Abstract
Objective
The outcome of traumatic brain injury (TBI) is impaired by hypotension and glutamate, and TBI associated release of endogenous tissue plasminogen activator (tPA) impairs cerebral autoregulation. Glucagon decreases CNS glutamate, lessens neuronal cell injury and improves neurological score in mice after TBI. Glucagon partially protects against impaired cerebrovasodilation during hypotension after TBI in piglets by upregulating cAMP which decreases release of tPA. Pial artery dilation during hypotension is due to release of cAMP dependent dilator prostaglandins (PG), such as PGE2 and PGI2. TBI impairs PGE2 and PGI2 mediated pial artery dilation, which contributes to disturbed cerebral autoregulation post insult, by upregulating mitogen activated protein kinase (MAPK). This study was designed to investigate relationships between tPA, prostaglandins, and MAPK as a mechanism to improve the efficacy of glucagon-mediated preservation of cerebrovasodilation during hypotension after TBI.
Methods
Lateral FPI was induced in piglets equipped with a closed cranial window. ERK and JNK MAPK concentrations in CSF were quantified by ELISA.
Results
CSF JNK MAPK was increased by FPI, but blunted by glucagon and the novel plasminogen activator inhibitor-1 derived peptide (PAI-1DP), Ac-RMAPEEIIMDRPFLYVVR-amide. FPI modestly increased, while glucagon and PAI-1DP decreased ERK MAPK. PGE2, PGI2, and hypotension induced pial artery dilation was blunted after FPI, partially protected by glucagon, and fully protected by glucagon + PAI-1DP, glucagon + JNK antagonist SP600125 or glucagon + ERK inhibitor U 0126.
Discussion
Glucagon + PAI-1DP act in concert to protect against impairment of cerebrovasodilation during hypotension after TBI via inhibition of ERK and JNK MAPK.
Keywords: newborn, cerebral circulation, TBI, plasminogen activators, signal transduction
Introduction
Traumatic brain injury (TBI) is a leading cause of death and morbidity in the US. While damage occurs from the primary insult, secondary injury that results from the release of a myriad of substances, such as excitatory amino acids, including glutamate, activated oxygen species, neurohormones, signaling molecules, and others are thought to play a key role in the ultimate outcome. Additional risk factors further exacerbate secondary brain damage, including hypotension, hypoxia, increased intracranial pressure, and hyperglycemia. Thus, intervention that mitigates these secondary pathways are important approaches to limit neurologic diability.
Glutamate can bind to any of three ionotropic receptor subtypes named after synthetic analogues: N-methyl-D-aspartate (NMDA), kainate, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA). Activation of NMDA receptors elicits cerebrovasodilation and might represent one mechanism by which local metabolism is coupled to blood flow1. All glutamate receptors have been implicated in neurotoxicity to some extent. However, the NMDA subtype is thought to play a crucial role in excitotoxic neuronal cell death2. Glutamatergic system hyperactivity has been demonstrated in animal models of TBI, while NMDA receptor antagonists have been shown to protect against TBI3,4. Although cerebral hemodynamics is thought to contribute to neurologic outcome, little attention has been given to the role played by NMDA-mediated alterations in vascular activity. We have observed that vasodilation in response to NMDA receptor activation is reversed to vasconstriction after fluid percussion brain injury (FPI) in the piglet5.
Glutamate release and activation of the NMDA receptor have long been recognized as key contributors to negative outcome after TBI. NMDA antagonists such as MK801 improve outcome after TBI in animal models. However, toxicity of NMDA antagonists is limiting in translating this approach to humans, though another NMDA antagonist, mementine, has shown some promise. Therefore, although the key role of excitotoxicity in outcome of TBI is widely appreciated, use of NMDA antagonists for treatment has not been successful to date.
Tissue plasminogen activator (tPA) can enhance excitotoxic neuronal cell death through interactions with the NMDA receptor by causing excessive increases in intracellular calcium, leading to apoptosis and necrosis6. However, engaging NMDA receptors may activate additional and reversible pathways that eventuate in neurotoxicity if left unchecked. In the context of the neurovascular unit, for example, impaired cerebral hemodynamics is thought to contribute to neuronal cell necrosis. tPA upregulation contributes to impaired cerebral hemodynamics, including disturbed cerebral autoregulation during hypotension, and cell damage after FPI7–9. tPA contributes to impaired NMDA mediated cerebrovasodilation via upregulation of mitogen activated protein kinase (MAPK)10, a family of at least 3 kinases (ERK, p38, and JNK) that are critically important in regulating hemodynamics after TBI8. EEIIMD, a peptide derived from the endogenous plasminogen activator inhibitor-1 (PAI-1), inhibits PA mediated vascular activity without compromising its catalytic function11,12 and also prevents impairment of NMDA receptor mediated vasodilation after FPI5.
