM. Variability in home-measured blood pressure and heart rate: associations with self-reported insomnia and sleep duration. J Hypertens. 2011;29:1897–905. doi: 10.1097/HJH.0b013e32834abccd. [DOI] [PubMed] [Google Scholar]
- 51.Barlow CE, LaMonte MJ, FitzGerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory Fitness Is an Independent Predictor of Hypertension Incidence among Initially Normotensive Healthy Women. Am J Epidemiol. 2006;163:142–50. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 52.Sawada SS, Lee I-M, Muto T, Matuszaki K, Blair SN. Cardiorespiratory Fitness and the Incidence of Type 2 Diabetes. Diabetes Care. 2003;26:2918–22. doi: 10.2337/diacare.26.10.2918. [DOI] [PubMed] [Google Scholar]
- 53.Rowland T, Goff D, Martel L, Ferrone L. Influence of cardiac functional capacity on gender differences in maximal oxygen uptake in children. Chest. 2000;117:629–35. doi: 10.1378/chest.117.3.629. [DOI] [PubMed] [Google Scholar]
- 54.Winsley RJ, Fulford J, Roberts AC, Welsman JR, Armstrong N. Sex difference in peak oxygen uptake in prepubertal children. J Sci Med Sport. 2009;12:647–51. doi: 10.1016/j.jsams.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Aspenes ST, Nilsen TIL, Skaug E, et al. Peak oxygen uptake and cardiovascular risk factors in 4631 healthy women and men. Med Sci Sports Exerc. 2011;43:1465–73. doi: 10.1249/MSS.0b013e31820ca81c. [DOI] [PubMed] [Google Scholar]
- 56.Littner M, Hirshkowitz M, Kramer M, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–60. doi: 10.1093/sleep/26.6.754. [DOI] [PubMed] [Google Scholar]
- 57.Saletu-Zyhlarz G, Anderer P, Gruber G, et al. Insomnia related to postmenopausal syndrome and hormone replacement therapy: sleep laboratory studies on baseline differences between patients and controls and double-blind, placebo-controlled investigations on the effects of a novel estrogen-progestogen combination (Climodien®, Lafamme®) versus estrogen alone. Journal of Sleep Research. 2003;12:239–54. doi: 10.1046/j.1365-2869.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- 58.Holmen J, Midthjell K, Kr̈uger Ø, et al. The Nord-Trøndelag Health Study 1995-97 (HUNT 2): objectives, contents, methods and participation. Norsk epidemiologi. 2003;13:19–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Least square means and 95% confidence intervals for the insomnia symptoms and VO2peak (mL/kg/min) when excluding participants reporting sleep disordered breathing and snoring several times per wk
Least square means and 95% confidence intervals for the cumulative insomnia symptoms and VO2peak (mL/kg/min) when excluding participants reporting sleep disordered breathing and snoring several times per wk
Least square means and 95% confidence intervals for the insomnia symptoms and VO2peak (mL/kg/min) when excluding participants with diabetes
Least square means and 95% confidence intervals for the cumulative insomnia symptoms and VO2peak (mL/kg/min) when excluding participants with diabetes
Least square means and 95% confidence intervals for the insomnia symptoms and VO2peak (mL/kg/min) when excluding participants that did not respond to all the insomnia questions