Release of excitatory amino acids such as glutamate and activation of the NMDA receptor also contribute to impaired cerebral autoregulation13. Recent approaches to limit elevation of glutamate after TBI in the mouse and pig using glucagon post insult prevent brain tissue damage and partially preserves autoregulation by elevating cAMP, which blunts tPA upregulation9,14. Based on these studies, we posit that glutamate and tPA act in concert to induce neurotoxicity. In absence of tPA (tPA null mice), even high levels of CNS glutamate occurring after brain injury are only weakly neurotoxic. In addition, exogenous tPA is not neurotoxic when glutamate levels are kept low. Based on this, we propose that tPA and glutamate create a vicious cycle wherein tPA increases the toxicity of glutamate by increasing the sensitivity of NMDA receptors to tPA and glutamate increases the neurotoxicity of tPA by signal transduction through NMDA receptors that have been activated by tPA10. Furthermore, neurotoxicity induced by tPA increases CSF levels of glutamate14 and neurotoxicity induced by glutamate increases the levels of tPA9, which further exacerbates injury. The corollary of this proposed feed forward cycle is that preventing activation of NMDA receptors by tPA will decrease the toxicity of glutamate as well. Since glucagon only partially protects against impairment of cerebral autoregulation during hypotension, we posit that co-administration of an inhibitor of tPA mediated signaling with improve its efficacy.
Pial artery dilation during hypotension is mediated by release of cAMP dependent dilator prostaglandins (PG), such as PGE2 and PGI215. TBI impairs PGE2 and PGI2 mediated pial artery dilation16, which contributes to disturbed cerebral autoregulation post insult. This study was designed to investigate relationships between tPA, prostaglandins, and MAPK as a mechanism for enhancing glucagon-mediated protection of cerebrovasodilation during hypotension after TBI.
Materials and Methods
Closed cranial window and brain injury procedures
Newborn pigs (1–5 days old, 1.2–1.6 Kg) of either sex were studied. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Animals were sedated with isoflurane (1–2 MAC). Anesthesia was maintained with a-chloralose (30–50 mg/kg. supplemented with 5 mg/kg/h i.v.). A catheter was inserted into a femoral artery to monitor blood pressure and to sample for blood gas tensions and pH. Drugs to maintain anesthesia were administered through a second catheter placed in a femoral vein. The trachea was cannulated, and the animals were ventilated with room air. A heating pad was used to maintain the animals at 37° – 39° C, monitored rectally.
A cranial window was placed in the parietal skull of these anesthetized animals. This window consisted of three parts: a stainless steel ring, a circular glass coverslip, and three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution, similar to CSF, of the following composition (in mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3. This artificial CSF was warmed to 37° C and had the following chemistry: pH 7.33, pco2 46 mm Hg, and po2 43 mm Hg, which was similar to that of endogenous CSF. Pial arterial vessel diameter was measured with a microscope, a camera, a video output screen and a video microscaler.
Methods for brain FPI have been described previously5,8. A device designed by the Medical College of Virginia was used. A small opening was made in the parietal skull contralateral to the cranial window. A metal shaft was sealed into the opening on top of intact dura. This shaft was connected to the transducer housing, which was in turn connected to the fluid percussion device. The device itself consisted of an acrylic plastic cylindrical reservoir 60 cm long, 4.5 cm in diameter, and 0.5 cm thick. One end of the device was connected to the transducer housing, while the other end had an acrylic plastic piston mounted on O-rings. The exposed end of the piston was covered with a rubber pad. The entire system was filled with 0.9 % saline. The percussion device was supported by two brackets mounted on a platform. FPI was induced by striking the piston with a 4.8 kg pendulum. The intensity of the injury (usually 1.9–2.3 atm. with a constant duration of 19–23 ms) was controlled by varying the height from which the pendulum was allowed to fall. The pressure pulse of the injury was recorded on a storage oscilloscope triggered photoelectrically by the fall of the pendulum. The amplitude of the pressure pulse was used to determine the intensity of the injury.
Protocol
Pial small arteries (resting diameter, 120–160 μm) were examined. Typically, 2–3 ml of artificial CSF were flushed through the window over a 30s period, and excess CSF was allowed to run off through one of the needle ports. For sample collection, 300 μl from the total cranial window volume of 500 μl was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side.
Nine experimental groups were studied (all n=5): (1) sham control, treated with vehicle (2) FPI, vehicle treated, (3) FPI treated with glucagon (25 μg/kg iv) (4) FPI, treated with glucagon and the novel plasminogen activator inhibitor-1 derived peptide (PAI-1DP), Ac-RMAPEEIIMDRPFLYVVR-amide (1 mg/kg iv), (5) FPI treated with glucagon and the JNK antagonist SP 600125 (1 mg/kg iv), (6) FPI treated with glucagon and the ERK antagonist U 0126 (1 mg/kg iv), (7) FPI treated with the PAI-1DP (8) FPI treated with SP 600125, and (9) FPI treated with U 0126. Hypotension was induced by the rapid withdrawal of either 5–8 or 10–15 ml blood/Kg to induce moderate or severe hypotension (decreases in mean arterial blood pressure of 25 and 45%, respectively)9. Decreases in blood pressure were maintained constant for 10 min by titration of additional blood withdrawal or blood reinfusion9. The vehicle for all agents was 0.9% saline, except for U 0126 and SP 600125, which used dimethyl sulfoxide (100 μl) diluted with 9.9 ml 0.9% saline. In sham control and FPI-vehicle animals, vascular responses to hypotension, NMDA, papaverine (10−8, 10−6 M), PGE2, and PGI2, (1, 10 ng/ml) were obtained initially and 60 min later in the presence of the agent vehicle. In drug treated animals, agents were administered 30 min post injury and vascular responses to vasodilators were obtained 60 min after FPI.
ELISA
Commercially available ELISA Kits were used to quantify total and phosphorylated isoforms of CSF ERK and JNK MAPK (Assay Designs, Ann Arbor, MI) concentration. Phosphorylated MAPK isoform enzyme values were normalized to total form and then expressed as percent of the control condition.
Statistical analysis
Pial artery diameter and CSF MAPK isoform values were analyzed using ANOVA for repeated measures. If the value was significant, the data were then analyzed by Fishers protected least significant difference test. An α level of p<0.05 was considered significant in all statistical tests. Values are represented as mean ± SEM of the absolute value or as percentage changes from control value.
Results
Combination of glucagon and PAI-1DP blocks elevation of CSF ERK and JNK MAPK after FPI
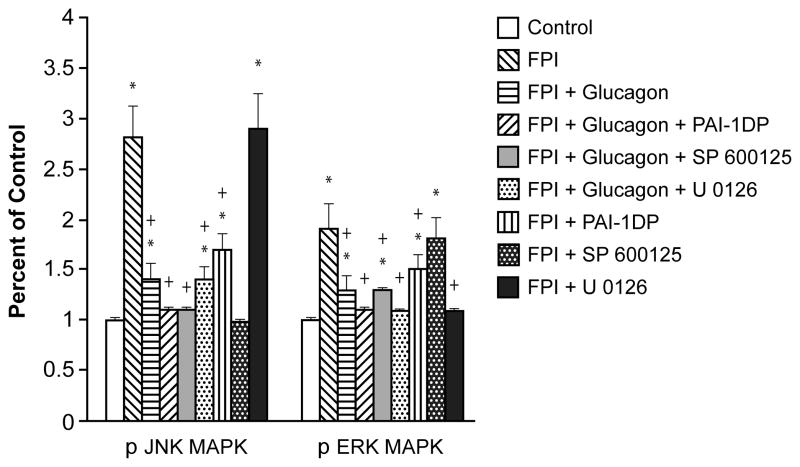
CSF concentrations of the two MAPK isoforms increased within 1h of FPI, the relative order of magnitude being JNK > ERK (Fig. 1). Glucagon (25 μg/kg iv), blunted the increase in JNK and ERK after FPI, but the combination of glucagon + PAI-1DP blocked such increases (Fig 1). Similarly, combining glucagon + SP 600125 blocked upregulation of JNK after FPI, while CSF ERK was unchanged (Fig 1). Combining glucagon + U 0126 similarly blocked increases in CSF ERK while JNK was unchanged after FPI. Finally, PAI-1DP alone blunted increases in CSF JNK and ERK. Cross selectivity experiments showed that SP 600125 blocked JNK with elevated ERK left unchanged and U 0126 blocked ERK with no change in the elevation of JNK after FPI (Fig 1). We next investigated the functional significance of the inhibition of JNK and ERK MAPK release by combined administration of glucagon + PAI-1DP on stimulus induced cerebrovasodilation after FPI.
Figure 1.
Phosphorylation of JNK and ERK MAPK in cortical periarachnoid CSF prior to FPI (Control) and 1h after FPI in vehicle, glucagon (25 μg/kg iv), glucagon + PAI-1DP, glucagon + SP 600125, glucagon + U 0126, PAI-1DP, SP 600125, and U 0126 (all 1 mg/kg iv) post-injury treated animals, n=5. Data expressed as percent of control by ELISA determination of phospho MAPK and total MAPK isoforms and subsequent normalization to total form. *p<0.05 compared with corresponding Control value +p<0.05 compared with corresponding FPI vehicle treated value.
Combination of glucagon and PAI-1DP preserves cerebrovasodilation mediated by NMDA receptor activation and hypotension
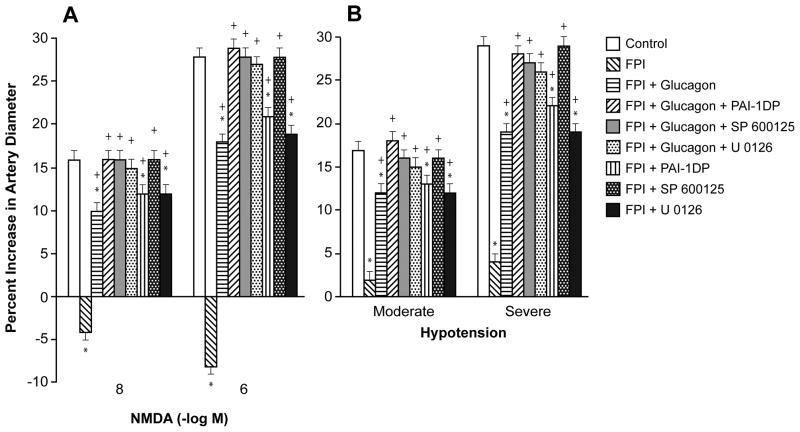
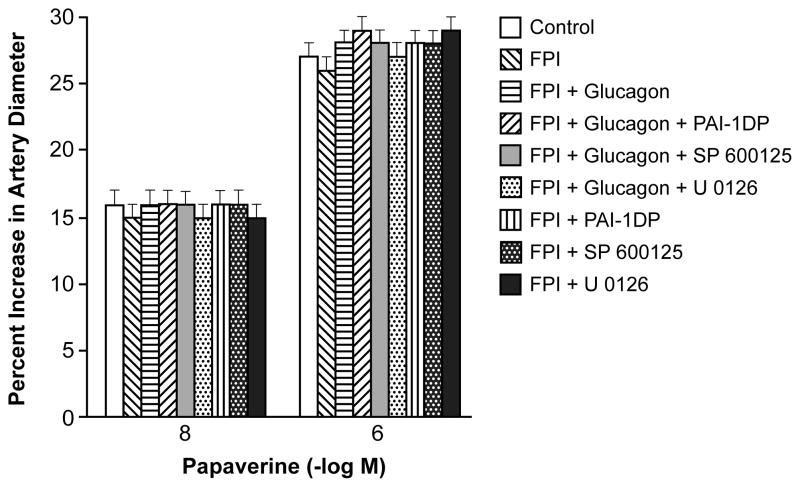
NMDA (10−8, 10−6 M) and hypotension (moderate and severe, 25 and 45 % decreased in mean arterial blood pressure, respectively) elicited reproducible pial artery dilation under sham control conditions (data not shown). Pial artery dilation in response to NMDA was reversed to vasoconstriction (Fig 2A), whereas dilation in response to hypotension was diminished after FPI (Fig 2B). tPA is upregulated after FPI and potentiates the reversal of NMDA receptor mediated dilation to constriction10, whereas the PAI-1 derived peptide EEIIMD re-reversed NMDA mediation vasoconstriction to vasodilation after FPI5. In this study, glucagon also re-reversed NMDA mediated vasoconstriction to vasodilation after FPI (Fig 2A) and partially protected pial artery dilation during hypotension (Fig 2B). Again, combined treatment with glucagon + PAI-1DP fully preserved cerebrovasodilation mediated by NMDA receptor activation and hypotension (Fig 2A). Similarly, combined treatment with glucagon + SP 600125 or glucagon + U 0126 also fully preserved NMDA- and hypotension-mediated cerebrovasodilation (Fig 2A, B). PAI-1DP and U 0126 by themselves provided only partial protection, but SP 600125 fully protected vasodilation induced by NMDA and hypotension (Fig 2A, B). Papaverine-induced pial artery vasodilation was unchanged by FPI and by the administration of glucagon or other agents (Fig 3).
Figure 2.
Influence of NMDA (Panel A) (10−8, 10−6 M) and hypotension (Panel B) (moderate, severe) on pial artery diameter before (control) and 1h after FPI in vehicle, glucagon (25 μg/kg iv), glucagon + PAI-1DP, glucagon + SP 600125, glucagon + U 0126, PAI-1DP, SP 600125, and U 0126 (all 1 mg/kg iv) post-injury treated animals, n=5. *p<0.05 compared with corresponding Control value +p<0.05 compared with corresponding FPI vehicle treated value.
Figure 3.
Influence of papaverine (10−8, 10−6 M) on pial artery diameter before (control) and 1h after FPI in vehicle, glucagon (25 μg/kg iv), glucagon + PAI-1DP, glucagon + SP 600125, glucagon + U 0126, PAI-1DP, SP 600125, and U 0126 (all 1 mg/kg iv) post-injury treated animals, n=5.
Combination of glucagon and PAI-1DP preserves cerebrovasodilation mediated by prostaglandins
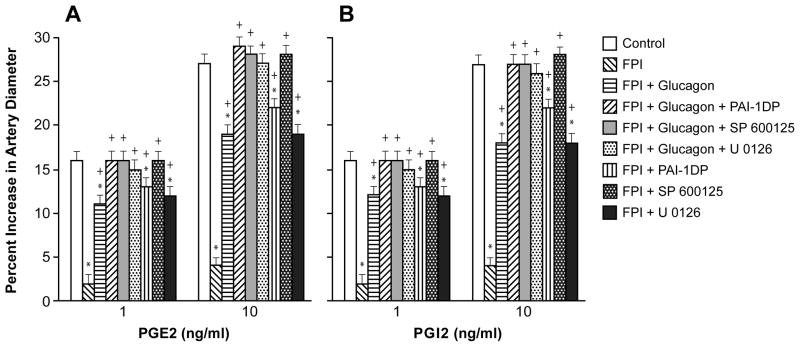
Pial artery dilation during hypotension, in part, is due to release of prostaglandins (PG), such as PGE2 and PGI215, which elicit cerebrovasodilation via cAMP. FPI impairs PGE2 and PGI2 mediated pial artery dilation16, which probably contributes to disturbed cerebral autoregulation after TBI. The relationship between NMDA and tPA in the context of impaired PG mediated vasodilation after TBI has not been explored to our knowledge. In particular, we were interested in the ability of glucagon to decrease CNS glutamate, prevent the reduction in cAMP and thereby prevent tPA upregulation after FPI and we wished to investigate how the latter affects vascular responsiveness to PGs after FPI. In this study, PGE2 and PGI2 induced pial artery dilation was blunted after FPI, partially protected by glucagon, but fully protected by co-administration of glucagon + PAI-1DP (Fig 4). Similar to what was observed with NMDA-receptor and hypotension-induced pial artery responses, co-administered glucagon + SP 600125 and glucagon + U 0126 fully protected PGE2 and PGI2 mediated cerebrovasodilation (Fig 4). Also similar to these observations involving NMDAR and hypotension, PGE2 and PGI2 induced pial artery vasodilation was only partially protected by PAI-1DP or U 0126, but fully protected by SP 600125 administered by themselves (Fig 4).
Figure 4.
Influence of PGE2 (Panel A) and PGI2 (1, 10 ng/ml) on pial artery diameter before (control) and 1h after FPI in vehicle, glucagon (25 μg/kg iv), glucagon + PAI-1DP, glucagon + SP 600125, glucagon + U 0126, PAI-1DP, SP 600125, and U 0126 (all 1 mg/kg iv) post-injury treated animals, n=5. *p<0.05 compared with corresponding Control value +p<0.05 compared with corresponding FPI vehicle treated value.
Glucagon, PAI-1DP, and MAPK isoform antagonist effects on pial artery diameter in sham control piglets
Administration of glucagon, PAI-1DP, SP 600125, U 0126, or combinations of the latter with glucagon had no effect on pial artery diameter. These drugs also had no effect on vascular responses to NMDA, hypotension, PGE2, or PGI2 in the absence of FPI.
Blood chemistry
There were no statistical differences in blood chemistry between sham control, FPI, and FPI antagonist treated animals before or after all experiments. For example, values for pH, pCO2, and pO2 were 7.45 ± 0.02, 36 ± 5, and 95 ± 10 vs 7.43 ± 0.03, 37 ± 4, and 85 ± 10 vs 7.43 ± 0.02, 35 ± 7, and 88 ± 9 mm Hg for sham control, FPI, and FPI + SP 600125 treated animals, n=5, respectively. The amplitude of the pressure pulse, used as an index of injury intensity, was equivalent in FPI-vehicle and FPI-antagonist animals (1.9 ± 0.1 atm).
Discussion
Several key new findings emerged from this study. First, it was observed that the combination of glucagon + PAI-1DP given after FPI fully prevented impairment of NMDA receptor and hypotension mediated pial artery vasodilation. Cerebrovasodilation induced by NMDA receptor activation that had been reversed to vasoconstriction by FPI was restored to vasodilation after FPI, but similar to hypotension, not fully protected by glucagon alone9. Glucagon administered post insult lowers CNS glutamate concentration, prevents brain tissue damage, and partially preserves autoregulation by elevating cAMP, which blunts tPA upregulation9,14. Based on these studies, we proposed that tPA and glutamate create a vicious cycle wherein tPA increases the toxicity of glutamate by increasing the sensitivity of NMDA receptors to tPA and glutamate increases the neurotoxicity of tPA by signal transduction through NMDA receptors that have been activated by tPA10. Because glucagon or PAI-1DP by itself failed to break this cycle, we hypothesized that combined administration of the PAI-1DP with glucagon might be more efficacious in yielding full protection, which was, in fact, observed. Possibilities for non maximal efficacy with single drug administration could relate to several factors, including choice of drug dose that was not high enough or activation of different pathways by these drugs, which, when combined, leads to an additive/synergistic intracellular response. By extension, the present data may also suggest that the re-reversal of NMDA receptor mediated vasoconstriction to dilation post FPI by glucagon and PAI-1DP is due to the activation of alternative pathways that override those that cause vasoconstriction and prevent it from occurring in the first place.
tPA is a serine protease that cleaves the zymogen plasminogen to produce the active serine protease plasmin. In the intravascular compartment, blood clots are formed by platelets and a fibrin meshwork. Intravascular fibrinolysis is mediated by plasmin generated chiefly through the action of tPA. Termination of tPA’s catalytic activity in blood is mediated by serine protease (serpin) inhibitors, chiefly plasminogen activator inhibitor I. Inactive tPA/plasminogen activator inhibitor I complexes are cleared from the circulation by low the density receptor-related protein (LRP). Plasminogen activators may also initiate intracellular signaling by binding to LRP17,18, through other pathways that are independent of its catalytic activity5,11. Signaling can be inhibited by a hexapeptide, EEIIMD, derived from plasminogen activator inhibitor-15,11,12. This peptide interacts with the plasminogen activator inhibitor-1 receptor docking site in tPA, which lies outside of its catalytic center and therefore does not affect its fibrinolytic activity7,11. This peptide also provides neuroprotection against endogenous and exogenous recombinant tPA (rtPA) in models of stroke and traumatic brain injury7,19. Recent studies show that a novel 18 amino acid plasminogen activator inhibitor-1 derivative based on this peptide, Ac-RMAPEEIIMDRPFLYVVR-amide (PAI-1 derived peptide), extends the therapeutic window in mechanical and thromboembolic models of stroke20,21. For this reason, this peptide was used in the present study.
Whereas it is well known that tPA aggravates excitotoxic neuronal cell death, little is known regarding the role of NMDA receptor mediated vascular activity in neuropathologic outcome. Activation of NMDA receptors elicits cerebrovasodilation and may represent one of the mechanisms that couple local metabolism to blood flow1. In healthy brain, tPA is critical for the full expression of the flow increase evoked by activation of the mouse whisker barrel cortex22. tPA promotes nitric oxide (NO) synthesis that follows NMDA receptor activation by modulating the phosphorylation state of neuronal nitric oxide synthase22. These findings suggest that tPA is a key mediator linking NMDA receptor activation to NO synthesis and functional hyperemia.
In contrast, in the injured brain our recent studies show that tPA aggravates FPI induced reversal of NMDA receptor mediated pial artery vasodilation to vasoconstriction by upregulating ERK and JNK isoforms of mitogen activated protein kinase (MAPK)5,10. A potential explanation for the differential role of tPA in normal and injured brain could relate to increased superoxide production after FPI23, which together with increased NO generates excessive peroxynitrite. Once formed, peroxynitrite could impair cerebrovasodilator systems post injury. However, the severity of constriction observed with NMDA after FPI + tPA is substantial and probably not the sole result of loss of a dilator, such as NO scavenging by superoxide, but also production of a vasoconstrictor. While the identity of this vasoconstrictor is not known with certainty, endothelin (ET-1) may play a role since it is upregulated and contributes to impaired dilation induced by NMDA receptor activation after FPI 24.
Reversal of NMDA induced vasodilation to vasoconstriction is important since administration of the NMDA antagonist MK801 protects against impairment of cerebral autoregulation after FPI in the pig13. However, the toxicity of MK 801 has limited translating this approach to the human condition, though another NMDA antagonist, mementine, has shown some promise. Therefore, despite the key role of excitotoxicity in outcome after TBI, use of NMDA antagonists to treat brain injury has not succeeded to date in the clinical setting. Combined administration of glucagon + PAI-1DP provides an alternative approach to limit tPA-NMDA interactions that avoids the potential toxicity associated with use of NMDA antagonists.
Notably, the data in the present study also identify another potential mechanistic target (MAPK) for prevention of tPA-NMDA receptor mediated loss of autoregulation. JNK and ERK concentrations in CSF are elevated after FPI, with the JNK isoform predominating. Increases in CSF concentration of MAPK isoforms were blocked with combined glucagon + PAI-1DP. SP 600125 blocked elevations of CSF JNK while CSF JNK was unchanged after U 0126. Similarly, elevated CSF ERK was blocked by U 0126, but unchanged by SP 600125. These data are supportive of efficacy and selectivity in the use of these MAPK isoform antagonists as probes in the investigation of the functional significance of interactions between tPA, MAPK, and the NMDA receptor. Glucagon and PAI-1DP by themselves only blunted, but did not block, elevations of CSF JNK and ERK MAPK. CSF concentrations reflect events in the brain parenchyma, as shown by the finding that changes in CSF ERK parallel those seen in parietal cortex after FPI and global cerebral hypoxia/ischemia10,25. A limitation of the closed cranial window to quantify substances in CSF is that neither the cellular site of origin nor the cellular site of action can be determined. Potential sources include neurons, glia, vascular smooth muscle, and endothelial sources.
Pial artery dilation during hypotension, in part, is due to release of prostaglandins, such as PGE2 and PGI215, which elicit cerebrovasodilation via cAMP. FPI impairs PGE2 and PGI2 mediated pial artery dilation16, which probably contributes to disturbed cerebral autoregulation after TBI. The relationship between NMDA and tPA in the context of impaired PG mediated vasodilation after TBI has not been explored to our knowledge. In particular, we were interested in the ability of glucagon to decrease CNS glutamate, prevent its lowering effect on CSF cAMP concentration and thereby prevent tPA upregulation after FPI and we asked how the latter may influence vascular reactivity to PGs after FPI. In this study, PGE2 and PGI2 induced pial artery dilation was blunted after FPI, partially protected by glucagons or PAI-1DP, but fully protected by their co-administration. Similar to what was observed with NMDA-receptor and hypotension-induced pial artery responses, co-administered glucagon + SP 600125 and glucagon + U 0126 also fully protected PGE2 and PGI2 mediated cerebrovasodilation. These data suggest that glucagon and PAI-1DP protect prostaglandin induced cerebrovasodilation via mechanisms that involve restoration of CSF cAMP and prevent tPA signaling involving JNK and ERK MAPK.
The present data, by showing the importance of maintaining cerebral hemodynamics, argue against the prevailing dogma that NMDA receptor toxicity is mediated exclusively through calcium dependent neuronal apoptosis and necrosis. We propose that, in the context of the neurovascular unit, the vicious cycle created by tPA and NMDA and its effect on CBF is a key element in determining outcome after brain injury. We propose that breaking this vicious cycle through combined administration of glucagon + PAI-1DP prevents the reduction in pial artery diameter, impairment of autoregulation, and neuronal cell necrosis after TBI. These studies hold the potential to re-engineer the standard of care for TBI.
Acknowledgments
This research was supported by grants from the National Institutes of Health, NS53410 and HD57355 (WMA), HL76406, CA83121, HL76206, HL07971, and HL81864 (DBC), HL77760 and HL82545 (AARH), the University of Pennsylvania Research Foundation (WMA), the University of Pennsylvania Institute for Translational Medicine and Therapeutics (DBC), and the Israeli Science Foundation (AARH).
Footnotes
Conflicts of Interest
Nothing to declare.
References
- 1.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 2.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 3.Katayama Y, Becker DP, Tamura T, et al. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 4.Merchant RE, Bullock MR, Carmack CA, et al. A double blind, placebo controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann NY Acad Sci. 1999;890:41–50. doi: 10.1111/j.1749-6632.1999.tb07979.x. [DOI] [PubMed] [Google Scholar]
- 5.Armstead WM, Cines DB, Higazi AAR. Plasminogen activators contribute to age dependent impairment of NMDA cerebrovasodilation after brain injury. Dev Brain Res. 2005;156:139–146. doi: 10.1016/j.devbrainres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Tsirka SE, Strickland S, et al. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 7.Armstead WM, Nassar T, Akkawi S, et al. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nature Neuroscience. 2006;9:1150–1157. doi: 10.1038/nn1757. [DOI] [PubMed] [Google Scholar]
- 8.Armstead WM, Cines DB, Bdeir K, et al. uPA modulates the age dependent effect of brain injury on cerebral hemodynamics through LRP and ERK MAPK. J Cereb Blood Flow Metab. 2009;29:524–533. doi: 10.1038/jcbfm.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstead WM, Kiessling JW, Cines DB, et al. Glucagon protects against impaired NMDA- mediated cerebrovasodilation and cerebral autoregulation during hypotension after brain injury by activating cAMP protein kinase A and inhibiting upregulation of tPA. J Neurotrauma. 2011;28:451–457. doi: 10.1089/neu.2010.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstead WM, Kiessling JW, Riley J, et al. tPA contributes to impaired NMDA cerebrovaso- dilation after traumatic brain injury through activation of JNK MAPK. Neurological Research. 2011;33:726–733. doi: 10.1179/016164110X12807570509853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassar T, Akkawi S, Shin A, et al. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897–202. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- 12.Akkawi S, Nassar T, Tarshis M, et al. LRP and avB3 mediate tPA- activation of smooth muscle cells. AJP. 2006;291:H1351–H1359. doi: 10.1152/ajpheart.01042.2005. [DOI] [PubMed] [Google Scholar]
- 13.Armstead WM. Age dependent NMDA contribution to impaired hypotensive cerebral hemo- dynamics following brain injury. Develop Brain Res. 2002;139:19–28. doi: 10.1016/s0165-3806(02)00511-4. [DOI] [PubMed] [Google Scholar]
- 14.Fanne RA, Nassar T, Mazuz A, et al. Neuroprotection by glucagons: role of gluconeogenesis. J Neurosurgery. 2011;114:85–91. doi: 10.3171/2010.4.JNS10263. [DOI] [PubMed] [Google Scholar]
- 15.Armstead WM. Age and cerebral circulation. Pathophysiology. 2005;12:5–1. doi: 10.1016/j.pathophys.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Armstead WM. Brain injury impairs prostaglandin cerebrovasodilation. J Neurotrauma. 1998;15:721–729. doi: 10.1089/neu.1998.15.721. [DOI] [PubMed] [Google Scholar]
- 17.Bu G, Williams S, Strickland DR, et al. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. PNAS. 1992;89:7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nassar T, Haj-Yehia A, Akkawi S, et al. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. Journal of Biological Chemistry. 2002;277:40499–404504. doi: 10.1074/jbc.M207172200. [DOI] [PubMed] [Google Scholar]
- 19.Tan Z, Li X, Kelly k, Rosen C, et al. Plasminogen activator inhibitor type I derived peptide, EEIIMD, diminishes cortical infarct but fails to improve neurological function in aged rats following middle cerebral artery occlusion. Brain Res. 2009;1281:84–90. doi: 10.1016/j.brainres.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanne RA, Nassar T, Yarovoi S, et al. Bloodbrain barrier permeability and tPA-mediated neurotoxicity. Neuropharm. 2010;58:972–980. doi: 10.1016/j.neuropharm.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstead WM, Riley J, Kiessling JW, et al. Novel plasminogen activator inhibitor -1 derived peptide protects against impairment of cerebrovasodilation after photothrombosis through inhibition of JNK MAPK. Am J Physiol. 2010;299:R480–R485. doi: 10.1152/ajpregu.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park L, Gallo EF, Anrather J, et al. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci USA. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni M, Armstead WM. Relationship between NOC/oFQ, dynorphin, and COX- activation in impaired NMDA cerebrovasodilation after brain injury. J Neurotrauma. 2002;19:965–973. doi: 10.1089/089771502320317113. [DOI] [PubMed] [Google Scholar]
- 24.Armstead WM. Age dependent endothelin contribution to NOC/oFQ induced impairment of NMDA cerebrovasodilation after brain injury. Peptides. 2001;22:39–46. doi: 10.1016/s0196-9781(00)00354-5. [DOI] [PubMed] [Google Scholar]
- 25.Armstead WM, Cines DB, Bdeir K, et al. uPA impairs cerebrovasodilation after hypoxia/ischemia through LRP and ERK MAPK. Brain Res. 2008;1231:121–131. doi: 10.1016/j.brainres.2008.06.115. [DOI] [PMC free article] [PubMed] [Google Scholar]